Abstract
The oocyte of the domestic dog is unique from that of other mammalian species studied to date. Ovulation occurs either once or twice per year, with the oocyte released at the germinal vesicle stage and then completing nuclear and cytoplasmic maturation within the oviduct under the influence of rising circulating progesterone. In vivo meiotic maturation of the bitch oocyte is completed within 48 to 72 h after ovulation, which is longer than 12 to 36 h required for oocytes from most other mammalian species. Due to these inherently novel traits, in vitro culture systems developed for maturing oocytes of other species have been found inadequate for maturation of dog oocytes. On average, only 15 to 20% of ovarian oocytes achieve the metaphase II stage after 48 to 72 h of in vitro culture. Thus far, no offspring have been produced in the dog (or other canids) by transferring embryos derived from in vitro matured oocytes. This review addresses current knowledge about dog reproductive physiology, specifically those factors influencing in vitro developmental competence of the oocyte. This summary lays a foundation for identifying the next steps to understanding the mechanisms regulating meiotic maturation and developmental competence of the dog oocyte.
Keywords: Dog oocyte, Folliculogenesis, Oogenesis, In vitro maturation
1. Introduction
For thousands of centuries, the domestic dog has served as a primary companion animal to humans (Vila et al., 1999; Leonard et al., 2002; Savolainen et al., 2002). There are more than 30 million families in the United States that own one or more pet dogs (Patronek, 1994) that in some cases also serve to help hunt, farm and even assist the disabled. Besides being ‘man’s best friend’, the domestic dog also has a historic and growing role as an animal research model for studying human diseases. There are more than 370 canine genetic disorders (half of which are breed-specific) and many of which resemble similar diseases and dysfunctions occurring in humans (Patterson et al., 1982; Ostrander et al., 2000; Ostrander and Kruglyak, 2000; Patterson, 2000; Kirkness et al., 2003). Retaining these genotypes requires high maintenance (i.e., cost to maintain a colony and veterinary care) and intensive breeding. However, the dividend is an array of canine genotypes that are invaluable for studying the mechanisms and etiology of human genetic diseases, especially rare recessive disorders with complex inheritance that are difficult to study in human populations (Patterson, 2000). The significance of the species as a model is best illustrated by the contemporary dog genome project that has received substantial attention and financial support from the National Institutes of Health (O’Brien and Murphy, 2003).
Although researchers have made significant progress in mapping dog genomic sequences, the scientific community has been oddly incapable of enhancing or controlling reproduction in this species. This is partially explained on the basis of the cost to maintain laboratory-based dog colonies (i.e., the lack of adequate numbers of accessible research colonies). However, our inability to regulate or promote reproductive success in the dog also is due to inherent physiological uniqueness of the species and to the lack of research attention (few laboratories study dog reproductive biology). Overall knowledge in this species compared to others is rudimentary at best, and especially for the oocyte. The latter is a particularly important target because an understanding of the mechanisms controlling oocyte maturation and development would be helpful to developing assisted breeding in this species, especially embryo production and transfer. Such technologies would be useful for managing specialized dog colonies (or breeds), as well as rescuing genetic material from individuals that fail to reproduce or are genetically under-represented in the population, geriatric, terminally ill or recently deceased. If embryo techniques become consistently effective in the domestic dog, such technologies can then be applied to rare canid species. Currently, there are 36 species in the family Canidae of which nine are formally listed as threatened or endangered by extinction (IUCN, 2002). Assisted breeding could be important for improving the genetic management of rare populations maintained ex situ (in captivity) as insurance for counterparts living in nature (Wildt et al., 1993; Wildt and Roth, 1997; Asher et al., 1999; Pope, 2000; Pukazhenthi and Wildt, 2004).
The first study on in vitro maturation (IVM) and fertilization (IVF) of dog oocytes was reported 30 years ago by Mahi and Yanagimachi (1976). In that study, approximately 25% of ovarian oocytes obtained from bitches at random stages of the reproductive cycle were able to resume meiosis and developed to metaphase I (MI) or to MII stages after being cultured for 48 to 72 h. In vitro insemination of cultured oocytes resulted in 70% of oocytes penetrated by spermatozoa and 20% containing decondensed sperm head(s). Since that report, little progress has been made, and, on average, only 20% of ovarian oocytes complete nuclear maturation in vitro (Cinone et al., 1992; Yamada et al., 1992, 1993; Nickson et al., 1993; Bolamba et al., 1998, 2002; Hewitt and England, 1998, 1999a, Hewitt and England, b; Otoi et al., 1999, 2000, 2001, 2002; Luvoni et al., 2001, 2003; Saint-Dizier et al., 2001a, b; Songsasen et al., 2002, 2003b; Rodrigues and Rodrigues, 2003a, b; Kim et al., 2004, 2005; Rodrigues et al., 2004; de los Reyes et al., 2005). As a consequence of this inability to develop a consistently effective IVM system, other techniques that require in vitro oocyte culture (e.g., IVF) also have met with limited success (Yamada et al., 1992; Fulton et al., 1998; Otoi et al., 2000; England et al., 2001; Songsasen et al., 2002; Rodrigues et al., 2004). For example, over the past 30 years, there has been only a handful of studies reporting embryonic development in the dog after IVM/IVF (Yamada et al., 1992; Otoi et al., 2000; England et al., 2001; Songsasen et al., 2002; Rodrigues et al., 2004). One reported producing a single blastocyst after in vitro insemination of more than 200 oocytes (Otoi et al., 2000), and another described a single, non-full term pregnancy after transferring close to 100 presumptive zygotes to recipient bitches (England et al., 2001). Although domestic dog puppies were recently produced from somatic cell nuclear transfer of in vivo matured oocytes (Lee et al., 2005), there has been no report on the production of live young from transferring IVM/IVF-derived embryos.
Here we discuss current relevant knowledge on dog reproductive physiology and folliculogenesis, largely because it is purported that species uniqueness has limited technology effectiveness to date (Luvoni et al., 2005). This is followed by reviewing oocyte growth and maturation, especially aspects of intracellular changes during these processes and those factors that influence oocyte developmental competency in vitro. This information also will be integrated to give the reader a perspective on high priority future research needs in this arena.
2. Species reproductive norms, including ovarian folliculogenesis
2.1. Fundamental reproductive cycle interrelationships
The reproductive physiology of the domestic bitch is distinct from other common species, as the dog generally is nonseasonal and monoestrous, ovulating only once or twice a year at a 5 to 12 month interval (reviewed by Concannon et al., 1989). The dog’s reproductive cycle is characterized by a protracted proestrus and estrus (each about 1 week in duration). Irrespective of whether pregnancy occurs, estrus is followed by diestrus (metestrus), a luteal phase of elevated circulating progesterone with an average duration of about 2 months (Concannon et al., 1989; Johnston et al., 2001). Toward the end of diestrus, corpus luteum function declines as the bitch enters a prolonged anestrus period of 2 to 10 months duration. Anestrus is characterized by the lack of any sexual behavior or gonadal activity, including nadir circulating progesterone concentrations. From mid- to late anestrus, there is an increase in hypothalamic gonadotropin releasing hormone (GnRH) secretion that elicits an increase in follicle stimulating hormone (FSH) and episodic LH release. FSH plays a crucial role in the initiation of folliculogenesis and the onset of proestrus in the bitch (Kooistra et al., 1999; Kooistra and Okkens, 2001; Beijerink et al., 2004). Toward the end of proestrus, the LH surge stimulates a rapid enlargement of mature follicles and preovulatory luteinization that leads to ovulation 40 to 50 h after the LH peak (Wildt et al., 1977; Concannon et al., 1989). Behavioral estrus and ovulation occur in the presence of declining circulating estrogen and significantly elevated progesterone (Wildt et al., 1979).
2.2. Ovarian folliculogenesis
Information on follicular development in the dog is rudimentary. Formation of oogonia can be recognized in the dog fetus around Day 42 post-coitum (Andersen and Simpson, 1973). Dog follicles can be categorized into five classes depending on morphology, size, type and number of follicle cell layers and the presence of follicular fluid. Data in Table 1 summarizes follicle classification and days in which each class is initially found in the ovaries. Primordial follicles form from Day 17 to 54 after birth (Andersen and Simpson, 1973; Tesoriero, 1981; Blackmore et al., 2004) and contain small oocytes (approx. 25 μm in diameter) with a single granulosa cell layer but no zona pellucida (ZP) at this stage (Durrant et al., 1998; Blackmore et al., 2004). Primary or early preantral follicles occur around Day 120 after birth and contain small, pale oocytes (78 ± 15 μm) with a distinctive ZP (Durrant et al., 1998; Barber et al., 2001). Sizes of primordial and primary follicles are not uniquely different from each other (Barber et al., 2001); however, these significantly increase in size during the transformation to secondary and tertiary stages due to proliferation and differentiation of follicle cells, as well as growth of the oocyte and ZP (Barber et al., 2001). Secondary or advanced preantral follicles contain fully grown oocytes (>100 μm) that are comprised of dark cytoplasmic lipid (Figure 1, Durrant et al., 1998; Barber et al., 2001). Synthesis of follicular fluid is known to occur in tertiary or early antral follicles which can be observed from Day 120 through 160 after birth (Andersen and Simpson, 1973; Durrant et al., 1998; Barber et al., 2001). Thus, the time required for the recruitment of primordial follicles to early antral follicles is around 70 to 150 days in prepubertal bitches (Andersen and Simpson, 1973), which is similar to the required 110 days for adult dogs (Spanel-Borowski and Calvo, 1982). Finally, advanced antral follicles are present in bitches as young as 6 months of age during mid-proestrus (Concannon et al., 1989). As a result of the LH surge, advanced antral follicles rapidly enlarge into 4 to 13 mm preovulatory follicles (Wildt et al., 1979; Concannon et al., 1989; England and Allen, 1989) and ovulate approximately 48 h later.
Table 1.
Follicle classification and diameter in relation to fetal (or bitch) age during which each class are found within the ovary
| Classifications | Age (days) | Follicle diameter (mm) | References |
|---|---|---|---|
| Primordial | 17–54 | 0.04 – 0.05 | Andersen and Simpson (1973); Tesoriero (1981); Barber et al. (2001); Blackmore et al. (2004) |
| Primary or preantral | 120 | 0.050 – 0.08 | Durrant et al. (1998); Barber et al. (2001); |
| Secondary or advanced preantral | NA | 0.148 – 0.211 | Durrant et al. (1998); Barber et al. (2001) |
| Tertiary or early antral | 120–160 | 0.350 – 0.360 | Durrant et al. (1998); Barber et al. (2001). |
| Advanced antral | 240 | 2–13 | Wildt et al. (1977); England and Allen (1989) |
Fig. 1.
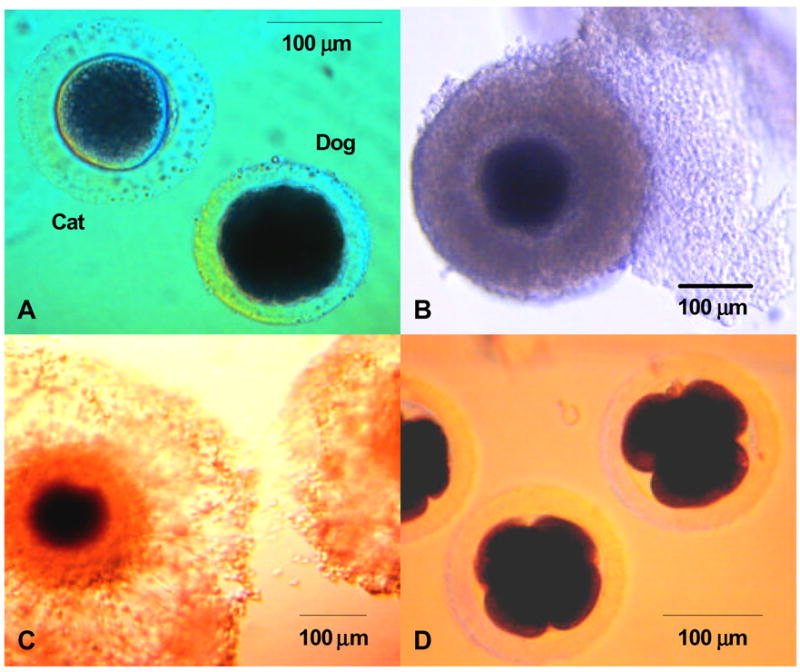
Photomicrographs of: (A) domestic dog and cat oocytes; (B) a dog oocyte surrounded by several layers of cumulus cells; (C). a dog oocyte after 48 h culture in a medium supplemented with 1 to 10 μg/ml bovine somatotropin; and (D). dog embryos (48 post insemination) derived from in vitro matured oocytes.
Most growing follicles in the dog undergo atresia before reaching the preovulatory stage. Secondary follicles (or advanced preantral follicles) exhibit two types of regression patterns. In Type A the degeneration is characterized by necrotic changes of the oocytes and the ZP, whereas Type B involves granulosa cell necrosis (Spanel-Borowski, 1981). Type B degeneration results in development of a pseudoantrum, a structure similar to that found in a tertiary follicle (Spanel-Borowski, 1981). It has been shown that condensation of chromosomes and wrinkling of the nuclear envelope of the oocyte (similar to that of germinal vesicle breakdown; GVBD) is indicative of atresia in growing follicles (Baker, 1982). This is important in making accurate assessments of oocyte quality. During the physical recovery of oocytes from recovered dog ovaries there is a high incidence of these cells that appear to be undergoing GVBD (Hewitt and England, 1998b; Bolamba et al., 2002). However, it is likely that the observation of many GVBD oocytes really represents an unusually greater incidence of degenerative processes rather than a true resumption of meiosis.
Bitch age significantly influences numbers of follicles within the ovary (Telfer and Gosden, 1987; McDougall et al., 1997). Ovaries of peripubertal bitches (i.e., 6 to 10 months of age) contain significantly more follicles than those of prepubertal (i.e., < 6 months old) and mature counterparts (i.e., > 10 months old; McDougall et al., 1997). Telfer and Gosden (1987) reported that follicle numbers declined from ~85,000 in 1 to 2 year old bitches to only 3,000 in 7 to 11 year old individuals. Therefore, there is a marked increase in follicular atresia before first estrus and then what is likely a continuous loss in viability for the first decade of life. The development of > 100 μm diameter follicles does not occur until shortly before the first estrus (McDougall et al., 1997). One intriguing feature of dog gamete biology is that the bitch produces an unusually high incidence (11%) of polyovular follicles (more than one oocyte/follicle), considerably greater than, for example, the cat (4%) human (3%) and rhesus monkey (2%) (Telfer and Gosden, 1987). The cause and significance of polyovular follicles remains unclear, but perhaps results from a greater density of primordial follicles in the ovarian cortex of the young dog so that some follicles actually merge with adjacent ones (Luvoni et al., 2005). Polyovular follicles do not appear until shortly before the first estrus (Andersen and Simpson, 1973), and their number within the ovary is not influenced by bitch age (McDougall et al., 1997). Interestingly, within the same follicle, the companion oocytes can be at different stages of development or simultaneously one can be viable whereas the other is atretic (Barber et al., 2001).
3. Oocyte growth
3.1. Structural aspects
The ultrastructure of dog oocytes at various stages has been described (Tesoriero, 1981). The oocyte of the primordial follicle contains a large, central nucleus with nucleolus, many large, rounded mitochondria, smooth endoplasmic reticulum and small Golgi bodies. At this early developmental stage, the interconnection between the oocyte and follicle cells is incompletely established (Tesoriero, 1981). The ZP in the oocyte of the primary follicle begins to form and becomes more evident with cell growth. A more recent ultrastructural study has demonstrated that the dog ZP is characterized by a mesh of fibrous network with numerous fenestrations of intermediate size (Ström Holst et al., 2000). The thickness of the ZP of a fully grown oocyte is ~10 μm, which interestingly is only two-thirds of that of another common carnivore, the cat (Figure 1A) (Barber et al., 2001).
Mitochondria are present in increasing numbers throughout the oocyte’s growth period, reflecting a natural increase in metabolic activity (Tesoriero, 1981). The cytoplasm of oocytes from primary follicles has granular matrices and contains strands or lamellae of smooth endoplasmic reticulum. Golgi bodies also increase in number. Lipid yolk bodies first appear in the primary oocyte of growing follicles and increase throughout the entire process of oogenesis (Tesoriero, 1981, 1982). Although there is evidence for oocyte-granulosa cell contact at this time, the two membranes are not fused (Tesoriero, 1981). In early antral follicles, the oocytes fill with large, rounded mitochondria, and numbers of lipid yolk bodies continue to increase. Cortical granules appear in the cortical region during this stage of oocyte development. Regions of oocyte cytoplasm adjacent to the granulosa cell contact areas contain large Golgi bodies and several mitochondria. Throughout development, the oocyte continues to increase in size with increasing numbers of lipid yolk bodies giving it a dark appearance that is markedly different from other noncarnivore mammalian species (Figure 1B) (Guraya, 1965; Tesoriero, 1982; Durrant et al., 1998). Thus far, it is unclear how this large amount of intracellular lipid influences dog oocyte maturation and development. Nevertheless, we speculate that some of the challenges associated with maturing dog oocytes using conventional media and culture conditions are related to the inherently high intracellular lipid. Bitch oocytes may require different culture conditions from those with lower lipid contents to allow them to successfully mature and develop in vitro.
3.2. Biochemical aspects
3.2.1. Lipid yolk
Precursors of lipid yolk are transported from follicle cells through the ZP (Guraya, 1965). In dogs of various breeds, analysis of total lipids extracted from oocytes has revealed that intracellular lipids include saturated fats, triglyceride, cholesterol, phospholipids and glycolipid. Furthermore, types and amounts of lipid fraction are consistent among individuals within and between breeds (Tesoriero, 1982).
3.2.2. Zona pellucida
The ZP is easily visible in oocytes of developing secondary follicles (although it is smaller in primary follicles and completely absent in primordial counterparts) (Barber et al., 2001; Blackmore et al., 2004). There is remarkable species-specific carbohydrate structure within the ZP and its cellular origin (Skutelsky et al., 1994; Barber et al., 2001; Blackmore et al., 2004). Unlike the mouse ZP that is exclusively synthesized by the oocyte (Rankin et al., 2000), dog ZP proteins originate from both granulosa cells and the oocyte and are synthesized and expressed in a sequential fashion (Blackmore et al., 2004). Additionally, expression of the ZP3 gene in dog oocytes cultured in vitro is dependent on the number of cumulus cell layers surrounding the oocyte (Nickson et al., 1993). Oocytes with one layer of cumulus cells show no (or little) ZP3 transcription during in vitro culture, and coincidentally none of these oocytes are able to complete nuclear maturation in vitro. However, oocytes with two or more layers of cumulus cells experience a rise in ZP3 expression with the peak of ZP3 transcripts occurring from 24 to 48 h after culture (Nickson et al., 1993). Moreover, oocytes surrounded by > 2 layers of cumulus cells complete nuclear maturation at a greater frequency than those surrounded by fewer layers (Nickson et al., 1993). This suggests that cumulus cells play a crucial role in modulating dog oocyte nuclear maturation.
4. Oocyte maturation
Ovulation occurs from 2 days before estrus through 7 days after estrus (Phemister et al., 1973) with most bitches ovulating by the third day after sexual receptivity (Holst and Phemister, 1971; Phemister et al., 1973; Tsutsui, 1989). The most distinctive feature of dog reproduction is that oocytes are ovulated at the onset of the first meiotic division (i.e., the germinal vesicle or GV stage; Pearson and Ender, 1943). Preovulatory follicles (4 to 13 mm diameter) release oocytes (118 to 135 μm diameter) into the oviduct where GVBD occurs within 48 h (Holst and Phemister, 1971; Farstad et al., 1989; Tsutsui, 1989; Renton et al., 1991; Reynaud et al., 2005). Neither breed nor age of the bitch influences maturational kinetics of dog oocytes (Reynaud et al., 2005). Nuclear maturation is completed 48 to 72 h post-ovulation in the presence of elevated circulating progesterone (Wildt et al., 1978; Concannon et al., 1989; Reynaud et al., 2005), when the oocyte reaches the mid-portion of the oviduct (Tsutsui, 1989). A detailed study of oocytes/embryos obtained from 17 to 138 h post-ovulation demonstrated that the first MII oocyte was observed at 54 h after oocytes were released from the follicle (Reynaud et al., 2005). Cumulus expansion has been observed as oocytes mature within the oviduct. However, the innermost layer of corona radiata remains attached to the ovum after fertilization and until embryonic development to the morula stage (Pearson and Enders, 1943; Holst and Phemister, 1971; Renton et al., 1991). Fertilized dog oocytes are sustained within the oviduct for 9 to 10 days and then enter the uterus at the morula stage (Holst and Phemister, 1971; Tsutsui, 1989; Renton et al., 1991) which is 3 to 5 days longer than reported for the cat (Swanson et al., 1994), cow (Gordon, 1994) and mouse (Hogan et al., 1986).
Because mating can occur as early as 3 days before ovulation (Renton et al., 1991), the immature dog oocyte and spermatozoon meet in the oviduct, and sperm penetration may be involved in inducing nuclear maturation, i.e., resumption of meiosis (Saint-Dizier et al., 2001a, b). However, in vivo studies have reported that fertilization does not occur until 44 to 120 h after ovulation when the oocyte has completed nuclear maturation (Tsutsui, 1989; Badinand et al., 1993; Reynaud et al., 2005). These observations in vivo contradict those made in vitro. For example, Mahi and Yanagimachi (1976) demonstrated that maturation of the dog oocyte is not a prerequisite for the spermatozoon to penetrate the ZP and undergo nuclear decondensation. Additionally, Saint-Dizier et al. (2001a, b) recently have reported that dog oocytes penetrated by spermatozoa in vitro resume meiosis and develop beyond MI at a greater proportion than those devoid of spermatozoa. Thus far, there is no clear explanation for the discrepancy between in vivo versus in vitro observations. It is known that oviductal cells can maintain longevity of dog sperm by inhibiting calcium flux that, in turn, prevents sperm from undergoing capacitation (Kawakami et al., 2001). Additionally, dog spermatozoa remain viable in vivo for as long as 11 days after mating (Doak et al., 1967). Based on these observations, it is possible that the discrepancy between in vivo and in vitro studies is related to delayed sperm capacitation within the oviduct rather than the inability of oocytes to be fertilized.
There have been few studies on structural and biochemical alterations during oocyte maturation in the dog. During meiosis, it is known that dog oocytes undergo cytoskeletal changes similar to those observed in other mammalian species (Verlhac et al., 1994; Dedieu et al., 1996; Kim et al., 1996; Velilla et al., 2005). Germinal vesicle-stage oocytes contain an extensive array of subcortical microtubules and perinuclear tubulins. However, no well organized microtubule network is detected at this developmental stage (Saint-Dizier et al., 2004). In GVBD oocytes, a clear network of microtubules appears, and small tubulin asters are visible throughout the ooplasm. At the MI stage, chromosomes are highly compacted, and microtubules are restricted to the meiotic spindle. Microtubule organization of MII oocytes is similar to, but smaller than that of MI oocytes (Saint-Dizier et al., 2004).
There also is a paucity of information on cell cycle control in canid oocytes. However, it has been shown that activation of M-phase promoting factor (MPF) and mitogen activating protein kinase (MAPK) is dependent on meiotic stage (Saint-Dizier et al., 2004). Kinase activities are minimal in GV and GVBD oocytes, but significantly increase when oocytes develop to MI and MII stages. Cumulus cells control MAPK activation and phosphorylation of blue fox (Alopex lagopus) oocytes: ova completely surrounded by several layers of cumulus cells exhibit higher MAPK activities than those surrounded only by the corona radiata (Kalab et al., 1997). Again, this confirms a significant role(s) of cumulus cells in regulating development and maturation of canid oocytes.
5. Factors influencing developmental competence of oocytes cultured in vitro
5.1. Meiosis
Dog oocytes, like those of other species, are able to resume meiosis in vitro, but are not fully competent to complete nuclear maturation. Since the first study reported by Mahi and Yanagimachi (1976), numerous studies have been conducted on the impact of several factors known to affect oocyte meiotic competency in other species.
5.2. Oocyte donor age, breed, stage of reproductive cycle and size of ovarian follicle
Age of the dog influences number of oocytes recovered from the ovary for maturation studies. Durrant et al. (1998) and Ström Holst et al. (2001) have demonstrated that follicle and oocyte yield were greatest in 1 to 6 year old bitches compared to younger (< 12 months) or older (≥ 7 years) counterparts. It has been suggested that intraovarian oocytes from prepubertal bitches (age 4 to 6 months) are incapable of resuming and completing meiosis (Haenisch et al., 2003). At this stage of the dog’s life cycle, there seems to be few cell-to-cell connections between the cumulus and either neighboring granulosa cells or the oocyte itself (Haenisch et al., 2003). Oocytes from prepubertal bitches also have low protein synthesis, yet high metabolic and transcription activities (Haenisch et al., 2003) indicating that these cells have not fully acquired developmental competence. In contrast, when bitches are 6 months (peripubertal) to 7 years (fully mature) of age, there is no influence of donor age on oocyte meiotic competence (Hewitt and England, 1998a; Songsasen et al., 2002). However, developmental abilities of the oocyte are decreased substantially in bitches beyond the latter age (Hewitt and England, 1998a). Although limited data are available, it does not appear that bitch breed influences in vitro nuclear maturational ability (Songsasen et al., 2002), although crossbred donors have yielded more ovarian follicles than purebred bitches (351.8 ± 52.4 versus 288.1 ± 43.6) (Durrant et al., 1998).
Published information on the relationship between stage of reproductive cycle and oocyte meiotic competence is contradictory. Some investigators have indicated no association (Cinone et al., 1992; Hewitt and England, 1997; Otoi et al., 2002; Rodrigues and Rodrigues, 2003b; Songsasen and Wildt, 2005), whereas others have demonstrated that reproductive cycle stage significantly impacts developmental capacity of the oocyte (Yamada et al., 1993; Luvoni et al., 2001; Otoi et al., 2001; Willingham-Rocky et al., 2003; Kim et al., 2004; Rodrigues et al., 2004). It does appear that oocytes obtained from preovulatory follicles and during the follicular (estrous/proestrous) phase complete nuclear maturation more successfully than those recovered during other reproductive stages (Yamada et al., 1993; Otoi et al., 2001; Kim et al., 2004). Willingham-Rocky et al. (2003) similarly found that more oocytes from estrous bitches develop to MII compared to those from proestrous and anestrous individuals. However, oocytes from diestrous females were as able to achieve MII in vitro as those from females in estrus. Stage of reproductive cycle also appears to influence fertilizing capacity because formation of pronuclei has been observed more frequently in intraovarian oocytes collected from bitches at the follicular compared to the luteal or anestrous stages (Rodrigues et al., 2004).
Differences in meiotic competence of oocytes across stages of the reproductive cycle are most likely due to size distribution of follicles and oocytes during a given stage. Otoi et al. (2001) have demonstrated that there are significant differences in mean (± SEM) diameter of follicular oocytes recovered during estrus, diestrus and anestrus (119.2 ± 0.7, 107.7 ± 0.7 and 103.6 ± 0.8 μm, respectively). Moreover, more ≥ 120 μm diameter oocytes are recovered in a follicular phase than in other reproductive stages (Otoi et al., 2001). This is important because we know that oocytes must be at least 100 to 120 μm (Otoi et al., 2000, 2001) to allow meiotic resumption, and nuclear maturation is enhanced in oocytes of 120 μm compared to smaller sizes (Otoi et al., 2001). Although oocyte size appears important, it does not seem to be the sole factor limiting IVM success. Only approximately 20% of large oocytes (i.e. > 120 μm in diameter) complete nuclear maturation in vitro (Otoi et al., 2000, 2001).
Recently, we demonstrated that follicle size significantly influences developmental competence of the dog oocyte (Songsasen and Wildt, 2005). As many as 80% of oocytes from follicles > 2 mm in diameter complete nuclear maturation in vitro compared to only 16 to 38% of those from smaller (0.5 to < 2 mm) source follicles (Songsasen and Wildt, 2005). Furthermore, we have discovered a relationship between stages of reproductive cycle and the distribution of different size follicles. For example, ovaries of anestrous and diestrous bitches contain more small follicles of < 0.5 mm diameter, whereas large follicles (> 2 mm) are mostly observed mostly during proestrus/estrus (Songsasen and Wildt, 2005). Nevertheless, small follicles can be observed within the ovaries of proestrous bitches, and occasionally a few large follicles can be found in some diestrous individuals. When data were pooled based on reproductive cycles, there were no significant differences in proportion of oocytes completing meiotic maturation among groups (Songsasen and Wildt, 2005). Therefore, it is apparent that the size of donor follicle is an important factor influencing developmental competency of the dog oocyte. Most investigators recover oocytes using a random slicing technique (Luvoni et al., 2005), thus resulting almost inevitably in a heterogeneous oocyte population that varies in developmental stage and ability to complete nuclear maturation. This no doubt at least partially explains differences in results among laboratories, across stages of the reproductive cycle and the overall lesser incidence of IVM success for the species as a whole.
In anestrous bitches, there is a lack of communication between the ooplasm and cumulus cells (Luvoni et al, 2001), and oocytes obtained during this reproductive stage may require different culture conditions from those obtained during other periods. For example, culture density significantly influences the ability of oocytes collected during anestrus to complete nuclear maturation in vitro (Otoi et al., 2002). At high density (20 oocytes/100 μl of culture medium), most oocytes obtained during anestrus (80%) arrest at the GV stage, while only 50% of those obtained during diestrus arrested. When the culture density was decreased to 10 oocytes/100 μl of culture medium, more oocytes obtained during anestrus resumed meiosis and completed nuclear maturation; these numbers were not significantly different from those of diestrous counterparts (Otoi et al., 2002). Therefore, it appeared that differences in meiotic competence between oocytes obtained from anestrous and diestrous bitches were eliminated by using optimal culture density.
5.3. Culture medium
Three culture media have been tested for effectiveness in supporting maturation of dog oocytes in vitro: TCM 199, synthetic oviductal fluid (SOF) and CMRL1066. Originally Hewitt and England (1999b) reported no differences in maturation success between canine oocytes cultured in a complex medium, TCM 199 versus SOF, a simple medium. But in a more recent study, TCM 199 was found to be superior to SOF based on numbers of oocytes resuming meiosis and developing to MI/AI/MII stages (Rota and Cabianca, 2004). Songsasen et al. (2002) compared two complex media, TCM 199 and CMRL 1066 (routinely used for IVM of rhesus monkey oocytes) and demonstrated that higher proportions of oocytes cultured in the former (7 to 16%) completed nuclear maturation compared to the latter (0–1%). From a constituency perspective, TCM 199 contains more vitamins and has a greater concentration of cysteine and ascorbic acid than CMRL. It has been shown that cysteine and ascorbic acid protect cells from oxidative stress and improve maturation of cow and mouse oocytes (de Matos et al., 1997; de Matos and Fernus, 2000; Eppig et al., 2000). Furthermore, Kim et al. (2004) showed that addition of a thiol compound, β-mercaptoethanol, to maturation media improved meiotic maturation of dog oocytes. Perhaps the presence of these antioxidants is crucial for maturation of dog oocytes that contain large amount of intracellular lipid and are likely to be susceptible to oxidative stress.
5.4. Culture interval
Dog oocytes require 48 to 72 h to complete nuclear maturation in vivo within the oviduct (Tsutsui, 1989; Reynaud et al., 2005), an inordinate time interval perhaps related to the inherent challenges of stimulating maturation in vitro. When recovered directly from ovarian follicles and incubated, some oocytes advance to the MII stage by as early as 24 h of culture (Robertson et al., 1992; Yamada et al., 1992, 1993; Nickson et al., 1993; Saint-Dizier et al., 2004). Increasing the culture period to 48 h has resulted in more oocytes completing nuclear maturation, but with few exceptions (Otoi et al., 2004; de los Reyes et al., 2005) a more protracted incubation (beyond 48 hours) fails to increase the total number of MII oocytes produced (Robertson et al., 1992; Yamada et al., 1992, 1993; Nickson et al., 1993; Songsasen et al., 2003b; Saint-Dizier et al., 2004) and even increases oocyte degeneration (Nickson et al., 1993; Luvoni et al., 2003; Songsasen et al., 2003b). Thus, the optimal interval for IVM of dog oocytes appears to be 48 h, which also correlates to best embryonic development after IVF (Otoi et al., 2004). Otoi et al. (2004) reported that more oocytes reached MII when they were cultured for 72 h than those cultured for only 48 h, but the optimum maturation period was found to be 48 h based on embryonic development after IVF.
5.5. Protein supplementation
The influence of protein supplementation on cultured dog oocytes has been examined extensively, albeit with conflicting results (Hewitt et al., 1998; Otoi et al., 1999; Bolamba et al., 2002; Songsasen et al., 2002; Rodrigues and Rodrigues, 2003a). Hewitt et al. (1998) demonstrated that supplementing culture media with 0.3% bovine serum albumin (BSA) or 10 to 20% fetal bovine serum (FBS) appeared optimal for dog oocyte maturation when TCM 199 was the culture medium. When SOF, a simple medium was used, there was a need for a greater concentration of BSA (4% w/v) to achieve a beneficial effect (Hewitt and England, 1999b). However, Bolamba et al. (2002) contradicted these results by finding that protein supplementation was nonessential for in vitro nuclear maturation of dog oocytes cultured in SOF. Likewise, Songsasen et al. (2002, 2003b) and Songsasen and Wildt (2005) reported greater proportions of MII oocytes after 48 h culture in a protein-free medium. Furthermore, in vitro insemination has resulted in 30% of these oocytes developing into early stage embryos (Songsasen et al., 2002).
Adding serum to maturation media results in greater percentages of oocytes with undistinguishable nuclear organization after maturation (Hewitt et al., 1998). Addition of BSA to media containing serum decreased the percentages of degenerated oocytes compared to serum alone (Bolamba et al., 2002). Yet most studies to date have used serum as the protein supplement in maturation media with the results being highly variable. Robertson et al. (1992) supplemented culture media with 20% estrous cow serum (ECS), estrous bitch serum (EBS) or FBS and found that more oocytes developed to MI/MII stage when cultured in the presence of FBS. Estrous bitch serum (which has greater concentrations of estradiol and progesterone; Otoi et al., 1999) has been found effective in supporting nuclear maturation in dog oocytes (Nickson et al., 1993; Otoi et al., 1999). However, (Rodrigues and Rodrigues, 2003a) demonstrated that there were no differences between BSA and ECS in supporting dog oocyte maturation, but ECS was superior to EBS. The reasons for the differences among studies are unclear, but may be due to how the bitch was classified at the time of blood collection (as a source for supplemental serum). For example, Otoi et al. (1999) categorized that status of the blood donor according to histological analysis of the ovaries. However, Rodrigues and Rodrigues (2003a) used clinical signs (i.e., receptivity to mounting) and vaginal cytology as criteria for classifying reproductive stage. Thus, it is likely that differences in progesterone and estradiol concentrations between the source of serum samples used to create the supplements may have contributed to the conflicting results between studies.
5.6. Hormonal supplementation
5.6.1. Estrogen and progesterone
It has been suggested that canine oocytes may require an environment with a greater estrogen concentration to stimulate nuclear maturation (Nickson et al., 1993). Kim et al. (2005) recently reported that adding estradiol-17β to maturation media significantly increased the proportion of dog oocytes developing to the MII stage in vitro. However, this effect only occurred when follicular phase oocytes were used, with minimal impact on those recovered during the anestrous or luteal phases. Progesterone or the combination of progesterone and estradiol also positively influence nuclear maturation of dog oocytes isolated during the follicular phase (Kim et al., 2005). In contrast, Willingham-Rocky et al. (2003) found no benefits of progesterone supplementation on meiotic maturation regardless of reproductive stage of the oocyte donor.
5.6.2. Gonadotropins
Unlike the domestic cat (Schramm and Bavister, 1995; Pope et al., 1997; Comizzoli et al., 2003), supplementation of culture medium with gonadotropins fails to promote meiotic maturation of the dog oocyte (Hewitt and England, 1999a). Furthermore, adding LH to IVM media for the entire culture period significantly reduces the proportion of dog oocytes developing to the MII stage (Songsasen et al., 2002). Although culturing oocytes with gonadotropins for the entire incubation period is not beneficial, short-term or temporary exposure of oocytes to FSH or LH may support nuclear maturation. For example, brief exposure of oocytes to FSH induces mouse cumulus cells to produce a meiosis activating agent (Byskov et al., 1997) and stimulates nuclear maturation and extrusion of the first polar body in mouse and pig oocytes (Byskov et al., 1997; Xia et al., 2000). Similar results also have been reported in the domestic dog wherein brief exposure of oocytes to equine chorionic gonadotropin (eCG) promotes resumption of meiosis and enhanced ability to develop to MI/MII stages (Songsasen et al., 2003b).
Because meiotic maturation occurs within the oviduct over a prolonged interval, the dog oocyte is exposed to a vastly fluctuating extracellular environment that includes substantial alterations in the endocrine milieu (Wildt et al., 1978; Concannon et al., 1989). Therefore, it could be that more successful nuclear maturation in the dog is dependent on a culture system that more effectively mimics the natural in vivo hormonal environment (Nickson et al., 1993; de los Reyes et al., 2005). Especially intriguing may be the role of LH in promoting early preovulatory follicular luteinization in the bitch (Wildt et al., 1978; Concannon et al., 1989). The induction of preovulatory follicular development using exogenous steroid (estrone) and gonadotropins (equine chorionic gonadotropin and human chorionic gonadotropin; hCG) is known to provoke IVM of the dog oocyte (Yamada et al., 1993). Furthermore, culturing dog oocytes in the presence of 10 IU of hCG for 48 h followed by an additional 48 h in its absence seems to enhance the incidence of MII formation (de los Reyes et al., 2005). In the latter study, supplementing culture medium with hCG promoted GVBD, probably by stimulating cumulus expansion and disassociation of gap junctions between the cumulus cells and oocytes. Interestingly, omitting hCG from the medium during the second half of the 96 h culture increased MII oocyte formation compared to those cultured with hCG for the entire period (or those cultured completely in its absence). In vivo, the dog oocyte completes nuclear maturation several days after the LH peak occurs (Concannon et al., 1989). Therefore, it is likely that LH exerts its influence on initiation of meiotic maturation through the cumulus cells (i.e., promotes cumulus expansion and disrupts communication between cumulus cells and the oocyte). However, its extended presence in vitro may not be essential or could even be detrimental to oocytes during their progression from GVBD to MII stages.
5.6.3. Growth hormone and growth factors
Supplementing IVM medium with bovine somatotropin (BST) enhances meiotic maturation in the blue fox oocyte in the absence of cumulus cell expansion (Sršeň et al., 1998). However, BST fails to improve the proportion of domestic dog oocytes developing to the MII stage, although it appears to enhance meiotic resumption (Songsasen et al., 2002). Furthermore, in contrast to observations in the blue fox (Srŝeň et al., 1998), exposure to growth hormone seems to promote cumulus cell expansion in the dog oocyte ( ure 1C) (Songsasen et al., 2002). Although expansion of the outer layer of cumulus granulosa cells has been observed, the corona radiata has remained tightly packed making it difficult to remove (Songsasen et al., 2002). Such patterns of cumulus expansion have been found in blue fox oocytes exposed to FSH, but in the absence of enhanced nuclear maturation (Srŝeň et al., 1998).
For several mammalian species, epidermal growth factor (EGF) in IVM medium is asserted to be beneficial to oocyte development (Gomez et al., 1993; Schramm and Bavister, 1995; Izadyar et al., 1998; Gall et al., 2005; Purohit et al., 2005; and see Farin et al., this volume). EGF activates MAP kinase and EGF-receptors in the cumulus cells causing cumulus cell expansion (Conti et al., 2006; Gall et al., 2005). In dogs, EGF influences meiotic maturation of oocytes in a dose-dependent manner. The greatest numbers of MII oocytes were obtained after 72 h of culture in the presence of 20 ng/ml of EGF (Kim et al., 2004). Furthermore, there appear to be synergistic effects between LH and EGF, and between EGF and estradiol on nuclear maturation (Bolamba et al., 2006). The proportion of oocytes developing to MI to MII stages increased when EGF was added to a medium containing LH or estradiol compared to those cultured with the gonadotropin or steroid alone. It has been suggested that LH enhances the meiotic promoting effect of EGF via the cumulus cells by stimulating EGF mRNA expression (Conti et al., 2006). Interestingly, adding EGF to medium containing FSH inhibits nuclear maturation in the dog (Bolamba et al., 2006), probably due to the inhibitory effect of EGF on differentiation of FSH-stimulated granulosa cells (Rusovici et al., 2005). Because of its possible interaction with gonadotropins and estrogen, it is not surprising that EGF has failed to promote canine oocyte maturation when FSH, LH and estrogen were present together in the culture medium (Rota and Cabianca, 2004).
5.7. Meiotic inhibitory substances
Dog oocytes from small follicles are inherently unable to easily achieve meiotic maturation in vitro (Bolamba et al., 1998; 2002; Songsasen and Wildt, 2005). Thus, a sequential culture system that allows the oocyte to initially arrest at the GV stage and complete transcription and undergo ultrastructural modifications essential for meiotic and cytoplasmic maturation could be an effective means of enhancing IVM. In other mammalian species, the temporary inhibition of meiosis using phosphodiesterase or a cyclin-dependent kinase inhibitor improves oocyte developmental competence, including those obtained from small follicles (Funahashi et al., 1997; Pavlok et al., 2000). Exposure of dog oocytes to the phosphodiesterase inhibitor, dbcAMP, temporarily inhibited meiosis resumption (Songsasen et al., 2003b). Roscovitine (a cyclin -dependent kinase inhibitor) has been found less effective than dbcAMP in maintaining dog oocytes in meiotic arrest. Despite its ability to temporarily inhibit meiotic resumption, dbcAMP fails to improve meiotic competency of dog oocytes after culturing them for an additional 48 h in its absence (Songsasen et al., 2003b). It is likely that the duration of dbcAMP exposure was insufficient for the oocytes to acquire the capability to complete nuclear maturation (Pavlok et al., 2000).
5.8. Other supplements
Substances known to protect cells from oxidative damage and to promote oocyte maturation (e.g., beta-mercaptoethanol or βME and insulin-transferrin-selenium combinations) also have been tested on dog oocytes. Supplementation of TCM 199 with insulin-transferrin and selenium increases the number of oocytes developing to the MI/AI/MII stages (Rota and Cabianca, 2004). Besides protecting cells from oxidative stress, βME increases the production of intracellular glutathione, which improves developmental competence of mammalian oocytes (Abeydeera et al., 1998; de Matos and Furnus, 2000; Songsasen and Apimeteetumrong, 2002). In the dog, βME enhances nuclear maturation of follicular phase oocytes in a dose-dependent fashion (Kim et al., 2004); the optimal concentration was 50 to 100 μM. At a lower concentration (25 μM), βME failed to exert an effect (Songsasen et al., 2002).
Although it is well known that energy substrates play an important role in regulating oocyte maturation (Downs and Mastropolo, 1994; Downs, 1995), few studies have examined their influence on dog oocyte maturation. Glucose alone may not be sufficient as an energy source for canine oocytes (Songsasen et al., 2002). It also appears that increased concentrations of glucose (11.0 mM compared to 5.5 mM) in the absence of pyruvate inhibits developmental competence of the dog oocyte cultured under high oxygen tension (~20%, Songsasen et al., 2002; Songasen et al. 2003a). Furthermore, although supplemental pyruvate has no impact, glutamine appears essential for ensuring nuclear maturation of the dog oocyte (Songsasen et al. 2003a). Most recently, it has been determined that the dog oocyte utilizes glucose predominantly, but that overall maturation in vitro is linked to both glucose and glutamine metabolism (Songsasen et al., 2005).
5.9. Co-culture
The oviductal environment is comprised of the luminal oviductal cell surface (that is available for cell-to-cell interaction between the sperm and ovum) and the oviductal fluid (Boatman, 1997; Verhage et al., 1997). Oviduct-specific proteins (high molecular weight glycoproteins) are the major products produced by the oviductal epithelial cells (Verhage et al., 1997). It has been suggested that the production of these oviductal proteins (OP) is regulated by the reproductive cycle and, specifically, circulating estrogen (Verhage et al., 1997). The proteins facilitate sperm capacitation and prevent polyspermic fertilization in the cow and pig (Verhage et al., 1997) and promote embryo development in cultured mouse embryos (Xu et al., 2001). Because dog oocytes complete maturation, undergo fertilization and develop up to the morula or blastocyst stage within the oviduct (Renton et al., 1991; Reynaud et al., 2005), it is possible that oviductal epithelial cells and proteins play a major role in successful maturation, fertilization and early preimplantation embryo development in this species.
Hewitt and England (1999b) co-cultured dog oocytes with conspecific oviductal epithelial cells and found no improved developmental competence. However, most of the epithelial cells were derived from diestrous bitches, which may well lack the proteins essential for promoting meiotic maturation because oviductal secretory cells are mostly active during estrus (not diestrus). Intraoviductal concentrations of glycosaminoglycans are higher in estrual compared to anestrous bitches (Kawakami et al., 2000). In fact, culturing dog oocytes with oviductal cells recovered from an estrual bitch improved the proportions of oocytes resuming meiosis and developing to the MII stage (Bogliolo et al., 2002; Luvoni et al., 2003).
Because the morphological and ultrastructural features of secretory cells can vary along the length of the oviduct (Abe, 1994), it makes sense that region of the oviduct where the cells are recovered could impact type and concentration of protein produced, thus dictating the ultimate effect on the developing oocyte. However, Bogliolo et al. (2002) has partially addressed this issue in the dog, discovering that oviductal cells from both the infundibulum and the ampulla are equally able to promote meiotic maturation in this species. It also is worth noting that physical interaction between the oocyte and the oviduct may positively influence oocyte maturation (Luvoni et al., 2003). Co-culture of oocytes within the ligated oviduct increased the proportions of GVBD and MI-MII oocytes compared to those cultured without oviduct or on the mucosal epithelium of the open oviduct. However, culturing oocytes in ligated oviducts for longer than 30 h resulted in degeneration of a greater proportion of oocytes (Luvoni et al., 2003), probably due to a hostile environment caused by degenerating somatic cells. In a subsequent study, Luvoni et al. (2003b) described a sequential system comprised of 24 h culture of oocyte in the presence of oviductal cells followed by 48 to 72 h in medium devoid of somatic cells. Compared to culturing oocytes with oviduct cells for the entire period, the sequential system improved the ability of dog oocytes to resume meiosis and reduced numbers of degenerating oocytes.
Thus far, production of blastocysts after IVF of in vitro matured oocytes has been reported in only one study (Otoi et al., 2000). In this case, the oocytes were cultured in a feeder layer of bovine cumulus cells that had been cultured with bovine embryos for 13 to 14 days. Although frequencies of oocyte maturation and development were low, successful production of in vitro-derived embryos might have resulted from embryotropic factors produced by bovine cumulus cells or embryos.
The influence of spermatozoa on oocyte maturation in the domestic dog still remains to be investigated. Dog spermatozoa are known to have the capacity to penetrate immature, conspecific oocytes in vitro and to form a male pronucleus (Mahi and Yanagimachi, 1976). Saint-Dizier et al. (2001a, b) recently reported that sperm penetration resulted in greater percentages of dog oocytes resuming meiosis and developing beyond the MI stage compared to nonpenetrated counterparts. In a contemporary investigation, Rodrigues et al. (2004) found that only 4% of dog oocytes cultured for 48 h developed to MII. However, when cultured for 48 h and inseminated in vitro, more oocytes fertilized (24% forming pronuclei) and developed to early stage embryos (13% becoming 4 to 8 cells). If and how the spermatozoon exerts its influence is unknown. However, during mammalian fertilization, spermatozoa induce oscillatory changes in the oocyte’s intracellular Ca2+ that are essential for completing the second meiotic division and proceeding with embryo development (Whitetaker and Partel, 1990). It has been suggested that extra- and intra-cellular Ca2+ may play an important role in resumption and completion of meiosis (Whitetaker and Partel, 1990; Kaufman and Homa, 1993; Homa, 1995). Thus, it is possible that penetration of the dog oocyte by the spermatozoon induces meiotic resumption via one or more Ca2+-dependent pathways.
6. In vitro fertilization and embryo production
In vitro embryo production has not been successfully and reliably applied to the dog. Mahi and Yanagimachi (1976) reported the first IVF attempt in this species and achieved approximately 20 to 30% fertilization judged by the presence of swelling sperm nuclei within the oocyte. Songsasen et al., (2002) inseminated in vitro cultured oocytes and found that 34% of these oocytes were penetrated by spermatozoa. Normal fertilization (judged by the presence of two pronuclei and a single sperm tail within the cytoplasm) was observed only in 4% of the oocytes. In the same study, these authors obtained 7 early stage embryos (2- to 12-cells) after IVF of 85 oocytes (Figure 1D). In a more recent study, Rodrigues et al. (2004) achieved 30% fertilization of oocytes, half of which were polyspermically fertilized. These authors also reported that overall fertilization rate was superior with oocytes derived from bitches at the follicular (42.5%) and anestrous (34.3%) than at the luteal stages (18.6%). Overall embryonic development in that study was 10% with none of the embryos developing beyond the 8-cell stage.
There has only been one study reporting blastocyst development after IVM/IVF of dog oocytes (Otoi et al., 2000). In that study, 217 cultured oocytes were inseminated, but only one blastocyst was produced. Thus far, there has been only one attempt at transferring IVM/IVF dog embryos. England et al. (2001) obtained oocytes from estrus-induced bitches and cultured them for 24 to 72 h before IVF. The authors transferred 88 1- and 2-cell embryos to recipients and reported a single pregnancy by ultrasound on Day 20 after embryo transfer. However, the pregnancy was lost two days later.
7. Conclusions and priorities for the future
Meiotic maturation is a biologically complex event that proceeds under the orchestrated influences of intrinsic and extrinsic factors. The foundation for successful oocyte maturation and fertilization is established during the gamete’s growth and differentiation when it is in intimate communication with the follicle. Although mechanisms regulating nuclear maturation have been extensively studied in the mouse and cow, direct application of knowledge from these two animals to other species, including the dog, has not been successful. Clearly, there are species-specificities in mechanisms regulating meiotic maturation.
Because there are no data on mechanisms controlling oocyte maturation in the dog, future research should be directed toward identifying intrinsic and extrinsic factors regulating oocyte maturation in this species. These include (1) molecular and cellular studies on activation and phosphorylation of protein kinase and expression of gene (mRNA and protein concentrations) controlling cell cycle during the processes of folliculogenesis and oogenesis and (2) identification of the role of the follicle, oviduct and spermatozoa on oocyte maturation. Furthermore, determining metabolic rates of the oocyte would be particularly useful for (1) establishing normative metabolic requirements, and (2) optimizing culture conditions for enhanced IVM and IVF.
We assert that the study of a unique species – the dog - will shed light on novel mechanisms that will increase our overall understanding about mammalian oocyte maturation. For dogs, this scholarly knowledge will be beneficial to advancing in vitro culture and fertilization systems targeting immature oocytes that eventually will be useful in the management and conservation of valuable canine genotypes.
Acknowledgments
The authors thank B.S. Pukazhenthi for critical reading of this manuscript. N. Songsasen is supported by the Sichel Endowment Fund and, more recently, by the National Center for Resource Resources of the National Institutes of Health (KO1 RR020564).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H. The mammalian oviduct epithelium: regional variations in cytological and functional aspects of the oviductal secretory cells. Histol Histopath. 1994;11:743–768. [PubMed] [Google Scholar]
- Abeydeera LR, Wang WH, Cantley TC, Prather RS, Day BN. Presence of β-mercaptoethanol can increase the glutathione content of pig oocytes matured in vitro and the rate of blastocyst development after in vitro fertilization. Theriogenology. 1998;50:747–756. doi: 10.1016/s0093-691x(98)00180-0. [DOI] [PubMed] [Google Scholar]
- Andersen AC, Simpson ME. The Ovary and Reproductive Cycle of the Dog (Beagle) Geron-X Inc.; Los Altos, USA: 1973. [Google Scholar]
- Asher GW, Monfort SL, Wemmer C. Comparative reproductive function in cervids: implications for management of farm and zoo populations. J Reprod Fertil. 1999;54(Suppl):143–156. [PubMed] [Google Scholar]
- Badinand F, Fontbonne A, Maurel MC, Siliart B. Fertilization time in the bitch in relation to plasma concentration of oestradiol, progestorone and luteinizing hormone and vaginal smears. J Reprod Fertil. 1993;47(Suppl):63–67. [PubMed] [Google Scholar]
- Baker TG. Oogenesis and ovulation. In: Austin CR, Short RV, editors. Reproduction in Mammals: Germ Cells and Fertilization. Cambridge University Press; Cambridge: 1982. pp. 17–45. [Google Scholar]
- Barber MR, Lee SM, Steffens WL, Ard M, Fayrer-Hosken RA. Immunolocalization of zona pellucida antigens in the ovarian follicle of dogs, cats, horses and elephants. Theriogenology. 2001;55:1705–1717. doi: 10.1016/s0093-691x(01)00514-3. [DOI] [PubMed] [Google Scholar]
- Beijerink NJ, Kooistra HS, Dieleman SJ, Okkens AC. Serotonin antagonist-induced lowering of prolactin secretion does not affect the pattern of pulsatile secretion of follicle stimulating hormone and luteinizing hormone in the bitch. Reproduction. 2004;128:181–188. doi: 10.1530/rep.1.00117. [DOI] [PubMed] [Google Scholar]
- Blackmore DG, Baillie LR, Holt JE, Dierkx L, Aitken RJ, McLaughlin EA. Biosynthesis of the canine zona pellucida requires the integrated participation of both oocytes and granulosa cells. Biol Reprod. 2004;71:661–668. doi: 10.1095/biolreprod.104.028779. [DOI] [PubMed] [Google Scholar]
- Boatman DE. Response of gametes to the oviductal environment. Hum Reprod. 1997;12(Suppl):133–149. [PubMed] [Google Scholar]
- Bogliolo L, Zedda MT, Ledda S, Leoni G, Naitana S, Pau S. Influence of co-culture with oviductal epithelial cells on in vitro maturation of canine oocytes. Reprod Nutr Dev. 2002;42:265–273. doi: 10.1051/rnd:2002024. [DOI] [PubMed] [Google Scholar]
- Bolamba D, Borden-Russ KD, Durrant BS. In vitro maturation of domestic dog oocytes cultured in advanced preantral and early antral follicles. Theriogenology. 1998;49:933–942. doi: 10.1016/s0093-691x(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Bolamba D, Russ KD, Olson MA, Sandler JL, Durrant BS. In vitro maturation of bitch oocytes from advanced preantral follicle in synthetic oviduct fluid medium: serum is not essential. Theriogenology. 2002;58:1689–1703. doi: 10.1016/s0093-691x(02)01080-4. [DOI] [PubMed] [Google Scholar]
- Bolamba D, Russ KD, Harper SA, Sandler JL, Durrant BS. Effects of epidermal growth factor and hormones on granulosa expansion and nuclear maturation of dog oocytes in vitro. Theriogenology. 2006;65:1037–1047. doi: 10.1016/j.theriogenology.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Andersen CY, Hossaini A, Guoliang X. Cumulus cells of oocyte-cumulus complexes secrete a meiosis-activating substance when stimulated with FSH. Mol Reprod Dev. 1997;46:296–305. doi: 10.1002/(SICI)1098-2795(199703)46:3<296::AID-MRD8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction. 2003;126:809–816. [PubMed] [Google Scholar]
- Concannon PW, McCann JP, Temple M. Biology and endocrinology of ovulation, pregnancy, and parturition in the dog. J Reprod Fertil. 1989;39(Suppl):3–25. [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ. Role of EGF network in ovarian follicles. Mol Endocrinl. 2006;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- de los Reyes M, de Lange J, Miranda P, Palominos J, Barros C. Effect of human chorionic gonadotrophin supplementation during different culture periods on in vitro maturation of canine oocytes. Theriogenology. 2005;64:1–11. doi: 10.1016/j.theriogenology.2004.11.008. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development: effect of β mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Furnus CC, Moses DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod. 1997;57:1420–1425. doi: 10.1095/biolreprod57.6.1420. [DOI] [PubMed] [Google Scholar]
- Dedieu T, Gall L, Crozet N, Sevellec C, Ruffini S. Mitogen activated protein kinase activity during goat oocyte maturation and acquisition of meiotic competence. Mol Reprod Dev. 1996;45:351–358. doi: 10.1002/(SICI)1098-2795(199611)45:3<351::AID-MRD12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Doak RL, Hall A, Dale HE. Longevity of spermatozoa in the reproductive tract of the bitch. J Reprod Fertil. 1967;13:51–58. doi: 10.1530/jrf.0.0130051. [DOI] [PubMed] [Google Scholar]
- Downs SM. The influence of glucose, cumulus cells, and metabolic coupling on ATP levels and meiotic control in the isolated mouse oocytes. Dev Biol. 1995;167:502–512. doi: 10.1006/dbio.1995.1044. [DOI] [PubMed] [Google Scholar]
- Downs SM, Mastropolo AM. The participation of energy substrates in the control of meiotic maturation in murine oocytes. Dev Biol. 1994;162:154–168. doi: 10.1006/dbio.1994.1075. [DOI] [PubMed] [Google Scholar]
- Durrant BS, Pratt NC, Russ KD, Bolamba D. Isolation and characterization of canine advanced preantral and early antral follicles. Theriogenology. 1998;49:917–932. doi: 10.1016/s0093-691x(98)00041-7. [DOI] [PubMed] [Google Scholar]
- England GCW, Allen WE. Real-time ultrasonic imaging of the ovary and uterus of the dog. J Reprod Fertil. 1989;39(Suppl):91–100. [PubMed] [Google Scholar]
- England GWW, Verstegen JP, Hewitt DA. Pregnancy following in vitro fertilisation of canine oocytes. Vet Rec. 2001;148:20–22. doi: 10.1136/vr.148.1.20. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Hosoe M, O’Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose, and ascorbic acid. Mol Cell Endocrinol. 2000;163:109–116. doi: 10.1016/s0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Farstad W, Mondain-Monval M, Hyttel P, Smith AJ, Markeng D. Periovulatory endocrinology and oocyte maturation in unmated mature blue fox vixens. Acta Vet Scand. 1989;30:313–319. doi: 10.1186/BF03548037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton RM, Keskintepe L, Durant BS, Fayrer-Hosken RA. Intracytoplasmic sperm injection (ICSI) for the treatment of canine infertility. Theriogenology. 1998;49:366. (Abstr.) [Google Scholar]
- Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod. 1997;57:49–53. doi: 10.1095/biolreprod57.1.49. [DOI] [PubMed] [Google Scholar]
- Gall L, Boulesteix C, Ruffini S, Germain G. EGF-induced EGF-receptor and MAP kinase phosphorylation in goat cumulus cells during in vitro maturation. Mol Reprod Dev. 2005;71:489–494. doi: 10.1002/mrd.20317. [DOI] [PubMed] [Google Scholar]
- Gomez E, Tarin JJ, Pellicer A. Oocyte maturation in humans: the role of gonadotropins and growth factors. Fertil Steril. 1993;60:40–46. [PubMed] [Google Scholar]
- Gordon I. Laboratory Production of Cattle Embryos. CAB International; Oxfordshire, United Kingdom: 1994. [Google Scholar]
- Guraya SS. A histochemical analysis of lipid yolk deposition in the oocytes of cat and dog. J Exp Zool. 1965;160:123–136. doi: 10.1002/jez.1401600111. [DOI] [PubMed] [Google Scholar]
- Haenisch A, Kolle S, Neumuller C, Sinowatz F, Braun J. Morphology of canine cumulus-oocyte complexes in pre-pubertal bitches. Anat Histol Embryol. 2003;32:373–377. doi: 10.1046/j.0340-2096.2003.00514.x. [DOI] [PubMed] [Google Scholar]
- Hewitt DA, England GCW. Effect of preovulatory endocrine events upon maturation of oocytes of domestic bitches. J Reprod Fertil. 1997;51(Suppl):83–91. [PubMed] [Google Scholar]
- Hewitt DA, England GCW. The effect of oocyte size and bitch age upon oocyte nuclear maturation in vitro. Theriogenology. 1998a;49:957–966. doi: 10.1016/s0093-691x(98)00044-2. [DOI] [PubMed] [Google Scholar]
- Hewitt DA, England GCW. Incidence of oocyte nuclear maturation within the ovarian follicle of the bitch. Vet Rec. 1998b;21:590–591. doi: 10.1136/vr.143.21.590. [DOI] [PubMed] [Google Scholar]
- Hewitt DA, England GCW. Influence of gonadotrophin supplementation on the in vitro maturation of bitch oocytes. Vet Rec. 1999a;144:237–239. doi: 10.1136/vr.144.9.237. [DOI] [PubMed] [Google Scholar]
- Hewitt DA, England GCW. Synthetic oviductal fluid and oviductal cell coculture for canine oocyte maturation in vitro. Anim Reprod Sci. 1999b;55:63–75. doi: 10.1016/s0378-4320(98)00162-6. [DOI] [PubMed] [Google Scholar]
- Hewitt DA, Watson PF, England GCW. Nuclear staining and culture requirements for in vitro maturation of domestic bitch oocytes. Theriogenology. 1998;49:1083–1101. doi: 10.1016/s0093-691x(98)00058-2. [DOI] [PubMed] [Google Scholar]
- Holst PA, Phemister RD. The prenatal development of the dog: preimplantation events. Biol Reprod. 1971;5:194–206. doi: 10.1093/biolreprod/5.2.194. [DOI] [PubMed] [Google Scholar]
- Homa ST. Calcium and meiotic maturation of the mammalian oocyte. Mol Reprod Dev. 1995;40:122–134. doi: 10.1002/mrd.1080400116. [DOI] [PubMed] [Google Scholar]
- International Union for Conservation of Nature and Natural Resource. IUCN Red List of Threatened Species. 2002 http://www.redlist.org.
- Izadyar F, Hage WJ, Colenbrander B, Bevers MM. The promotory effect of growth hormone on the developmental competence of in vitro matured bovine oocytes is due to improved cytoplasmic maturation. Mol Reprod Dev. 1998;49:444–453. doi: 10.1002/(SICI)1098-2795(199804)49:4<444::AID-MRD12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Johnston SD, Root Kustritz MV, Olson PNS. Canine and Feline Theriogenology. W.B. Saunders Company; Philadelphia, USA: 2001. [Google Scholar]
- Kalab P, Sršeò V, Farstad W, Krogenaes A, Motlik J, Hafne AL. MAP kinase activation and RAF-1 synthesis in blue fox oocytes is controlled by cumulus granulosa cells. Theriogenology. 1997;47:400. Abstr. [Google Scholar]
- Kaufman ML, Homa ST. Defining a role for calcium in the resumption and progression of meiosis in the pig oocyte. J Exp Zool. 1993;265:69–76. doi: 10.1002/jez.1402650110. [DOI] [PubMed] [Google Scholar]
- Kawakami E, Kashiwagi C, Hori T, Tsutsui T. Effects of canine oviduct cells on movement and capacitation of homologous spermatozoa in vitro. Anim Reprod Sci. 2001;68:121–131. doi: 10.1016/s0378-4320(01)00135-x. [DOI] [PubMed] [Google Scholar]
- Kawakami E, Arai T, Oishi I, Hori T, Tsutsui T. Induction of dog sperm capacitation by glycosaminoglycans and glycosaminoglycan amounts of oviductal and uterine fluids in bitches. J Vet Med Sci. 2000;62:65–68. doi: 10.1292/jvms.62.65. [DOI] [PubMed] [Google Scholar]
- Kim MK, Fibrianto YH, Oh HJ, Jang G, Kim HJ, Lee KS, Kang SK, Lee BC, Hwang WS. Effect of β-mercaptoethanol or epidermal growth factor supplementation on in vitro maturation of canine oocyte collected from dogs with different stage of the estrus cycle. J Vet Sci. 2004;5:253–258. [PubMed] [Google Scholar]
- Kim MK, Fibrianto YH, Oh HJ, Jang G, Kim HJ, Lee KS, Kang SK, Lee BC, Hwang WS. Effects of estradiol-17β and progesterone supplementation on in vitro nuclear maturation of canine oocytes. Theriogenology. 2005;63:1342–1353. doi: 10.1016/j.theriogenology.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kim NH, Chung HM, Cha KY, Chung KS. Microtubule and microfilament organisation in maturing human oocytes. Hum Reprod. 1996;13:2217–2222. doi: 10.1093/humrep/13.8.2217. [DOI] [PubMed] [Google Scholar]
- Kirkness EF, Bafna V, Halpern AL, Levy S, Remington K, Rusch DB, Delcher AL, Pop M, Wang W, Fraser CM, Venter JC. The dog genome: survey sequencing and comparative analysis. Science. 2003;301:1898–1903. doi: 10.1126/science.1086432. [DOI] [PubMed] [Google Scholar]
- Kooistra HS, Okkens AC. Role of changes in the pulsatile secretion pattern of FSH in initiation of ovarian folliculogenesis in bitches. J Reprod Fertil. 2001;57(Suppl):11–14. [PubMed] [Google Scholar]
- Kooistra HS, Okkens AC, Bevers MM, Popp-Snijders C, van Haaften B, Dieleman SJ, Schoemaker J. Concurrent pulsatile secretion of luteinizing hormone and follicle stimulating hormone during different phases of the estrous cycle and anestrus in Beagle bitches. Biol Reprod. 1999;60:65–71. doi: 10.1095/biolreprod60.1.65. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Shamin MH, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005;436:641. doi: 10.1038/436641a. [DOI] [PubMed] [Google Scholar]
- Leonard JA, Wayne RK, Wheeler J, Valadez R, Guillén S, Vilà C. Ancient DNA evidence for old world origin of new world dogs. Science. 2002;298:1613–1616. doi: 10.1126/science.1076980. [DOI] [PubMed] [Google Scholar]
- Luvoni GC, Luciano AM, Modina S, Gandolfi F. Influence of different stages of the oestrous cycle on cumulus-oocyte communications in canine oocytes: effects on the efficiency of in vitro maturation. J Reprod Fertil. 2001;57(Suppl):141–146. [PubMed] [Google Scholar]
- Luvoni GC, Chigioni S, Allievi E, Macis D. Meiosis resumption of canine oocytes cultured in the isolated oviduct. Reprod Dom Anim. 2003;38:410–414. doi: 10.1046/j.1439-0531.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- Luvoni GC, Chigioni S, Allievi E, Macis D. Factors involved in vivo and in vitro maturation of canine oocytes. Theriogenology. 2005;63:41–59. doi: 10.1016/j.theriogenology.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Luvoni GC, Chigioni S, Allievi E, Macis D, Perego L. Proc 3rd EVSSAR Annu Congr. 2003b. Extension of incubation time in a two step culture system for the maturation of canine oocytes; pp. 121–122. [Google Scholar]
- Mahi CA, Yanagimachi R. Maturation and sperm penetration of canine ovarian oocytes in vitro. J Exp Zool. 1976;196:189–196. doi: 10.1002/jez.1401960206. [DOI] [PubMed] [Google Scholar]
- McDougall K, Hay MA, Goodrowe KL, Gartley CJ, King WA. Changes in the number of follicles and of oocytes in ovaries of prepubertal, peripubertal and mature bitches. J Reprod Fertil. 1997;51(Suppl):25–31. [PubMed] [Google Scholar]
- Nickson DA, Boyd JS, Eckersall PD, Ferguson JM, Harvey MJA, Renton JP. Molecular biological methods for monitoring oocyte maturation and in vitro fertilization in bitches. J Reprod Fertil. 1993;47(Suppl):231–240. [PubMed] [Google Scholar]
- O’Brien SJ, Murphy WJ. A dog’s breakfast? Science. 2003;301:1854–1855. doi: 10.1126/science.1090531. [DOI] [PubMed] [Google Scholar]
- Ostrander EA, Kruglyak L. Unleashing the canine genome. Genome Res. 2000;10:1271–1274. doi: 10.1101/gr.155900. [DOI] [PubMed] [Google Scholar]
- Ostrander EA, Galibert F, Patterson DF. Canine genetics comes of age. Trends Genet. 2000;16:117–124. doi: 10.1016/s0168-9525(99)01958-7. [DOI] [PubMed] [Google Scholar]
- Otoi FM, Tanaka M, Ooka A, Suzuki T. Effect of serum on the in vitro maturation of canine oocytes. Reprod Fertil Dev. 1999;11:387–390. doi: 10.1071/rd00012. [DOI] [PubMed] [Google Scholar]
- Otoi T, Fujii M, Tanaka M, Ooka A, Suzuki T. Effect of serum on the in vitro maturation of canine oocytes. Reprod Fertil Dev. 1999;11:387–390. doi: 10.1071/rd00012. [DOI] [PubMed] [Google Scholar]
- Otoi T, Shin T, Kraemer DC, Westhusin ME. Influence of maturation culture period on the development of canine oocytes after in vitro maturation and fertilization. Reprod Nutr Dev. 2004;44:631–637. doi: 10.1051/rnd:2004065. [DOI] [PubMed] [Google Scholar]
- Otoi T, Fujii M, Tanaka M, Ooka A, Suzuki T. Canine oocyte diameter in relation to meiotic competence and sperm penetration. Theriogenology. 2000;54:535–542. doi: 10.1016/s0093-691x(00)00368-x. [DOI] [PubMed] [Google Scholar]
- Otoi T, Ooka A, Murakami M, Kurniani Karja NW, Suzuki T. Size distribution and meiotic competence of oocytes obtained from bitch ovaries at various stages of the oestrous cycle. Reprod Fertil Dev. 2001;13:151–155. doi: 10.1071/rd00098. [DOI] [PubMed] [Google Scholar]
- Otoi T, Willingham L, Shin T, Kraemer DC, Westhusin M. Effects of oocyte culture density on meiotic competence of canine oocytes. Reproduction. 2002;124:775–781. [PubMed] [Google Scholar]
- Otoi T, Murakami M, Fujii M, Tanaka M, Ooka A, Une S, Suzuki T. Development of canine oocytes matured and fertilised in vitro. Vet Rec. 2000;146:52–53. doi: 10.1136/vr.146.2.52. [DOI] [PubMed] [Google Scholar]
- Patronek GI. Development of a model for estimating the size and dynamics of the pet dog population. Anthrozoos. 1994;7:25–41. [Google Scholar]
- Patterson DF. Companion animal medicine in the age of medical genetics. J Vet Intern Med. 2000;14:1–9. [PubMed] [Google Scholar]
- Patterson DF, Haskins ME, Jezyk PF. Models of human genetic disease in domestic animals. Adv Hum Genet. 1982;12:263–339. doi: 10.1007/978-1-4615-8315-8_4. [DOI] [PubMed] [Google Scholar]
- Pavlok A, Kanka J, Motlik J, Vodicka P. Culture of bovine oocytes from small antral follicles in meiosis-inhibiting medium with butyrolactone I: RNA synthesis, nucleolar morphology and meiotic competence. Anim Reprod Sci. 2000;64:1–11. doi: 10.1016/s0378-4320(00)00189-5. [DOI] [PubMed] [Google Scholar]
- Pearson OP, Enders RK. Ovulation, maturation and fertilization in the fox. Anat Rec. 1943;85:69–83. [Google Scholar]
- Phemister RD, Holst PA, Spano JS, Hopwood ML. Time of ovulation in the Beagle Bitch. Biol Reprod. 1973;8:74–82. doi: 10.1093/biolreprod/8.1.74. [DOI] [PubMed] [Google Scholar]
- Pope CE. Embryo technology in conservation efforts for endangered felids. Theriogenology. 2000;53:163–174. doi: 10.1016/s0093-691x(99)00249-6. [DOI] [PubMed] [Google Scholar]
- Pope CE, McRae MA, Plair BL, Keller GL, Dresser BL. In vitro and in vivo development of embryos produced by in vitro maturation and in vitro fertilization of cat oocytes. J Reprod Fertil. 1997;51(Suppl):69–82. [PubMed] [Google Scholar]
- Pukazhenthi GN, Wildt DE. Which reproductive technologies are most relevant to studying, managing and conserve wildlife? Reprod Fertil Dev. 2004;16:33–46. doi: 10.10371/RD03076. [DOI] [PubMed] [Google Scholar]
- Purohit GN, Brady MS, Sharma SS. Influence of epidermal growth factor and insulin-like growth factor 1 on nuclear maturation and fertilization of buffalo cumulus oocyte complexes in serum free media and their subsequent development in vitro. Anim Reprod Sci. 2005;87:229–239. doi: 10.1016/j.anireprosci.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Rankin T, Soyal S, Dean J. The mouse zona pellucida: folliculogenesis, fertility, and preimplantion development. Mol Cell Endocrinol. 2000;163:21–25. doi: 10.1016/s0303-7207(99)00236-1. [DOI] [PubMed] [Google Scholar]
- Renton JP, Boyd JS, Eckersall PD, Ferguson JM, Harvey MJA, Mullaney J, Perry B. Ovulation, fertilization and early embryonic development in the bitch (Canis familiaris) J Reprod Fertil. 1991;93:221–231. doi: 10.1530/jrf.0.0930221. [DOI] [PubMed] [Google Scholar]
- Reynaud K, Fontbonne A, Marseloo N, Thoumire S, Chebrout M, de Lesegno CV, Chastant-Maillard S. In vivo meiotic resumption, fertilization and early embryonic development in the bitch. Reproduction. 2005;130:193–201. doi: 10.1530/rep.1.00500. [DOI] [PubMed] [Google Scholar]
- Rodrigues BA, Rodrigues JL. Meiotic response of in vitro matured canine oocytes under different proteins and heterologous hormone supplementation. Reprod Dom Anim. 2003a;38:58–62. doi: 10.1046/j.1439-0531.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues BA, Rodrigues JL. Influence of reproductive status on in vitro oocyte maturation in dogs. Theriogenology. 2003b;60:59–66. doi: 10.1016/s0093-691x(02)01301-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues BA, Dos Santos LC, Rodrigues JL. Embryonic development of in vitro matured and in vitro fertilized dog oocytes. Mol Reprod Dev. 2004;67:215–223. doi: 10.1002/mrd.10394. [DOI] [PubMed] [Google Scholar]
- Rota A, Cabianca G. In vitro maturation rates of canine oocytes from anoestrous bitches in simple media. Reprod Nutr Dev. 2004;44:105–109. doi: 10.1051/rnd:2004018. [DOI] [PubMed] [Google Scholar]
- Rusovici R, Hui YY, LaVoie HA. Epidermal growth factor-mediated inhibition of follicle-stimulating hormone-stimulated StAR gene expression in porcine granulosa cells is associated with reduced histone H3 acetylation. Biol Reprod. 2005;72:862–871. doi: 10.1095/biolreprod.104.034298. [DOI] [PubMed] [Google Scholar]
- Saint-Dizier M, Renard JP, Chastant-Maillard S. Induction of final maturation by sperm penetration in canine oocytes. Reproduction. 2001a;121:97–105. [PubMed] [Google Scholar]
- Saint-Dizier M, Salomon JF, Petit C, Renard JP, Chastant-Maillard S. In vitro maturation of bitch oocytes: effect of sperm penetration. J Reprod Fertil. 2001b;57(Suppl):147–150. [PubMed] [Google Scholar]
- Saint-Dizier M, Reynaud K, Chastant-Maillard S. Chromatin, microtubules and kinases activities during meiotic resumption in bitch oocytes. Mol Reprod Dev. 2004;68:205–212. doi: 10.1002/mrd.20062. [DOI] [PubMed] [Google Scholar]
- Savolainen P, Zhang Y, Luo J, Lundeberg J, Leitner T. Genetic evidence of an east Asian origin of domestic dogs. Science. 2002;298:1610–1613. doi: 10.1126/science.1073906. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. Effects of gonadotrophins, growth hormone and prolactin on developmental competence of domestic cat oocytes matured in vitro. Reprod Fertil Dev. 1995;7:1061–1066. doi: 10.1071/rd9951061. [DOI] [PubMed] [Google Scholar]
- Skutelsky E, Ranen E, Shalgi R. Variations in the distribution of sugar residues in the zona pellucida as possible species-specific determinants of mammalian oocytes. J Reprod Fertil. 1994;100:35–41. doi: 10.1530/jrf.0.1000035. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Apimeteetumrong M. Effect of β-mercaptoethanol on formation of pronuclei and developmental competence of swamp buffalo oocytes. Anim Reprod Sci. 2002;72:193–202. doi: 10.1016/s0378-4320(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Wildt DE. Size of the donor follicle, but not stage of reproductive cycle or seasonality, influences meiotic competency of selected domestic dog oocyte. Mol Reprod Dev. 2005;72:113–119. doi: 10.1002/mrd.20330. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Yu I, Leibo SP. Nuclear maturation of canine oocytes cultured in protein-free media. Mol Reprod Dev. 2002;62:407–415. doi: 10.1002/mrd.10130. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Leibo SP, Wildt DE. Effect of energy substrates on nuclear maturation of domestic dog oocytes. Biol Reprod. 2003a;68(Suppl):352. Abstr. [Google Scholar]
- Songsasen N, Yu I, Gomez M, Leibo SP. Effects of meiosis-inhibiting agents and equine chorionic gonadotropin on nuclear maturation of canine oocytes. Mol Reprod Dev. 2003b;65:435–445. doi: 10.1002/mrd.10321. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Spindler RE, Wildt DE. Impact of nuclear status and maturation period on energy substrate use by in vitro dog oocytes. Biol Reprod. 2005;634(Special Iss) Abstr. [Google Scholar]
- Spanel-Borowski K. Morphological investigations on follicular atresia in canine ovaries. Cell Tissue Res. 1981;214:155–168. doi: 10.1007/BF00235153. [DOI] [PubMed] [Google Scholar]
- Spanel-Borowski K, Calvo W. Short- and long-term response of the adult dog ovary after 1200 R whole body X-irradiation and transfustion of mononuclear Leukocytes. Int J Radiat Biol. 1982;41:657–670. doi: 10.1080/09553008214550751. [DOI] [PubMed] [Google Scholar]
- Sršeň V, Kalous J, Nagyova E, Šutovsky P, King WA, Motlik J. Effects of follicle -stimulating hormone, bovine somatotrophin and okadaic acid on cumulus expansion and nuclear maturation of blue fox (Alopex lagopus) oocytes in vitro. Zygote. 1998;6:299–309. doi: 10.1017/s0967199498000252. [DOI] [PubMed] [Google Scholar]
- Ström Holst B, Larsson B, Linde-Forsberg C, Rodriguez-Martinez H. Sperm binding capacity and ultrastructure of the zona pellucida of stored canine oocytes. J Reprod Fertil. 2000;119:77–83. doi: 10.1530/jrf.0.1190077. [DOI] [PubMed] [Google Scholar]
- Ström Holst B, Larsson B, Rodriguez-Martinez H, Lagerstedt AS, Linde-Forsberg C. Prediction of the oocyte recovery rate in the bitch. J Vet Med A Physiol Pathol Clin Med. 2001;48:587–592. doi: 10.1046/j.1439-0442.2001.00388.x. [DOI] [PubMed] [Google Scholar]
- Swanson WF, Roth TL, Wildt DE. In vivo embryogenesis, embryo migration, and embryo mortality in the domestic cat. Biol Reprod. 1994;51:452–464. doi: 10.1095/biolreprod51.3.452. [DOI] [PubMed] [Google Scholar]
- Telfer E, Gosden RG. A quantitative cytological study of polyovular follicles in mammalian ovaries with particular reference to the domestic bitch (Canis familiaris) J Reprod Fertil. 1987;81:137–147. doi: 10.1530/jrf.0.0810137. [DOI] [PubMed] [Google Scholar]
- Tesoriero JV. Early ultrastructural changes of developing oocytes in the dog. J Morphol. 1981;168:171–179. doi: 10.1002/jmor.1051680206. [DOI] [PubMed] [Google Scholar]
- Tesoriero JV. A morphologic, cytochemical, and chromatographic analysis of lipid yolk formation in the oocytes of the dog. Gamete Res. 1982;6:267–279. [Google Scholar]
- Tsutsui T. Gamete physiology and timing of ovulation and fertilization in dogs. J Reprod Fertil. 1989;39(Suppl):269–275. [PubMed] [Google Scholar]
- Velilla E, Rodriguez-Gonzalez E, Vidal F, Paramio MY. Microtubule and microfilament organization in immature, in vitro matured and in vitro fertilized prepubertal goat oocytes. Zygote. 2005;13:155–165. doi: 10.1017/s0967199405003229. [DOI] [PubMed] [Google Scholar]
- Verhage HG, Fazleabas AT, Mavrogianis PA, O’Day-Bowman MB, Schmidt A, Arias EB, Jaffe RC. Characteristics of an oviductal glycoprotein and its potential role in fertility control. J Reprod Fertil. 1997;(Suppl):217–226. [PubMed] [Google Scholar]
- Verhage HG, Mavrogianis PA, Boomsma RA, Schmidt A, Brenner RM, Slayden OV, Jaffe RC. Immunologic and molecular characterization of an estrogen-dependent glycoprotein in the rhesus (Macaca mulatta) oviduct. Biol Reprod. 1997;57:525–531. doi: 10.1095/biolreprod57.3.525. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Clarke HJ, Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Vilà C, Maldonado JE, Wayne RK. Phylogenetic relationships, evolution, and genetic diversity of the domestic dog. J Hered. 1999;90:70–77. doi: 10.1093/jhered/90.1.71. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Albertini DF. The mammalian ovum. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 79–123. [Google Scholar]
- Whitetaker M, Partel R. Calcium and cell cycle control. Development. 1990;108:525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Roth TL. Assisted reproduction for managing and conserving threatened felids. Int Zoo Yearbook. 1997;35:164–172. [Google Scholar]
- Wildt DE, Levinson CJ, Seager WJ. Laparoscopic exposure and sequential observation of the ovary of the cycling bitch. Anat Rec. 1977;189:443–450. doi: 10.1002/ar.1091890305. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Seal US, Rall WF. Genetic resource banks and reproductive technology for wildlife conservation. In: Cloud JG, Thorgaard GH, editors. Genetic Conservation of Salmonid Fishes. Plenum Press; New York: 1993. pp. 159–173. [Google Scholar]
- Wildt DE, Chakraborty PK, Panko WB, Seager SWJ. Relationship of reproductive behavior, serum luteinizing hormone and time of ovulation in the bitch. Biol Reprod. 1978;18:561–570. doi: 10.1095/biolreprod18.4.561. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Panko WB, Chakraborty P, Seager SWJ. Relationship of serum estrone, estradiol-17β and progesterone to LH, sexual behavior and time of ovulation in the bitch. Biol Reprod. 1979;20:648–658. doi: 10.1095/biolreprod20.3.648. [DOI] [PubMed] [Google Scholar]
- Willingham-Rocky LA, Hinrichs K, Westhusin ME, Kraemer DC. Effects of stage of oestrous cycle and progesterone supplemention during culture on maturation of canine oocytes in vitro. Reproduction. 2003;126:501–508. doi: 10.1530/rep.0.1260501. [DOI] [PubMed] [Google Scholar]
- Xia GL, Kikuchi K, Noguchi J, Izaike Y. Short time priming of pig cumulus-oocyte complexes with FSH and forskolin in the presence of hypoxanthine stimulates cumulus cells to secrete a meiosis-activating substance. Theriogenology. 2000;53:1807–1815. doi: 10.1016/s0093-691x(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Xu JS, Cheung TM, Chan STH, Ho PC, Yeung WSB. Temporal effect of human oviductal cell and its derived embryotropic factors on mouse embryo development. Biol Reprod. 2001;65:1481–1488. doi: 10.1095/biolreprod65.5.1481. [DOI] [PubMed] [Google Scholar]
- Yamada S, Shimazu Y, Kawaji H, Nakazawa M, Naito K, Toyoda Y. Maturation, fertilization, and development of dog oocytes in vitro. Biol Reprod. 1992;46:853–858. doi: 10.1095/biolreprod46.5.853. [DOI] [PubMed] [Google Scholar]
- Yamada S, Shimazu Y, Kawano Y, Nakazawa M, Naito K, Toyoda Y. In vitro maturation and fertilization of preovulatory dog oocytes. J Reprod Fertil. 1993;47(Suppl):227–229. [PubMed] [Google Scholar]


