Abstract
The African clawed frog, Xenopus laevis, is a valuable model system for studies of vertebrate heart development. In the following review, we describe a range of embryological and molecular methodologies that are used in Xenopus research and discuss key discoveries relating to heart development that have been made using this model system. We also discuss how the sequence of the Xenopus tropicalis genome provides a valuable tool for identification of orthologous genes and for identification of evolutionarily conserved promoter elements. Finally, both forward and reverse genetic approaches are currently being applied to Xenopus for the study of vertebrate heart development.
Keywords: explants, mis-expression, antisense oligonucleotides, transgenic animals, promoter analysis
1. Introduction
Heart development is a beautifully orchestrated process that is highly conserved in all vertebrate organisms. This fact is most clearly illustrated by the observation that the heart of all vertebrates is practically indistinguishable through the linear heart tube stage and into the early stages of looping morphogenesis and chamber formation. This conservation has allowed researchers in the field of vertebrate heart development to utilize numerous animal models including zebrafish, Amphibia, chicken and rodents to further our understanding of the molecular and morphological aspects of human heart development and congenital heart disease. While researchers might argue the advantages of their particular model system, it is important to realize that the complementary experimental approaches offered by different systems has greatly increased our rate of progress in understanding the overall process of cardiogenesis. Here we will review a series of studies illustrating the ways in which the frog, Xenopus laevis and the related species Xenopus tropicalis, have been used to further our understanding of vertebrate heart development. In addition, we will discuss recent resources and methodologies that have been made possible by the genomic revolution and which further increase the usefulness of Xenopus as an animal model.
Studies of heart development in the early Twentieth Century typically used frog and Amphibian species other than Xenopus. Amphibia had become a favorite model system of embryologists due to the large size of the eggs, the large number of embryos, which develop outside of the mother and the fact that the embryos exhibit a remarkable ability to heal after microsurgery. In the past, these studies where often hindered by their dependence on wild-caught embryos. The observation in the 1930’s that Xenopus could be easily and reliably induced to ovulate large numbers of eggs year round [1, 2] combined with the fact that they could be raised and housed easily within the lab made this species of frog a favorite for many developmental biologists around the world.
2. Heart development in Xenopus
Through a combination of lineage tracing [3–5] and transplant experiments [6, 7], the following picture of the earliest events in normal Xenopus heart development has emerged. A timeline of significant steps of Xenopus heart development is outlined in Table 1. The heart, which ultimately occupies a ventral position within the chest of the mature tadpole/frog, starts out as two bilateral patches of specified mesoderm on the dorsal side of the embryo at the onset of gastrulation. Gastrulation movements cause the heart patches to move dorso-anteriorly until, during neurulation, the heart progenitors begin to migrate ventrally where they meet in the anterior region of the embryo at the ventral midline. Expression of numerous RNA and protein markers of the cardiac muscle lineage is first detected at the bilateral heart patch stage prior to fusion of the heart primordia. At the ventral midline the two heart patches fuse to form a simple linear tube, before undergoing the looping and remodeling processes of cardiac morphogenesis.
Table 1.
Timing of significant stages of heart development in Xenopus laevis (adapted from [66]).
| Morphological event | Stage of development [67] | Hours post-fertilization at room temperature |
|---|---|---|
| Specification | 12–14 | 15–17 |
| Migration to heart progenitors to ventral midline | 26–28 | 30–32 |
| Heart tube formation | 31–33 | 32–35 |
| Coordinated muscle contraction | 35 | 46 |
| Looping | 33–36 | 44–50 |
| Chamber differentiation | 39–40 | 60 |
| Valve formation | 41–44 | 70–90 |
| Atrial septation | 44–45 | 92 |
| Mature heart | 46 | 106 |
The availability of molecular markers has greatly facilitated detailed analysis of the morphological events involved in development of the mature frog heart. This has been achieved through in situ hybridization of cardiac markers to histological sections followed by 3D reconstruction [8] and also by immunohistological detection of heart tissue followed by whole mount confocal microscopy [9]. Through use of these methods we now have an excellent description of Xenopus heart development from the heart patch stage to chamber formation. In general the events of frog heart development closely resemble the equivalent processes of heart development in higher vertebrates including a leftward bend of the outflow tract, the presence of an atrioventricular valve to separate the atria and ventricle, asymmetric division of the atria early in development (with the right side being larger), and the presence of trabeculae within the thickened wall of the ventricular myocardium. Of course some differences do exist and Xenopus ultimately possesses a three-chambered heart (1 ventricle and 2 atria) rather than the four-chambered heart of birds and mammals. These differences in the Xenopus heart need not be viewed as a hindrance but may present valuable opportunities for investigation of the molecular mechanisms underlying formation of a heart that is an evolutionary intermediate between the two-chambered heart of fish and the four-chambered heart of birds and mammals.
3. Methods for studying heart development in Xenopus
3.1 Microinjections
The large size of the Xenopus embryo and its development outside of the mother make Xenopus extremely well suited for manipulations of gene activity via microinjection. Typically microinjection studies are used to examine the function of a gene of interest by over-expression of the wild type or mutant sequence or by loss of function approaches. Furthermore, the extremely reproducible cleavage patterns of the frog embryo (detailed by fate mapping studies [3–5]) facilitate targeting of injected material to restricted lineages of the developing embryo.
3.1.1 Over-expression/mis-expression
Over-expression/mis-expression studies in Xenopus are easily achieved by microinjection of in vitro synthesized mRNA, or in some cases plasmid DNA [10], into the single cell Xenopus egg before the first cleavage or into selected blastomeres. Injection of mRNA into a fertilized Xenopus egg prior to first cleavage generally results in global over-expression because the injected mRNA diffuses fairly broadly. Since the plane of first cleavage typically separates the left and right sides of the embryo, microinjection into one cell of a two-cell embryo results in overexpression on one side of the embryo only. One-sided injection is particularly useful for studies of early heart development, prior to fusion of the heart patches, because the uninjected side of the embryo serves as a stage-matched control for comparison. Injections after the two-cell stage (typically 8 and 16 cell embryos) can also be useful because the injected mRNA can be targeted so that it is overexpressed preferentially in the cardiac lineage. An example of the usefulness of this type of experiment is provided by investigations into the role of the homeodomain transcription factor Nkx2.5 in heart development. Nkx2-5 is the vertebrate orthologue of the Drosophila tinman gene and studies in the fly had raised the possibly that Nkx2-5 might act as master regulator for the cardiac lineage [11]. However, over-expression of Nkx2-5 in the frog embryo showed that this sequence was not sufficient to induce precocious or ectopic cardiac gene expression [12]. On the other hand, examination of embryos over-expressing Nkx2-5 revealed a thickening of the myocardium due to an increase in the overall number of myocardial cells. These experiments suggested that Nkx2-5 might function in recruitment of cells to the cardiac lineage or in regulation of myocardial proliferation. Overexpression studies have also been used for studies of left/right asymmetries of the heart and have been reviewed previously [13].
Expression cloning, which is a variant of the overexpression method, is also very conveniently carried out using Xenopus embryos. In this procedure, large pools of synthetic mRNA, derived from cDNA libraries, are injected into the fertilized egg. By coupling this technique to appropriate assays, it is possible to identify novel genes involved in regulation of development processes. A screen for modulators of heart development has been carried out by looking for sequences that altered the developmental expression pattern of Nkx2-5 [14]. The recent establishment of a unique, full-length cDNA set from Xenopus tropicalis [15] plus use of additional molecular markers will facilitate further studies of heart development using this approach.
3.1.2 Loss of function experiments
Loss of function experiments in Xenopus can be achieved through injection of mRNAs encoding appropriate protein constructions or by injection of antisense oligonucleotides. As with overexpression, the loss of function constructions can be expressed globally or in restricted regions of the embryo. One of the most widely used approaches involves the application of natural antagonist proteins to interfere with the normal function of a signaling protein or a protein family. An example is provided by the secreted proteins crescent and dickkopf, which are endogenous inhibitors of the Wnt signaling pathway. Overexpression of either crescent or dickkopf is sufficient to induce cardiac differentiation in tissues that would not normally form heart [16]. It is also possible to use dominant negative versions of proteins to inhibit function. Dominant negative variants of BMP receptors (lacking the cytoplasmic serine/threonine kinase domain) have been used very effectively to demonstrate an essential role for the BMP signaling for maintenance of the cardiogenic lineage (though not initial induction of the lineage) [17]. Similarly, dominant negative constructions that inhibit Notch signaling [18] have demonstrated a function for Notch in regulating the size of the heart field. In addition, dominant negative forms of transcriptions factors including Nkx2-3 and Nkx2-5 [19], Tbx5 [20] and myocardin [21] have been used to interfere with endogenous transcription regulatory programs. Unfortunately, due to the very nature of the method, dominant negative constructions tend to be promiscuous and often interfere with the function of related family members or possible interacting proteins. For this reason, the results of dominant negative studies must be interpreted with caution.
Unlike dominant negative approaches, the microinjection of antisense oligonucleotides into the Xenopus embryo provides gene specific inhibition of expression. While numerous types of modified antisense oligonucleotides exist (reviewed in [22]), morpholino oligomers (MOs) are non-toxic, stable and highly efficient and therefore have become widely used by Xenopus researchers. MOs function to knock-down the expression levels of a target gene by interfering with either translation of the target mRNA or splicing of the target pre-mRNA. The resistance of MOs to endogenous nucleases results in efficient and sustained reduction of the targeted message within the injected cell and all of its progeny, with a gradual loss of activity at later stages of development, which may be due to dilution [23]. Use of MOs, especially for analysis of function of uncharacterized genes has been greatly facilitated by the availability of the Xenopus tropicalis genome sequence and by the wealth of ESTs for both tropicalis and laevis transcripts (approximately 1.6 million ESTs for the two species). Numerous studies have employed MOs to knockdown specific gene expression during embryonic heart development, including depletion of various transcription factors [24–29] and signaling pathways [16, 30–34]. For example, this approach has been successful in investigating the requirement of GATA6 in correct maturation of cardiac mesoderm [24]. Similarly, MO studies have demonstrated that Tbx5 and Tbx20 act synergistically to regulate embryonic heart development [25] and that myocardin is required both for expression of cardiac markers and for establishment of correct heart tube morphology ([27] and Figure 1).
Figure 1.
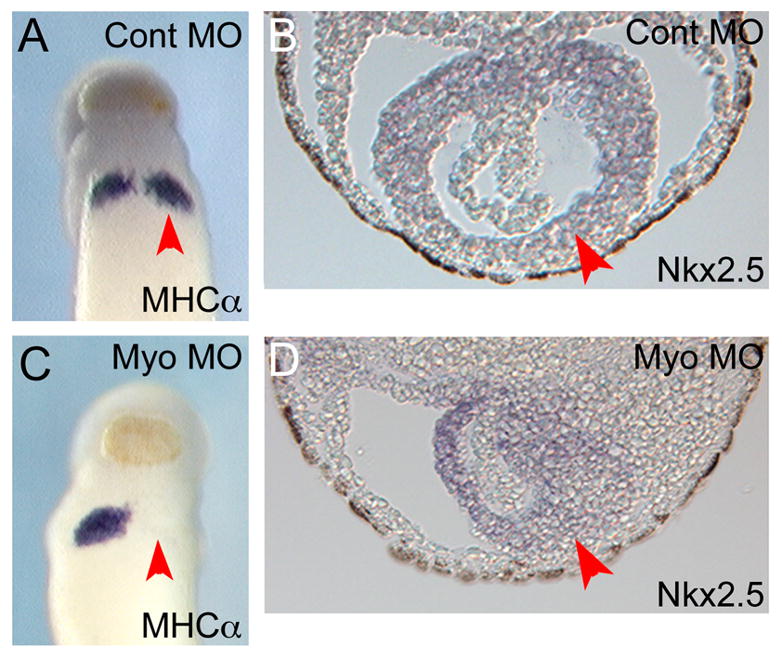
MO inhibition of myocardin expression results in reduced cardiac gene expression and defective cardiac morphogenesis. (A) Ventral view of embryo injected with control MO and then assayed by in situ hybridization for MHCα expression. The paired heart patches are clearly visible. Injected side is indicated with an arrow. (B) Section through heart of embryo injected with control MO. Heart tissue has been stained for Nkx2-5 transcripts. At this stage the myocardium is a simple linear tube surrounding the endocardial precursor cells. (C) Ventral view of embryo injected with MO directed against myocardin and assayed for MHCα expression. Note that no MHCα expression is detected on the injected side of the embryo (arrow). (D) Section through heart of embryo injected with myocardin MO. Heart tissue has been stained for Nkx2-5 transcripts. Note that the morphology of the developing heart is disrupted on the injected side (arrow). Adapted from [27] with permission from Elsevier.
3.2 Microsurgery and explants
Amphibian embryos have long been used to study the very interesting questions relating to induction and specification of the cardiac lineage [35–37]. Although most of the original studies used species other than Xenopus (newt embryos were particularly popular) many of the results have subsequently been confirmed for the Xenopus system. The central technique of these classical embryological studies involved explants of specific tissue from the embryo or microsurgery of the intact embryo. These experimental approaches have shown that both the dorsal lip/organizer of the gastrula stage embryo [7] and the underlying deep dorso-anterior endoderm [38] play inductive roles in heart formation. Further studies of the inductive process at the molecular level, using a mRNA expression in explant tissues, have demonstrated a central role for Wnt antagonism in vertebrate heart induction because expression of the Wnt antagonists dickkopf or crescent in non-cardiogenic tissues (i.e. a ventral marginal zone explant) can induce the formation of a beating heart (27). In agreement with this model, increased expression of the canonical Wnt ligands, Wnt3a or Wnt8 within tissue that would normally form heart, (i.e. a dorsal marginal zone explant) is able to block cardiogenesis [16]. While our knowledge of heart induction via endogenous Wnt antagonism remains incomplete, recent studies using marginal zone explants have suggested that Wnt antagonists may regulate the expression of the transcription factor Hex within the dorso-anterior endoderm. It is believed that Hex then regulates production of a diffusible factor that acts to induce the heart lineage in adjacent precardiac mesoderm [26].
Microsurgery techniques and explant assays have also been applied to embryos at later stages of heart development. These studies have provided significant insight into the location of endogenous signals that dictate the size and location of the developing heart [39–41]. Following up on these microsurgery approaches, experiments using dominant negative and constitutively active constructions have implicated Notch signaling as a key player in regulating the size of the heart field and ultimately the size of the heart [18].
3.2.1 The Xenopus animal cap explant model
The Xenopus animal cap has proven to be a powerful and versatile system for analysis of the ability of transcription factors and signaling molecules to influence patterns of gene expression. The use of animal cap explants is approximately equivalent to the use of mammalian cell culture systems for analysis of gene expression. The cap itself is simply a region of undifferentiated ectoderm that lies above the blastocoel of the blastula stage embryo. The animal cap explant protocol is illustrated in Figure 2. After fertilization but prior to first cleavage, mRNA encoding the regulatory protein of interest is injected into the animal region of the developing egg. As the egg continues to develop the injected mRNA is translated into protein and, at the blastula stage, (i.e. prior to mesoderm induction) the animal cap region of the embryo is excised and placed into culture. In a non-induced state the animal cap will develop into a simple mass of epithelial cells. The ability of the introduced factor to alter gene expression within the explant can be easily determined using a variety of molecular techniques including RT-PCR, in situ hybridization, microarray analysis and immunocytochemistry. The animal cap assay has proven to be particularly useful in the study of transcriptional regulation of cardiogenesis and numerous transcription factors including Nkx2-5 [27], Tbx5 [27], GATA4 [27], myocardin [27], members of the MEF2 family [42] and Sox family [28] have been assayed for their ability to regulate cardiac marker expression in this system. Under certain conditions animal caps expressing GATA4 can go beyond expression of cardiac markers, all the way to the presence of beating heart tissue [43].
Figure 2.
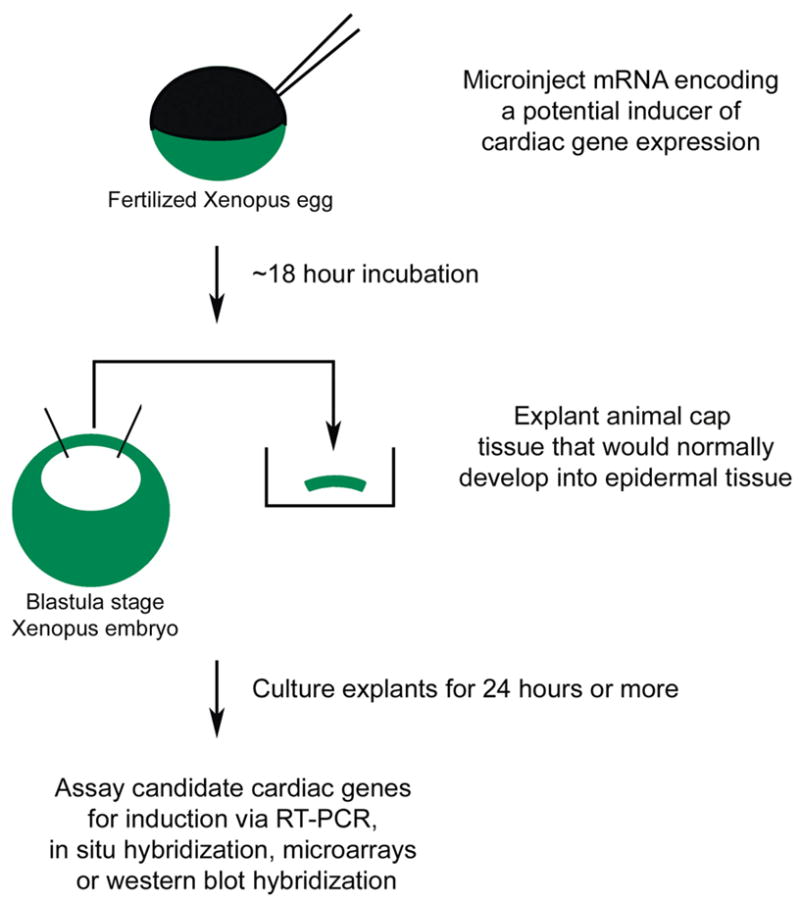
The Xenopus animal cap explant procedure. Synthetic mRNA encoding a potential regulator of cardiac development is injected into the fertilized Xenopus egg at the one cell stage. When the embryo has reached the blastula stage, the animal cap (ectodermal tissue overlying the blastocoel cavity) is carefully removed using fine forceps and it placed into explant culture. After an appropriate period in culture, the cap tissue and is subjected to appropriate assays for gene expression. Reprinted from [68] with permission from Elsevier.
3.3 Xenopus transgenesis
One of the most important advances for Xenopus developmental studies has been the development of a simple and efficient transgenesis procedure [44]. Modifications to the original procedure have been developed [45] and also additional methods involving the use of ΦC31 integrase [46] and I-SceI meganuclease [47, 48]. Regardless of the protocol used, a single researcher can generate hundreds of transgenic embryos in a single day. The application of Xenopus transgenesis to the study of heart development has so far been limited to expression of regulatory proteins under control of a cardiac specific promoter, or to in vivo analysis of gene regulatory regions.
3.3.1 Cardiac specific transgene expression
One of the primary applications of transgenesis is the ability to tightly regulate both the time and the place that a transgene is expressed in the developing embryo. Currently a number of cardiac promoters have been demonstrated to drive robust expression of a transgene in the developing myocardium of Xenopus (see Table 2). Transgenic approaches been used to demonstrate a role for BMP in heart looping [49]. Over-expression of BMP4 under control of the myosin light chain-2 promoter (MLC2) causes a randomization in the direction of heart looping while over-expression of the BMP inhibitor noggin, or a dominant negative BMP receptor, results in an unlooped heart. Transcription regulatory properties of proteins can also be addressed by utilizing promoters to mis-express a molecule outside of the heart-forming region. For example, expression of the transcription factor myocardin in neural tissues, under control of the neural specific β-tubulin promoter activated transcription of the cardiac specific myosin heavy chain α (MHCα) gene [27]. In addition, a number of inducible expression constructions have also been shown to work with Xenopus. So far however, the fact that the promoters are ubiquitously expressed [50], or the lack of a cardiac specific activator line for use with inducible binary systems [51, 52] has limited their usefulness for the study of heart development.
Table 2.
Useful promoters for tissue specific transgenic studies of heart development in Xenopus (modified from [68] with permission from Elsevier).
| Promoter | Sites of Expression | References |
|---|---|---|
| Nkx2-5 | Throughout the developing heart and pharyngeal endoderm. One of the earliest promoters expressed in the precardiac mesoderm | [53] |
| MLC1v | Cardiac and a subset of early skeletal muscle (i.e. somite). Expression in the heart restricts to the ventricle as development proceeds | [60] |
| MLC2 | Cardiac specific in the embryo | [56] |
| Rana catesbeiana (American bullfrog) cardiac troponin I | Cardiac specific | [58] |
| ANF | Cardiac specific and then atrial specific as development proceeds | [55] |
| MHCα | Cardiac and a subset of skeletal muscles(i.e. jaw muscles) | [59] |
| Cardiac α-actin | Cardiac and skeletal muscle | [44, 54] |
| Mouse Creatine kinase | Cardiac and skeletal muscle | [57] |
| Hex | Anterior endoderm | Aaron Zorn, personal communication |
3.3.2 In vivo analysis of promoters
One application of Xenopus transgenesis that offers particular promise is the in vivo analysis of cis-regulatory elements involved in regulating cardiac gene expression [53–60]. The entire transgenesis procedure is quick, inexpensive and extremely easy and very large numbers of transgenic embryos can be analyzed for each promoter construction, thus offering the potential for detailed in vivo analysis of gene regulation. Typically the promoter of interest is inserted upstream of a reporter gene, usually a variant of green fluorescent protein (GFP) because these reporters permit real-time monitoring of expression within living embryos. An example of transgenic analysis of cardiac promoter expression is illustrated by a study of the mechanisms regulating chamber specific expression atrial natriuretic factor (ANF), a gene that is initially expressed throughout the developing heart but restricts to the atria later in development. Mutation of putative cis-regulatory elements within the ANF promoter revealed that a GATA site and a NKE site (Nkx2-5 binding site) both play important roles in restricting ANF expression to the atria ([55] and Figure 3).
Figure 3.
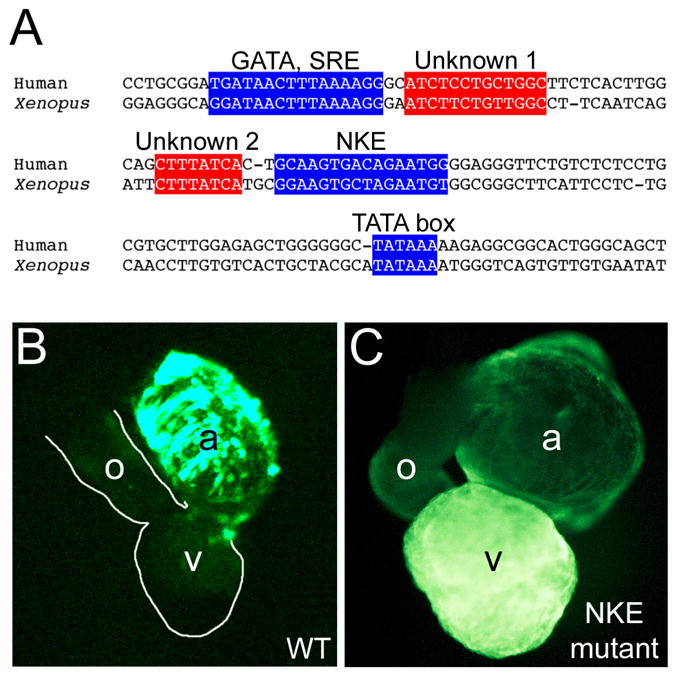
Mutational analysis of the ANF promoter using Xenopus transgenesis. (A) Alignment of the proximal promoter region of the human (top) and Xenopus (bottom) promoter sequences, showing conservation of sequence, spacing and orientation of transcription regulatory sequences. Two conserved sequences that do not correspond to binding sites for known transcription factors are indicated (Unknown 1 and 2). (B) Fluorescent image of the heart of Xenopus embryo expressing GFP reporter under control of the wild type ANF promoter. Note that GFP expression is restricted to the atrial chambers (a). (C). Fluorescent image of the heart of a Xenopus embryo expressing GFP under control of an ANF promoter in which the NKE (Nkx2-5 binding site) has been mutated. Note that mutation of the NKE prevents restriction of expression to the atria, with prominent expression persisting in the ventricular myocardium. Part A is adapted from [68] with permission from Elsevier, and Parts B and C are reprinted from [55] with permission from Elsevier.
Studies of the cardiac α-actin promoter in transgenics have shown that the sequences required for skeletal muscle expression and those for cardiac expression are separable [54]. Other transgene studies have identified an evolutionarily conserved module that is sufficient for cardiac specific expression of the Nkx2-5 gene in both the frog and mouse embryo [53]. Although each of these studies have used frog promoters, the regulatory elements required for tissue specific expression are often conserved across evolution, therefore it is also possible to study the cis-elements regulating expression of mammalian promoters in transgenic Xenopus embryos [57, 61].
4. The Xenopus genome as tool for studying heart development
Currently more than one million EST reads have been deposited in GenBank from Xenopus laevis and Xenopus tropicalis, emphasizing embryonic stages, but also representing adult organs including the heart. This wealth of information facilitates identification of cDNA sequences, which can then be ordered directly from a provider or can be cloned by RT-PCR using the sequence information from the ESTs. If the frog orthologue for a gene of interest is missing from the EST database it can often be identified by performing a BLAST search of the Xenopus tropicalis genome (accessible via http://www.jgi.doe.gov/xenopus). In most cases the orthology of genes can be confirmed by examination of the linear arrangement of genes (synteny) in different genomes.
The availability of the Xenopus tropicalis genome offers great promise for promoter analysis via phylogenetic footprinting. A simple search of the non-coding regions of a gene for putative cis-regulatory sequences using a program that identifies transcription factor binding sites typically results in a plethora of results, most of which are meaningless because they arise by chance alone. However, comparison of promoters from evolutionary diverse organisms can reveal which elements are conserved and are therefore likely to be important. In many cases, both the primary sequence of regulatory elements and the orientation and spacing of elements are conserved between Xenopus and human. Once identified in computer alignments, the transcriptional function of the conserved regulatory can be rapidly assayed using transgenic methods.
The Xenopus tropicalis genome is diploid and offers great promise for the application of forward and reverse genetics to examine gene function. A recent pilot screen using the chemical mutagen N-ethyl-N-nitrosourea (ENU) demonstrated that forward genetic screening is practical [62]. This screen identified 77 candidate mutations, 12 of which exhibited defects in heart development and/or function. Furthermore, the study showed that mutations in specific target genes could be identified by genomic TILLING. Finally, the ability of FLP and Cre recombinase [63–65] to function in Xenopus tissues suggests that targeted disruption of Xenopus genes may become a reality in the near future.
Acknowledgments
A.S.W. is the recipient of a Postdoctoral Fellowship Award from the American Heart Association. P.A.K. is the Allan C. Hudson and Helen Lovaas Endowed Professor of the Sarver Heart Center at the University of Arizona College of Medicine. Work in the Krieg lab is funded by the Sarver Heart Center and a National Institutes of Health research grant from the National Heart, Lung and Blood Institute (R01 HL074184).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellerby CW. A rapid test for the diagnosis of pregnancy. Nature. 1934;133:494–5. [Google Scholar]
- 2.Shapiro HA, Zwarenstein H. A rapid test for pregnancy on Xenopus laevis. Nature. 1934;133:762. [Google Scholar]
- 3.Keller RE. Vital dye mapping of the gastrula and neurula of Xenopus laevis. II. Prospective areas and morphogenetic movements of the deep layer. Dev Biol. 1976;51(1):118–37. doi: 10.1016/0012-1606(76)90127-5. [DOI] [PubMed] [Google Scholar]
- 4.Moody SA. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol. 1987;122(2):300–19. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- 5.Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99(4):527–51. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- 6.Sater AK, Jacobson AG. The specification of heart mesoderm occurs during gastrulation in Xenopus laevis. Development. 1989;105(4):821–30. doi: 10.1242/dev.105.4.821. [DOI] [PubMed] [Google Scholar]
- 7.Sater AK, Jacobson AG. The role of the dorsal lip in the induction of heart mesoderm in Xenopus laevis. Development. 1990;108(3):461–70. doi: 10.1242/dev.108.3.461. [DOI] [PubMed] [Google Scholar]
- 8.Mohun TJ, Leong LM, Weninger WJ, Sparrow DB. The morphology of heart development in Xenopus laevis. Dev Biol. 2000;218(1):74–88. doi: 10.1006/dbio.1999.9559. [DOI] [PubMed] [Google Scholar]
- 9.Kolker SJ, Tajchman U, Weeks DL. Confocal imaging of early heart development in Xenopus laevis. Dev Biol. 2000;218(1):64–73. doi: 10.1006/dbio.1999.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleaver O, Krieg PA. Expression from DNA injected into Xenopus embryos. Methods Mol Biol. 1999;127:133–53. doi: 10.1385/1-59259-678-9:133. [DOI] [PubMed] [Google Scholar]
- 11.Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118(3):719–29. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- 12.Cleaver OB, Patterson KD, Krieg PA. Overexpression of the tinman-related genes XNkx-2.5 and XNkx-2.3 in Xenopus embryos results in myocardial hyperplasia. Development. 1996;122(11):3549–56. doi: 10.1242/dev.122.11.3549. [DOI] [PubMed] [Google Scholar]
- 13.Mercola M. Embryological basis for cardiac left-right asymmetry. Semin Cell Dev Biol. 1999;10(1):109–16. doi: 10.1006/scdb.1998.0280. [DOI] [PubMed] [Google Scholar]
- 14.Grammer TC, Liu KJ, Mariani FV, Harland RM. Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev Biol. 2000;228(2):197–210. doi: 10.1006/dbio.2000.9945. [DOI] [PubMed] [Google Scholar]
- 15.Gilchrist MJ, Zorn AM, Voigt J, Smith JC, Papalopulu N, Amaya E. Defining a large set of full-length clones from a Xenopus tropicalis EST project. Dev Biol. 2004;271(2):498–516. doi: 10.1016/j.ydbio.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15(3):304–15. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Katsev S, Cai C, Evans S. BMP signaling is required for heart formation in vertebrates. Dev Biol. 2000;224(2):226–37. doi: 10.1006/dbio.2000.9802. [DOI] [PubMed] [Google Scholar]
- 18.Rones MS, McLaughlin KA, Raffin M, Mercola M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development. 2000;127(17):3865–76. doi: 10.1242/dev.127.17.3865. [DOI] [PubMed] [Google Scholar]
- 19.Grow MW, Krieg PA. Tinman function is essential for vertebrate heart development: elimination of cardiac differentiation by dominant inhibitory mutants of the tinman-related genes, XNkx2-3 and XNkx2-5. Dev Biol. 1998;204(1):187–96. doi: 10.1006/dbio.1998.9080. [DOI] [PubMed] [Google Scholar]
- 20.Horb ME, Thomsen GH. Tbx5 is essential for heart development. Development. 1999;126(8):1739–51. doi: 10.1242/dev.126.8.1739. [DOI] [PubMed] [Google Scholar]
- 21.Wang DZ, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105(7):851–62. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 22.Dagle JM, Weeks DL. Oligonucleotide-based strategies to reduce gene expression. Differentiation. 2001;69(2–3):75–82. doi: 10.1046/j.1432-0436.2001.690201.x. [DOI] [PubMed] [Google Scholar]
- 23.Nutt SL, Bronchain OJ, Hartley KO, Amaya E. Comparison of morpholino based translational inhibition during the development of Xenopus laevis and Xenopus tropicalis. Genesis. 2001;30(3):110–3. doi: 10.1002/gene.1042. [DOI] [PubMed] [Google Scholar]
- 24.Peterkin T, Gibson A, Patient R. GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation. Embo J. 2003;22(16):4260–73. doi: 10.1093/emboj/cdg400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown DD, Martz SN, Binder O, Goetz SC, Price BM, Smith JC, et al. Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development. 2005;132(3):553–63. doi: 10.1242/dev.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19(3):387–96. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small EM, Warkman AS, Wang DZ, Sutherland LB, Olson EN, Krieg PA. Myocardin is sufficient and necessary for cardiac gene expression in Xenopus. Development. 2005;132(5):987–97. doi: 10.1242/dev.01684. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Basta T, Klymkowsky MW. SOX7 and SOX18 are essential for cardiogenesis in Xenopus. Dev Dyn. 2005;234(4):878–91. doi: 10.1002/dvdy.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goetz SC, Brown DD, Conlon FL. TBX5 is required for embryonic cardiac cell cycle progression. Development. 2006;133(13):2575–84. doi: 10.1242/dev.02420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branford WW, Yost HJ. Lefty-dependent inhibition of Nodal- and Wnt-responsive organizer gene expression is essential for normal gastrulation. Curr Biol. 2002;12(24):2136–41. doi: 10.1016/s0960-9822(02)01360-x. [DOI] [PubMed] [Google Scholar]
- 31.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418(6898):636–41. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 32.Garriock RJ, D’Agostino SL, Pilcher KC, Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev Biol. 2005;279(1):179–92. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Brott BK, Sokol SY. A vertebrate homolog of the cell cycle regulator Dbf4 is an inhibitor of Wnt signaling required for heart development. Dev Cell. 2005;8(5):703–15. doi: 10.1016/j.devcel.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Onuma Y, Yeo CY, Whitman M. XCR2, one of three Xenopus EGF-CFC genes, has a distinct role in the regulation of left-right patterning. Development. 2006;133(2):237–50. doi: 10.1242/dev.02188. [DOI] [PubMed] [Google Scholar]
- 35.Copenhaver WM. Experiments on the development of the heart of Amblystoma punctatum. J Exp Zool. 1926;43:321–71. [Google Scholar]
- 36.Jacobson AG. Heart determination in the newt. J Exp Zool. 1961;146:139–51. doi: 10.1002/jez.1401460204. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson AG. Influences of ectoderm and endoderm on heart differentiation in the newt. Dev Biol. 1960;2:138–54. doi: 10.1016/0012-1606(60)90003-8. [DOI] [PubMed] [Google Scholar]
- 38.Nascone N, Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Development. 1995;121(2):515–23. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- 39.Sater AK, Jacobson AG. The restriction of the heart morphogenetic field in Xenopus laevis. Dev Biol. 1990;140(2):328–36. doi: 10.1016/0012-1606(90)90083-u. [DOI] [PubMed] [Google Scholar]
- 40.Raffin M, Leong LM, Rones MS, Sparrow D, Mohun T, Mercola M. Subdivision of the cardiac Nkx2.5 expression domain into myogenic and nonmyogenic compartments. Dev Biol. 2000;218(2):326–40. doi: 10.1006/dbio.1999.9579. [DOI] [PubMed] [Google Scholar]
- 41.Garriock RJ, Drysdale TA. Regulation of heart size in Xenopus laevis. Differentiation. 2003;71(8):506–15. doi: 10.1046/j.1432-0436.2003.7108005.x. [DOI] [PubMed] [Google Scholar]
- 42.Chambers AE, Logan M, Kotecha S, Towers N, Sparrow D, Mohun TJ. The RSRF/MEF2 protein SL1 regulates cardiac muscle-specific transcription of a myosin light-chain gene in Xenopus embryos. Genes Dev. 1994;8(11):1324–34. doi: 10.1101/gad.8.11.1324. [DOI] [PubMed] [Google Scholar]
- 43.Latinkic BV, Kotecha S, Mohun TJ. Induction of cardiomyocytes by GATA4 in Xenopus ectodermal explants. Development. 2003;130(16):3865–76. doi: 10.1242/dev.00599. [DOI] [PubMed] [Google Scholar]
- 44.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122(10):3173–83. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 45.Sparrow DB, Latinkic B, Mohun TJ. A simplified method of generating transgenic Xenopus. Nucleic Acids Res. 2000;28(4):E12. doi: 10.1093/nar/28.4.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen BG, Weeks DL. Transgenic Xenopus laevis embryos can be generated using phiC31 integrase. Nat Methods. 2005;2(12):975–9. doi: 10.1038/nmeth814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mech Dev. 2006;123(2):103–13. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev Dyn. 2006;235(1):247–52. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- 49.Breckenridge RA, Mohun TJ, Amaya E. A role for BMP signalling in heart looping morphogenesis in Xenopus. Dev Biol. 2001;232(1):191–203. doi: 10.1006/dbio.2001.0164. [DOI] [PubMed] [Google Scholar]
- 50.Wheeler GN, Hamilton FS, Hoppler S. Inducible gene expression in transgenic Xenopus embryos. Curr Biol. 2000;10(14):849–52. doi: 10.1016/s0960-9822(00)00596-0. [DOI] [PubMed] [Google Scholar]
- 51.Chae J, Zimmerman LB, Grainger RM. Inducible control of tissue-specific transgene expression in Xenopus tropicalis transgenic lines. Mech Dev. 2002;117(1–2):235–41. doi: 10.1016/s0925-4773(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 52.Das B, Brown DD. Controlling transgene expression to study Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2004;101(14):4839–42. doi: 10.1073/pnas.0401011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sparrow DB, Cai C, Kotecha S, Latinkic B, Cooper B, Towers N, et al. Regulation of the tinman homologues in Xenopus embryos. Dev Biol. 2000;227(1):65–79. doi: 10.1006/dbio.2000.9891. [DOI] [PubMed] [Google Scholar]
- 54.Latinkic BV, Cooper B, Towers N, Sparrow D, Kotecha S, Mohun TJ. Distinct enhancers regulate skeletal and cardiac muscle-specific expression programs of the cardiac alpha-actin gene in Xenopus embryos. Dev Biol. 2002;245(1):57–70. doi: 10.1006/dbio.2002.0639. [DOI] [PubMed] [Google Scholar]
- 55.Small EM, Krieg PA. Transgenic analysis of the atrial natriuretic factor (ANF) promoter: Nkx2-5 and GATA-4 binding sites are required for atrial specific expression of ANF. Dev Biol. 2003;261(1):116–31. doi: 10.1016/s0012-1606(03)00306-3. [DOI] [PubMed] [Google Scholar]
- 56.Latinkic BV, Cooper B, Smith S, Kotecha S, Towers N, Sparrow D, et al. Transcriptional regulation of the cardiac-specific MLC2 gene during Xenopus embryonic development. Development. 2004;131(3):669–79. doi: 10.1242/dev.00953. [DOI] [PubMed] [Google Scholar]
- 57.Lim W, Neff ES, Furlow JD. The mouse muscle creatine kinase promoter faithfully drives reporter gene expression in transgenic Xenopus laevis. Physiol Genomics. 2004;18(1):79–86. doi: 10.1152/physiolgenomics.00148.2003. [DOI] [PubMed] [Google Scholar]
- 58.Warkman AS, Atkinson BG. Amphibian cardiac troponin I gene’s organization, developmental expression, and regulatory properties are different from its mammalian homologue. Dev Dyn. 2004;229(2):275–88. doi: 10.1002/dvdy.10434. [DOI] [PubMed] [Google Scholar]
- 59.Garriock RJ, Meadows SM, Krieg PA. Developmental expression and comparative genomic analysis of Xenopus cardiac myosin heavy chain genes. Dev Dyn. 2005;233(4):1287–93. doi: 10.1002/dvdy.20460. [DOI] [PubMed] [Google Scholar]
- 60.Smith SJ, Ataliotis P, Kotecha S, Towers N, Sparrow DB, Mohun TJ. The MLC1v gene provides a transgenic marker of myocardium formation within developing chambers of the Xenopus heart. Dev Dyn. 2005;232(4):1003–12. doi: 10.1002/dvdy.20274. [DOI] [PubMed] [Google Scholar]
- 61.Beck CW, Slack JM. Gut specific expression using mammalian promoters in transgenic Xenopus laevis. Mech Dev. 1999;88(2):221–7. doi: 10.1016/s0925-4773(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 62.Goda T, Abu-Daya A, Carruthers S, Clark MD, Stemple DL, Zimmerman LB. Genetic screens for mutations affecting development of Xenopus tropicalis. PLoS Genet. 2006;2(6):e91. doi: 10.1371/journal.pgen.0020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryffel GU, Werdien D, Turan G, Gerhards A, Goosses S, Senkel S. Tagging muscle cell lineages in development and tail regeneration using Cre recombinase in transgenic Xenopus. Nucleic Acids Res. 2003;31(8):e44. doi: 10.1093/nar/gng044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werdien D, Peiler G, Ryffel GU. FLP and Cre recombinase function in Xenopus embryos. Nucleic Acids Res. 2001;29(11):E53–3. doi: 10.1093/nar/29.11.e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waldner C, Sakamaki K, Ueno N, Turan G, Ryffel GU. Transgenic Xenopus laevis strain expressing cre recombinase in muscle cells. Dev Dyn. 2006 doi: 10.1002/dvdy.20880. [DOI] [PubMed] [Google Scholar]
- 66.Lohr JL, Yost HJ. Vertebrate model systems in the study of early heart development: Xenopus and zebrafish. Am J Med Genet. 2000;97(4):248–57. doi: 10.1002/1096-8628(200024)97:4<248::aid-ajmg1275>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 67.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis development (Daudin) New York, NY: Garland Publishing, Inc; 1994. [Google Scholar]
- 68.Warkman AS, Meadows SM, Small EM, Cox CM, Krieg PA. Cardiovascular genomics: the promise of Xenopus. Drug Discov Today: Disease Models. 2004;1(3):249–55. [Google Scholar]


