Abstract
The protein L-isoaspartyl-O-methyltransferase, coded by the pcm-1 gene in Caenorhabditis elegans, participates in the repair of age-damaged proteins. We tested the ability of pcm-1-deficient nematodes to survive starvation stress as developmentally-arrested L1 larvae. We found that pcm–1 mutant L1 larvae do not survive as well as wild-type L1 larvae when incubated in M9 medium without nutrients. We then tested whether the starved L1 larvae could continue development when allowed access to food in a recovery assay. A loss of recovery ability with age was observed for all larvae, with little or no difference between the pcm-1 mutant and wild-type N2 larvae. Interestingly, when L1 larvae were starved in cholesterol-containing S medium or M9 medium supplemented with cholesterol, the survival rates of both mutant and wild-type animals nearly doubles, with pcm-1 larvae again faring more poorly than N2 larvae. Furthermore, L1 larvae cultured in these cholesterol-containing media show an increase in Sudan Black staining over animals cultured in M9 medium. The longevity defects of pcm-1 mutants previously seen in dauer larvae and here in L1 larvae suggest a defect in the ability of pcm-1 mutants to recycle and reuse old cellular components in pathways such as autophagy. Using an autophagosomal marker, we found evidence suggesting that the pcm-1 mutation may inhibit autophagy during dauer formation, suggesting that the absence of protein repair may also interfere with protein degradation pathways.
Keywords: Protein L-isoaspartyl-O-methyltransferase, L1 larval life span, autophagy, aging, cholesterol, Sudan Black, fat, LGG-1, dauer formation
Introduction
The nematode Caenorhabditis elegans is an ideal system for the study of aging. Many studies have been done on mutants that alter adult life span (Kenyon et al., 1993; Morris et al., 1996). There are a large number of genes that regulate adult life span in C. elegans, including 95 where mutations extend life span and 11 where mutations decrease life span (SAGE KE gene database; http://sageke.sciencemag.org/cgi/genesdb). Additionally, some 100 new candidate longevity genes have been recently identified by RNAi screens (Hamilton et al., 2005; Hansen et al., 2005). C. elegans can also survive for extended periods of time as L1 and dauer larvae, which can be as long-lived as or longer-lived than the adult stage (Múnoz and Riddle, 2003). There are sets of genes where mutations affect both dauer and L1 larval survival but not adult survival, while there are other genes where mutations affect all three stages (Múnoz and Riddle, 2003). In this study, we describe a mutation in a C. elegans gene that has no effect on adult aging but that does affect aging in the L1 and dauer stages.
The isolated mutation affects the protein L-isoaspartyl-O-methyltransferase gene, pcm-1. This protein repair methyltransferase has been conserved through evolution and enzymes from prokaryotes and eukaryotes share a high degree of sequence similarity (Kagan et al., 1997a). Proteins are targets for a number of inappropriate spontaneous covalent modifications that can lead to decreased or aberrant protein function (Clarke, 2003a). L-Aspartic acid and L-asparagine residues are particularly susceptible to these undesirable reactions, forming L-isoaspartyl, D-aspartyl, or D-isoaspartyl residues (Brennan and Clarke, 1995). The protein L-isoaspartyl-O-methyltransferase can initiate the conversion of L-isoaspartyl residues to L-aspartyl residues and may reduce the content of D-aspartyl residues as well (Chavous et al., 2001; Doyle et al., 2003; Ingrosso et al., 2000; Lanthier and Desrosiers, 2004; Young et al., 2001).
In C. elegans, pcm-1-mutant animals are similar to the wild-type N2 animals in morphology, fertility, and adult life span (Kagan et al., 1997b). Interestingly, the absence of the enzyme does not appear to cause a marked accumulation of damaged proteins (Niewmierzycka and Clarke, 1999). The pcm-1-null animals have two phenotypes: they are selected against in long-term competitive population studies and they have a reduced dauer life span (Kagan et al., 1997b).
Recently, it was shown that C. elegans with mutations in genes involved in autophagy had dauer-defective phenotypes (Melendez et al., 2003). Autophagy is the major degradative pathway for long-lived proteins and cytoplasmic organelles, and may be important for survival under starvation and stress conditions (Levine and Klionsky, 2004).
Here, we show that L1 larvae, when forced to use their existing stores of energy, do not survive as well without the repair methyltransferase, which suggests that protein repair may be important in autophagy. In addition, we found that both the wild-type and pcm-1-deficient L1 larvae survive longer during starvation when cholesterol was present in the media. It is known that exogenous sterols are necessary for the growth, development, and reproduction of C. elegans (Hieb and Rothstein, 1968; Shim et al., 2002), but the role of sterols in resistance to starvation has not been previously established. We demonstrate an increase in Sudan Black staining of animals maintained in cholesterol-containing media in comparison to non-supplemented media, indicating potential increases in fat stores in the presence of cholesterol. Finally, we found that autophagosomal bodies, as measured by the LGG-1::GFP autophagosome marker, accumulate as L2d larvae approach the dauer molt and that the pcm-1 mutation interferes with this process.
Results
pcm-1-delete L1 larvae show survival defects during starvation stress
To test the importance of the pcm-1 encoded L-isoaspartyl-O-methyltransferase in L1 larval longevity, we prepared synchronous cultures of mutant pcm-1(qa201) and wild-type N2 larvae and assayed for survival in various media without food. Larvae were cultured at a density that would otherwise promote growth, provided there was a food source. As expected (Johnson et al., 1984; Múnoz and Riddle, 2003), larvae in these experiments did not develop beyond the first L1 larval stage (data not shown). Using a movement assay to distinguish alive from dead animals, we found that pcm-1 deficient larvae did not survive as well as the wild-type L1 larvae in either minimal M9 medium, a cholesterol and trace metal-containing S medium, or in M9 medium containing cholesterol (Fig. 1; Supplemental Table S1, Supplemental Table S2). In three experiments, the average median survival time decreased from the N2 to the pcm-1 mutant strain from 11.8 days to 10.8 days in M9 medium, from 22.9 to 18.3 days in S medium, and from 30.7 to 27.9 days in cholesterol-containing M9 medium (Supplemental Table S2). Similar decreases were found in maximal survival time for pcm-1 compared to N2 L1 larvae (Fig. 1; Supplemental Table S2). The overall difference in survival between the mutant and wild-type strains were found to be significant at the level of p < 0.05 in seven of the eight cases where a direct comparison could be made (Supplemental Table S2). pcm-1 deficient larvae also showed a decrease in mobility with time; they migrated less well than the N2 larvae to the bacterial lawn after seven days and only moved in response to touch stimulus more often than the N2 L1 larvae.
Fig. 1.
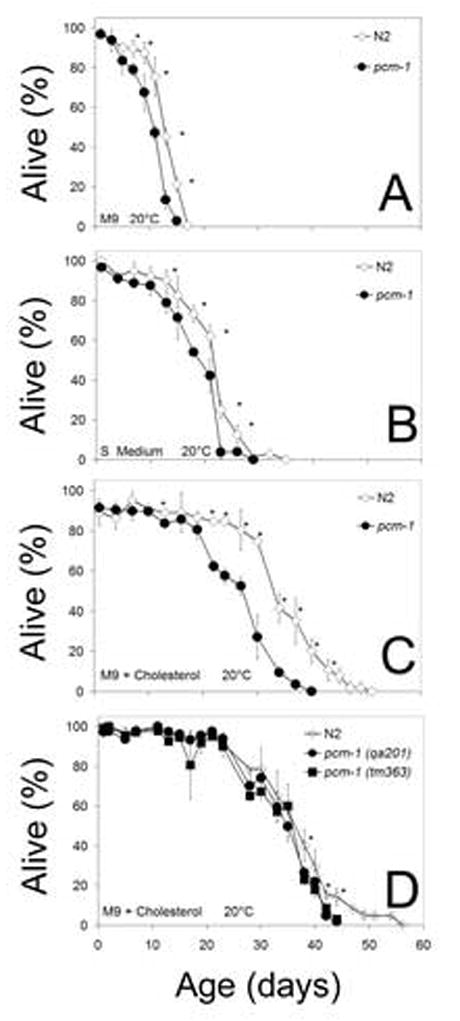
Survival of L1 larvae under starvation conditions. Larvae were aged at 20°C in various media. Panels A–C show N2 (open diamonds) and pcm-1(q201) (closed circles) in M9 medium (A), S medium (B), and M9 medium with cholesterol (C). Panel D shows a representative replicate experiment of N2 and pcm-1(q201) L1 larvae in M9 + cholesterol along with the additional mutant allele strain pcm-1(tm363) (D). Survival was measured by assaying motility or the ability to move in response to touch. Error bars represent the standard deviation of three replicate samples from each culture medium. All experiments were repeated at least once and showed similar results. Asterisks denote a p-value less than 0.05 when pcm-1 is compared to N2.
The pcm-1 gene overlaps a gene of unknown function, C10F3.4, in an antiparallel arrangement. Because the pcm-1(qa201) deletion removes potential promoter elements and an alternatively-spliced exon from the C10F3.4 gene, we confirmed the L1 longevity defects in an independent pcm-1(tm363) deletion mutant that only removes intronic portions of C10F3.4. The pcm-1(tm363) survival curve is nearly identical to the pcm-1(qa201) survival curve, suggesting that the L1 survival defect is due to the specific disruption of the pcm-1 gene (Fig. 1D).
L1 larvae survive longer in culture media containing cholesterol
M9 minimal medium lacks cholesterol, a molecule essential for growth (Hieb and Rothstein, 1968). We found that the survival of both N2 and pcm-1 deficient L1 larvae was greatly reduced in this medium compared to M9 medium supplemented with cholesterol or cholesterol-containing S-medium (Fig. 1; Supplemental Table S2). Mean survival was extended from 11–12 days in the cholesterol-deficient M9 medium to 18–31 days in the two cholesterol-containing media, and maximal survival similarly increased from 17–19 days to 29–49 days (Supplemental Table S2, p < 0.02). L1 larvae grown in both of the cholesterol-containing media also appeared to be more active than animals grown in M9 medium, showing increased mobility and appearing to thrash more rapidly.
The ability to recover from L1 arrest decreases as the larvae age
In addition to scoring survival by the movement assay (Fig. 1), the ability of the same starved L1 larvae to develop when food was presented was also measured (Fig. 2; Supplemental Table S3). Here, we considered the possibility that some larvae that were “alive” by the movement assay might not be able to recover and go on the developmental pathway towards adulthood. We thus asked what fraction of the larvae judged to be initially alive on the plates could migrate to the bacterial lawn and proceed to at least the L2 larval stage within 48 h. No recovery was seen from animals originally scored as dead. Most recovered animals developed to the L4 or young adult stage. We observed no dead larvae beyond the L1 stage, suggesting that no differential mortality occurred. For animals incubated in M9 medium, we found a rapid loss of the ability to recover after 10 days with the pcm-1 mutant larvae doing as well or more poorly than the N2 wild-type larvae at each time point (Fig. 2A). A similar situation was found in S medium or M9 medium with cholesterol (Fig. 2B and 2C, Supplemental Table S3). These results suggest that the loss of protein repair has little to no effect on their ability to move and utilize food sources when they become available. It is clear that as L1 larvae age, their ability to mature to late larval stages dramatically decreases despite their ability to survive to that age. Overall, the recovery data and the survival data show a compounded decrease with time in the ability of animals to overcome L1 starvation.
Fig. 2.
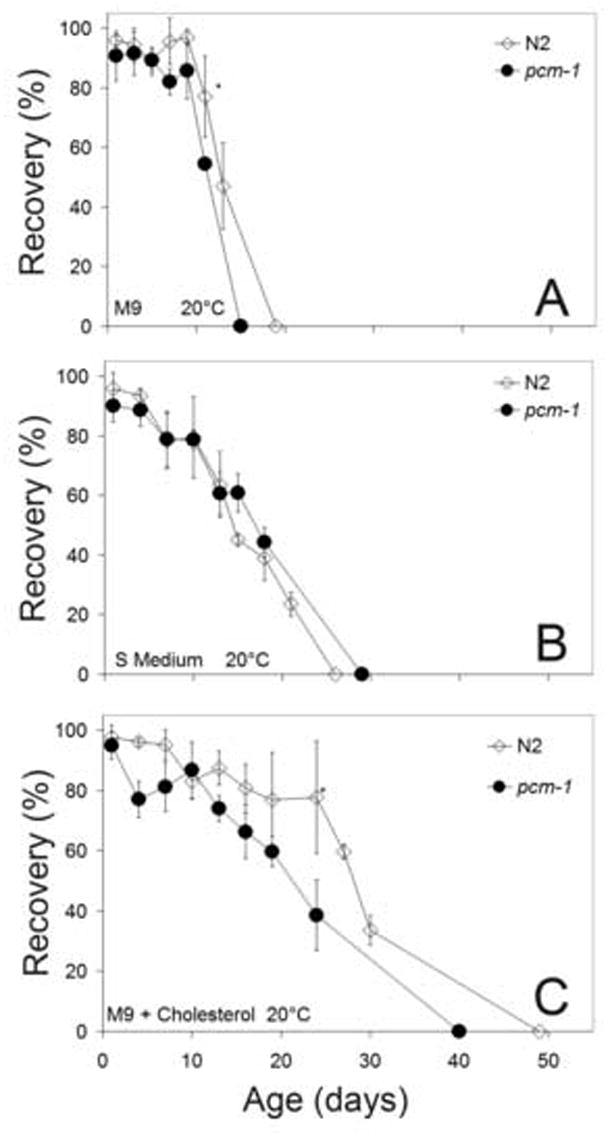
Ability of L1 larvae to recover after incubations under starvation conditions. Starved L1 larvae were placed on OP50-seeded, NGM plates at various ages and incubated at 20°C for two days. Recovery was defined as the ability to exit L1 arrest and is expressed as a percentage of the number of animals originally scored as alive. Results are shown for M9 (A), S Medium (B) and M9 medium with cholesterol (C). Asterisks denote a p-value less than 0.05 when pcm-1 is compared to N2. The values given represent the average percent recovery for samples in which the number of L1 larvae scored alive on the plates was greater than 15. The error represents the standard deviation of three replicate plates.
Size, morphology, and Sudan Black staining of N2 and pcm-1 L1 larvae
We confirmed by microscopy that pcm-1 mutant and wild-type animals grown in M9 medium and cholesterol-containing media did not develop past the L1 larval stage (Supplemental Fig. S1). At day 1, after hatching overnight without food, the larvae measured approximately 220–240 μm in length. The larvae remained in this range over the course of the life span studies in all tested media (data not shown). These measurements also show that the pcm-1-mutant larvae are initially larger than N2 larvae; they are wider by 1.2 to 1.7 μm and longer by about 16 to 17 μm at day 1 (Table 1). At later times of incubation, however, no difference was seen.
Table 1.
Size of wild-type and pcm-1 mutant L1 larvae on day 1 of L1 arrest.
| Experiment I: Day 1 L1 Measurements
|
||||
|---|---|---|---|---|
| Width (μm) | Length (μm) | Area (μm2) | N | |
| N2 | 15.8 ± 1.1 | 224 ± 24 | 2440 ± 325 | 42 |
| pcm-1 | 17.0 ± 1.0** | 240 ± 15* | 2850 ± 135** | 33 |
|
| ||||
| Experiment 2: Day 1 L1 Measurements
|
||||
| Width (μm) | Length (μm) | Area (μm2) | N | |
|
|
||||
| N2 | 17.4 ± 1.5 | 238 ± 27 | 2890 ± 420 | 30 |
| pcm-1 | 19.1 ± 1.4* | 255 ± 21** | 3350 ± 230** | 30 |
denotes 0.0001 <p< 0.001 when comparing N2 to pcm-1
denotes p< 0.0001 when comparing N2 to pcm-1
When live L1 larvae were examined under high magnification, animals incubated in cholesterol-containing media were found to be distinct from those incubated in M9 medium (Supplemental Fig. S1, panels A, E). The animals cultured in cholesterol-supplemented media appeared denser and darker with an accumulation of dark spots and granules in the head and body (Supplemental Fig. S1). No obvious differences were observed between the morphology of pcm-1 L1 animals and N2 L1 animals cultured in any media.
The granules noted in the L1 microscopy appeared similar to those characteristic of dauer larvae that stain with Sudan Black dye (Kimura et al., 1997; Cassada and Russell, 1975; Ogg et al., 1997; Wolkow et al., 2000). Sudan Black stains cholesterol esters, triglycerides, and to a lesser extent, phospholipids and free fatty acids (Bayliss High, 1981). Sudan Black does not normally stain cholesterol because it is usually found in a crystalline state in normal cells and is impenetrable to Sudan dyes (Bayliss High, 1981). Wild-type animals had little to no staining after hatching (Fig. 3, panel A) and continued to have low staining when cultured in M9 medium (Fig. 3, panels B, E, H). Animals cultured in cholesterol-containing media, however, dramatically accumulated staining from hatching to day 12 (Fig. 3, panels C, D, F, G, I, J). In cholesterol-containing media, the appearance of the larvae was darkened overall with stained granules out of the focal plane contributing to the intensity observed. The pcm-1 mutant’s staining pattern was similar to that of N2 (data not shown). These results suggest that the presence of cholesterol in the medium induces the formation of fat granules for energy storage.
Fig. 3.
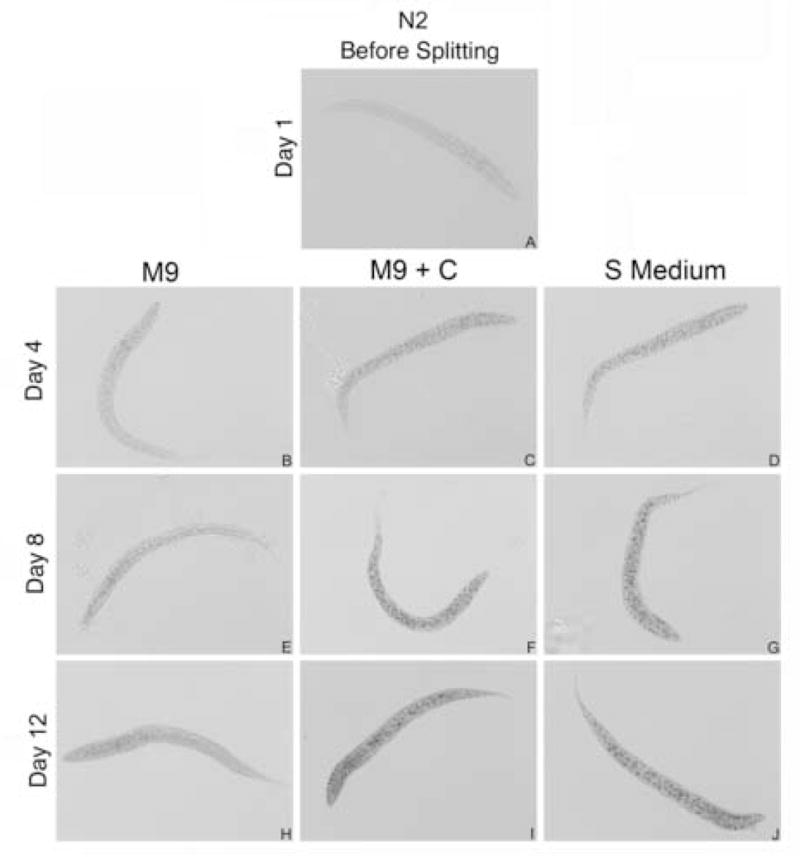
Sudan Black staining of N2 L1 larvae. L1 larvae stained for fat accumulation with Sudan Black are shown for each medium with age.
Autophagy defects in pcm-1 mutant L2d larvae
Survival as L1 larvae may depend on how well animals utilize their internal energy stores. One process for this utilization is autophagy, the engulfment of cytosol and organelles in autophagosomes for energy-generating catabolism and reshaping protein makeup. Autophagy has been shown to be important in temperature-induced dauer larvae formation in the dauer constitutive mutant (daf-c), daf-2 (Melendez et al., 2003). Since pcm-1 larvae have L1 and dauer larvae survival defects (Kagan et al., 1997b), we hypothesized that pcm-1 animals may have autophagy defects that affect survival in both the L1 larval stage and the dauer larval stage.
To observe autophagosomes, we monitored the fluorescence of a GFP-LGG-1 fusion protein in transgenic animals (Melendez et al., 2003). The worm LGG-1 protein is the ortholog of the yeast Atg8 and rat LC3 proteins that associate with autophagosomal membranes and have been used as fusion protein-based markers (Kabeya et al., 2000; Kirisako et al., 1999, Melendez et al., 2003; Mizushima, 2004). Autophagosomes in transgenic GFP::LGG-1 worms have been quantified as punctate GFP positive areas in seam cells (Melendez et al., 2003). We examined wild-type and pcm-1 deficient L2d larvae at 48, 52 and 56 h after egg fertilization on dauer-inducing pheromone plates (Fig. 4A). These time points span the 12 h of development before the L2d larvae molts to become a dauer larva. The diffuse fluorescence seen throughout the seam cells at 48 h becomes distinctly punctate at 56 h. After 48 h of development, both N2 and pcm-1 mutant larvae exhibit a low number of autophagosomes as measured by the GFP signal. At 52 h of development, the diffuse dispersion of the fluorescent marker becomes more concentrated in small areas, and after 56 h little diffuse spreading of fluorescence is seen (Fig. 4A).
Fig. 4.
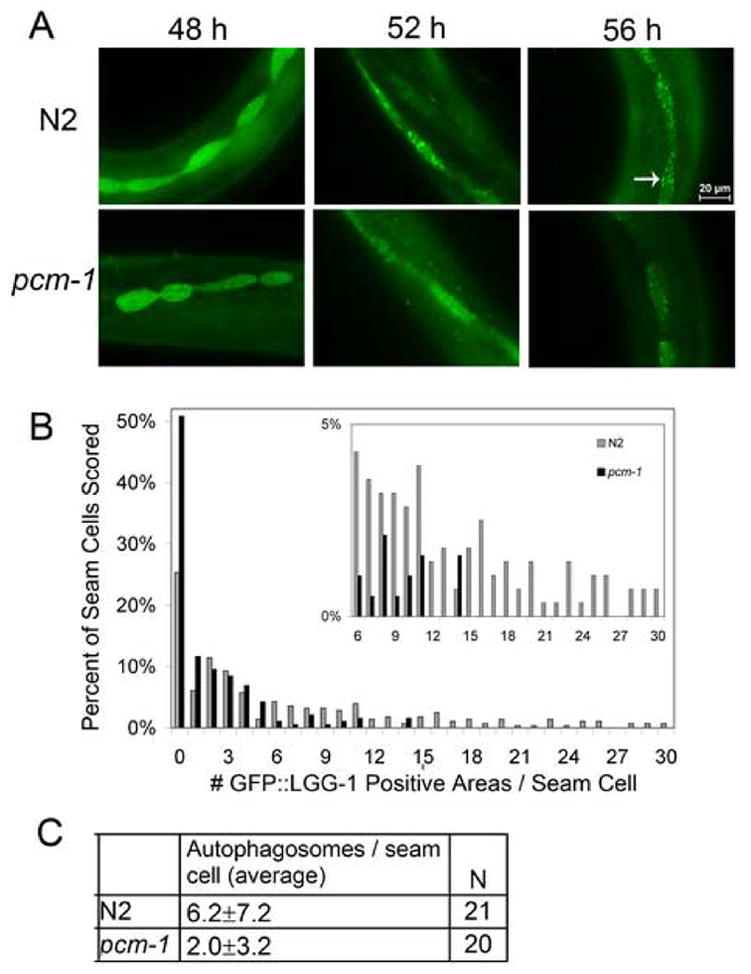
Autophagy in L2d larvae. GFP::LGG-1 positive areas/seam cell in N2 Ex[GFP::LGG-1] and pcm-1 Ex[GFP::LGG-1] L2d larvae were observed at 48, 52 and 56 h post fertilization. Panel A, fluorescence microscopy of seam cells from L2d transgenic larvae. The arrow indicates a representative autophagosome. The scale bar is 20 microns. Panel B, distribution of GFP::LGG-1 positive areas/seam cells seen at 52 h. Panel C, quantification of autophagosomes at 52 h. We employed a two-sample Kolmogorov-Smirnov statistical approach to show statistical significance (p = 0.009) for equality of distributions in the distributions of GFP::LGG-1 positive areas/seam cell in N2 and pcm-1 larvae. This experiment was replicated on two separate populations and similar results were gathered.
Although these changes were seen in both the N2 and pcm-1 mutant larvae, the pcm-1 mutant has fewer GFP::LGG-1 positive areas at all time points (Fig. 4A). Quantitation of the number of fluorescent punctate spots showed that N2 larval seam cells have a maximum of 30 spots/seam cell, while pcm-1 mutant L2d larvae have a maximum of 14 spots/seam cell (Fig. 4B). At 52 h of development, N2 L2d larvae have an average of 6.2 spots/seam cell, while pcm-1 larvae have an average of only 2.0 spots/seam cell (Fig. 4C). These results suggest a defect in autophagy in animals lacking the pcm-1 repair methyltransferase, although we cannot rule out the possibilities that the pcm-1 mutation can affect the behavior of the LGG-1 marker protein.
Discussion
This study highlights the importance of the protein repair L-isoaspartyl-O-methyltransferase in stress survival in nematodes. The decrease in the survival of L1 larvae during starvation parallels the decrease in life span of pcm-1-deficient dauer larvae and is consistent with the loss of mutant animals in long-term competitive growth experiments (Kagan et al., 1997b). These results in nematodes are similar to those found in previous studies in bacteria, where the deletion of this methyltransferase in Escherichia coli causes the most dramatic effects only when the organism is under survival stress (Visick et al., 1998).
An important finding in this study is that L1 larval life span is nearly doubled when cholesterol is added to the media for both mutant and wild-type animals. It is well known that free-living nematodes have a dietary sterol requirement for growth, development, and reproduction (Hieb and Rothstein, 1968; Lu et al., 1977; Shim et al., 2002). The dietary requirement for cholesterol is absolute, but the amount required for growth and reproduction is very low (Merris et al., 2003), suggesting that its effects are not due to changes in membrane structure (Chitwood, 1999; Kurzchalia and Ward, 2003). Cholesterol does function as a precursor to the steroid hormones involved in endocrine signaling (Entchev and Kurzchalia, 2005; Motola et al., 2006; Rottiers et al., 2006). In C. elegans, reproductive growth as opposed to dauer formation is dependent upon steroids whose biosynthesis is catalyzed by the daf-9 and daf-36 gene products and that become ligands for the nuclear hormone receptor encoded by the daf-12 gene (Entchev and Kurzchalia, 2005; Motola et al., 2006; Rottiers et al., 2006). C. elegans also make the unusual modification of cholesterol via methylation at the 4th ring position (Kurzchalia and Ward, 2003; Merris et al., 2003). The presence of unique biosynthetic enzymes in C. elegans indicates the possibility of novel cholesterol derived hormones and developmental effectors (Entchev and Kurzchalia, 2005; Kurzchalia and Ward, 2003; Merris et al., 2003).
A previous report showed that “Cholegans”, a transgenic cholesterol-producing C. elegans strain has an extended adult life-span in comparison to wild-type animals (Lee et al., 2005). The authors also showed that cholegans has a survival advantage under heat and UV stress, leading them to conclude that cholesterol confers longevity by helping animals resist stress. Those findings are in line with the current findings in this study where L1 larval survival is diminished when cholesterol is not supplemented.
In the dauer larval stage, animals rely on fat stores for survival (Kimura et al., 1997), which can be seen as a dark accumulation of granules in the animal and can be observed directly by Sudan Black staining (Cassada and Russell, 1975; Ogg et al., 1997; Wolkow et al., 2000). The correlation seen here of longer life with increased Sudan Black staining in L1 larvae cultured in cholesterol-containing media versus M9 media suggests that similar fat storage may also be important in L1 survival.
This study provides evidence that the repair methyltransferase may function in survival by ensuring the efficient use of the organism’s cellular components. When the newly hatched larvae are put under starvation stress, it forces the animals to depend solely on their initial internal resources. The decreased survival of the newly hatched pcm-1-mutant larvae under starvation stress indicates that the animals may have an inability to recycle their existing cellular components. Due to the accumulation of isomerized proteins, the methyltransferase-delete animals may be unable to properly degrade and reuse these proteins to make other components necessary for survival.
Protein repair and degradation can thus represent parallel pathways for the regeneration of stress-damaged proteins. We were interested to find a defect in this study in autophagosome formation in pcm-1 deficient L2d larvae during dauer formation, suggesting that protein repair might be needed to allow protein turnover. The exact defect or defects responsible for this mutant phenotype is unknown; perhaps the presence of unrepaired isoaspartyl-containing proteins or peptides inhibits one or more of the degradation reactions in autophagy. In conclusion, it is possible that pcm-1 not only affects protein repair but also directly or indirectly protein degradation and recycling.
In mammals, the loss of the repair methyltransferase results in more severe phenotypes. Pcmt −/− mice have seizures resulting in early death (Kim et al., 1999) that have not been seen in pcm-1 mutants of C. elegans. One possible explanation is that there may be more functional redundancy in the pathways for removing age-damaged proteins either by repair or degradation to amino acids in worms. We note that there is a homolog of the pcm-1 gene in C. elegans (R119.5) that may also bind damaged L-isoaspartyl residues, although there is no evidence that it is a repair methyltransferase. In mammals, where preserving post-translational modifications such as those that may lead to memory and learning is important, the repair pathway may be allowed to predominate over degradation pathways (Clarke, 2003). On the other hand, nematodes may have very active proteolytic pathways that work in conjunction with the repair process and that may largely mask the loss of repair (Niewmierzycka and Clarke, 1999). The autophagy phenotype observed here may come from the intersection of the repair and proteolytic pathways.
Supplementary Material
Acknowledgments
We are extremely grateful to Pamela Larsen for her continuing advice on this project. We also want to thank Brad McEvoy for biostatistical analysis of the autophagy experiments, and to Sean Curran, Carla Koehler and the Koehler lab for microscopy assistance. We would also like to thank Alicia Mèlendez and Beth Levine for kindly providing the strain N2 Ex[GFP::LGG-1] as well as Shohei Mitani for providing the pcm-1(tm363) strain. Some strains used in this study were obtained from the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This work was supported in part by the United States Public Service Institutional Award T32 GM07185 to K. L. B., National Institutes of Health grants AG18000 and GM26020 to S. C., and T.A.G. was supported by a MARC U*STAR traineeship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Banfield KL. Regulatory roles of the S-Adenosylmethionine-dependent-O-methyltransferases, PhD Thesis. UCLA; Los Angeles: 2004. [Google Scholar]
- Bayliss High OB. The histochemical versatility of Sudan Black B. Acta Histochem Suppl. 1981;24:247–55. [PubMed] [Google Scholar]
- Brennan TV, Clarke S. Deamidation and isoaspartate formation in model synthetic peptides: the effects of sequence and solution environment. In: Aswad DW, editor. Deamidation and isoaspartate formation in peptides and proteins. CRC Press; Boca Raton: 1995. pp. 65–90. [Google Scholar]
- Byerly L, Cassada RC, Russell RL. The life cycle of the nematode Caenorhabditis elegans I. Wild-type growth and reproduction. Dev Biol. 1976;51:23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–42. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Chavous DA, Jackson FR, O’Connor CM. Extension of the Drosophila lifespan by overexpression of a protein repair methyltransferase. Proc Natl Acad Sci U S A. 2001;98:14814–8. doi: 10.1073/pnas.251446498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DJ. Biochemistry and function of nematode steroids. Crit Rev Biochem Mol Biol. 1999;34:273–84. doi: 10.1080/10409239991209309. [DOI] [PubMed] [Google Scholar]
- Clarke S. Aging as war between chemical and biochemical processes: Protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–285. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Doyle HA, Gee RJ, Mamula MJ. A failure to repair self-proteins leads to T cell hyperproliferation and autoantibody production. J Immunol. 2003;171:2840–7. doi: 10.4049/jimmunol.171.6.2840. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Kurzchalia TV. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin Cell Dev Biol. 2005;16:175–82. doi: 10.1016/j.semcdb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:119–28. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieb WF, Rothstein M. Sterol requirement for reproduction of a free-living nematode. Science. 1968;160:778–80. doi: 10.1126/science.160.3829.778. [DOI] [PubMed] [Google Scholar]
- Ingrosso D, D’Angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267:4397–405. doi: 10.1046/j.1432-1327.2000.01485.x. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Mitchell DH, Kline S, Kemal R, Foy J. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech Ageing Dev. 1984;28:23–40. doi: 10.1016/0047-6374(84)90150-7. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan RM, McFadden HJ, McFadden PN, O’Connor CM, Clarke S. Molecular phylogenetics of a protein repair methyltransferase. Comp Biochem Physiol. 1997a;117B:379–85. doi: 10.1016/s0305-0491(96)00333-1. [DOI] [PubMed] [Google Scholar]
- Kagan RM, Niewmierzycka A, Clarke S. Targeted gene disruption of the Caenorhabditis elegans L-isoaspartyl protein repair methyltransferase impairs survival of dauer stage nematodes. Arch Biochem Biophys. 1997b;348:320–8. doi: 10.1006/abbi.1997.0362. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kim E, Lowenson JD, Clarke S, Young SG. Phenotypic analysis of seizure-prone mice lacking L-isoaspartate (D-aspartate) O-methyltransferase. J Biol Chem. 1999;274:20671–8. doi: 10.1074/jbc.274.29.20671. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–6. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147:435–46. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia TV, Ward S. Why do worms need cholesterol? Nat Cell Biol. 2003;5:684–8. doi: 10.1038/ncb0803-684. [DOI] [PubMed] [Google Scholar]
- Lanthier J, Desrosiers RR. Protein L-isoaspartyl methyltransferase repairs abnormal aspartyl residues accumulated in vivo in type-I collagen and restores cell migration. Exp Cell Res. 2004;293:96–105. doi: 10.1016/j.yexcr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–83. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Shim YH, Chitwood DJ, Hwang SB, Lee J, Paik YK. Cholesterol-producing transgenic Caenorhabditis elegans lives longer due to newly acquired enhanced stress resistance. Biochem Biophys Res Commun. 2005;328:929–36. doi: 10.1016/j.bbrc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Lee SS. Whole genome RNAi screens for increased longevity: Important new insights but not the whole story. Experimental Gerontology. doi: 10.1016/j.exger.2006.06.048. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Lu NC, Newton C, Stokstad ELR. The requirement of sterol and various sterol precursors in free-living nematodes. Nematologica. 1977;23:57–61. [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–91. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Merris M, Wadsworth WG, Khamrai U, Bittman R, Chitwood DJ, Lenard J. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4alpha-methyl sterols. J Lipid Res. 2003;44:172–81. doi: 10.1194/jlr.m200323-jlr200. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2000;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–9. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–23. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Múnoz MJ, Riddle DL. Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics. 2003;163:171–80. doi: 10.1093/genetics/163.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewmierzycka A, Clarke S. Do damaged proteins accumulate in Caenorhabditis elegans L-isoaspartate methyltransferase (pcm-1) deletion mutants? Arch Biochem Biophys. 1999;364:209–18. doi: 10.1006/abbi.1999.1114. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–9. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, Antebi A. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev Cell. 2006;10:473–82. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Shim YH, Chun JH, Lee EY, Paik YK. Role of cholesterol in germ-line development of Caenorhabditis elegans. Mol Reprod Dev. 2002;61:358–66. doi: 10.1002/mrd.10099. [DOI] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Vol. 1. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 587–606. [Google Scholar]
- Visick JE, Cai H, Clarke S. The L-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses. J Bacteriol. 1998;180:2623–9. doi: 10.1128/jb.180.10.2623-2629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee M, Ruvkun G. Regulation of C. elegans lifespan by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Young AL, Carter WG, Doyle HA, Mamula MJ, Aswad DW. Structural integrity of histone H2B in vivo requires the activity of protein L-isoaspartate O-methyltransferase, a putative protein repair enzyme. J Biol Chem. 2001;276:37161–5. doi: 10.1074/jbc.M106682200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


