Abstract
Background
There are few investigations of the potential recovery of neurocognitive function in chronic alcoholic samples after very long-term abstinence. The current study examined cognitive abilities in middle-aged, (mean age 46.8 years) long-term abstinent alcoholics (LTAA). Twenty-five LTAA men and 23 LTAA women abstinent for an average of 6.7 years were compared to an equal number of gender and age comparable normal controls (NC). We examined the association of neurocognitive variables with age, duration of abstinence, alcohol use measures, and the density of family history of problem drinking.
Methods
LTAA and NC underwent comprehensive neuropsychological assessment. Performance was measured in the following nine domains: abstraction/cognitive flexibility, attention, auditory working memory, immediate memory, delayed memory, psychomotor function, reaction time, spatial processing, and verbal skills.
Results
LTAA performed similarly to NC, except for deficits in the spatial processing domain. The spatial processing results must be interpreted with caution because of multiple comparison issues; however, spatial processing deficits are among the impairments most often reported in abstinent alcoholics. None of the cognitive measures was associated with length of abstinence, any alcohol use variable, or family history measure.
Conclusions
Very long-term abstinence resolves most neurocognitive deficits associated with alcoholism, except for the suggestion of lingering deficits in spatial processing.
Keywords: Alcoholism, long-term abstinence, cognition, neuropsychology, aging
Introduction
The deleterious effects of alcoholism on cognitive functioning were reported in the literature as early as the 1880s by Wernicke (Wernicke, 1881) and Korsakoff (Korsakoff, 1887), followed by Hamilton in 1906 (Hamilton, 1906), and Fisher in 1910 (Fisher, 1910). By the 1960s, the studies by Fitzhugh and coworkers introduced the clinical neuropsychological model to the study of cognitive function in alcoholism, and marked the beginning of systematic research in this area (Fitzhugh et al., 1960; Fitzhugh et al., 1965). The current literature reflects a developing understanding of the time course of the recovery of cognitive functions during abstinence, and the multiple factors that may influence the presence and severity of cognitive deficits in specific patients (Bates et al., 2005; Brady and Sinha, 2005; Fein et al., 1990; Munro et al., 2000; Oscar-Berman, 2000; Parsons et al., 1987; Rosenbloom et al., 2005; Rosenbloom et al., 2004; Sullivan et al., 2002; Sullivan et al., 2000a; Sullivan et al., 2000b).
The presence of neuropsychological deficits in alcoholics after abstinence is not a trivial issue; at least some deficits are present in a majority of abstinent alcoholics after acute detoxification (Fein et al., 1990). Although researchers in the field of alcoholism have made great headway in the study of cognitive function in abstinence, there are several limitations in the current literature. First, there have been no large-scale epidemiologic studies establishing the prevalence of cognitive deficits in the alcoholic population at large. Most of the investigations of neuropsychological function in abstinent alcoholics are studies of relatively early abstinence only. In addition, the great majority of these studies used convenience samples from inpatient or outpatient treatment settings. We have shown that bias is present in such treated samples because they have more severe early alcohol use trajectories (Fein and Landman, 2005). Bias in treated samples may also be present with regard to the presence and severity of cognitive impairments, psychiatric co-morbidity, and other factors that may contribute to the decision to seek treatment.
These caveats aside, the available data illustrate the tremendous extent to which neuropsychological problems are exhibited in alcoholics entering treatment and in early abstinence. Fortunately, the evidence also reports clinically significant recovery of function in most cognitive domains over the first months to one year of abstinence (Munro et al., 2000; Sullivan et al., 2000a; Sullivan et al., 2000b). These investigators found that age was an important modifying variable, with older alcoholics showing more severe cognitive deficits and less recovery of function in early abstinence. However, there is a dearth of data on the extent of recovery of cognitive abilities in long-term abstinence. The current study addresses this gap in our understanding by investigating cognitive function in very long-term abstinent alcoholics. We examined cognitive performance in middle-aged (average age 46.8 years) alcoholic men and women abstinent for an average of 6.7 years, compared to age and gender comparable normal controls. In long-term abstinence, we expected to find: 1) further recovery of cognitive abilities (compared to the literature reporting cognitive deficits in alcoholics with short-term abstinence), 2) that cognitive performance was correlated with increasing abstinence, and 3) that increasing age was an important variable modifying the magnitude and time course of recovery of cognitive abilities with abstinence.
Methods
Subjects
A total of 96 participants were recruited from the community by postings at AA meetings, mailings, newspaper advertisements, a local Internet site, and subject referrals. The study consisted of two subject groups, long-term abstinent alcoholics (LTAA) and age and gender comparable light/non-drinking normal controls (NC). The LTAA group (n = 48) contained 25 men and 23 women, ranging from 35 to 57 years of age (mean = 46.8 years), abstinent from 6 months to 13 years. The inclusion criteria for the LTAA group were: 1) met lifetime DSM-IV-R (American Psychiatric Association, 2000) criteria for alcohol dependence 2) had a lifetime drinking average of at least 100 standard drinks per month for men, and 80 standard drinks per month for women, and 3) were abstinent for at least 6 months. A standard drink was defined as 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor. The control group also consisted of 25 men and 23 women, ranging in age from 34 to 59 years of age (mean = 45.6 years). The inclusion criteria for the NC group was a lifetime drinking average of less than 30 standard drinks per month, with no periods of drinking more than 60 drinks per month.
Exclusion criteria for both groups were: 1) lifetime or current diagnosis of schizophrenia or schizophreniform disorder (c-DIS) (Robins et al., 1998), 2) history of drug abuse or dependence or current substance abuse or dependence (other than nicotine or caffeine), 3) significant history of head trauma or cranial surgery, 4) history of significant neurological disease, 5) history of diabetes, stroke, or hypertension that required medical intervention, 6) laboratory evidence of hepatic disease, or 7) clinical evidence of Wernicke-Korsakoff syndrome.
Procedures
All subjects were fully informed of the study’s procedures and aims, and signed a consent form prior to their participation. Subjects completed four sessions that lasted between an hour and a half and three hours, and included clinical, neuropsychological, electrophysiological, and neuroimaging assessments. Normal controls were asked to abstain from consuming alcohol for at least 24 hours prior to any lab visit. A Breathalyzer (Intoximeters, Inc., St. Louis, MO) test was administered to all subjects. A 0.000 alcohol concentration was required of all participants in all sessions. We had one positive Breathalyzer test result, on an “abstinent alcoholic” subject; that subject was excluded from the study. Subjects were compensated for time and travel expenses upon completion of each session. Subjects who completed the entire study were also given a completion bonus.
General Assessment
All subjects participated in the following assessments: 1) psychiatric status regarding exclusionary criteria was assessed using the c-DIS (Robins et al., 1998), 2) subjects were interviewed on their drug and alcohol use using the lifetime drinking history methodology (Skinner and Allen, 1982; Skinner and Sheu, 1982; Sobell and Sobell, 1990; Sobell et al., 1988), 3) medical histories were reviewed, 4) blood was drawn to test liver functions, and 5) the Family Drinking Questionnaire was administered based on the methodology of Mann et al. (Mann et al., 1985; Stoltenberg et al., 1998).
Family Drinking Density
Family Drinking Density was assessed using the Family History Drinking Questionnaire (Mann et al., 1985; Stoltenberg et al., 1998), participants were asked to rate their biological relatives as being alcohol abstainers, alcohol users with no problem, or problem drinkers. The proportion of first-degree relatives that were identified as problem drinkers was determined from this questionnaire. Table 1 presents a summary of the demographic, alcohol use, and family drinking density variables.
Table 1.
Demographic and Alcohol Use Variables
| Abstinent Alcoholics | Controls | Group Effect Sizea | |||
|---|---|---|---|---|---|
| Variables | Male (n=25) | Female (n=23) | Male (n=25) | Female (n=23) | |
| Age | 45.6 ± 7.0 | 48.1 ± 6.4 | 43.4 ± 6.3 | 48.0 ± 6.6 | 0.8 |
| Yrs of Ed | 15.5 ± 2.0 | 15.5 ± 2.4 | 16.3 ± 2.2 | 16.0 ± 1.9 | 2.5 |
| Proportion of 1st Degree Relative Problem Drinkers | 0.41 ± 0.25 | 0.45 ± 0.30 | 0.14 ± 0.22 | 0.17 ± 0.21 | 24.2b*** |
| Alcohol Use Variables | |||||
| Alcohol Use (months) | 245.4 ± 88.8 | 268.1 ± 102.8 | 232.2 ± 130.3 | 294.0 ± 130.1 | c |
| Avg Dose (drinks/mo) | 176.3 ± 135.0 | 128.1 ± 79.1 | 6.9 ± 8.3 | 6.8 ± 7.6 | c |
| Peak Use (months) | 51.0 ± 30.9 | 92.1 ± 81.2 | 115.2 ± 154.9 | 113.8 ± 112.5 | c |
| Peak Dose (drinks/mo) | 361.8 ± 257.2 | 264.0 ± 202.8 | 15.1 ± 14.5 | 16.7 ± 22.4 | c |
| Abstinence Duration (yrs) | 6.9 ± 6.2 | 6.5 ± 5.6 | N/A | N/A | c |
Percent of variance of dependent variable accounted for by group membership
The proportions were fist normalized using the arcsin transformation
Statistical comparisons between groups on the alcohol use variables are not appropriate since amount of alcohol use was a selection criteria
Effect is significant:
= p≤0.05,
= p≤0.01,
= p≤0.001
Neuropsychological Assessment
The neuropsychological assessments were administered in one session. The battery began with the administration of the following individual tests: Rey-Osterrieth Complex Figure (copy, immediate, and 20 minute delayed) (Osterrieth, 1944), Trail Making Test A and B (Reitan and Wolfson, 1985), Symbol Digit Modalities Test (written administration only) (Smith, 1968), American version of the Nelson Adult Reading Test (AMNART) (Grober and Sliwinski, 1991), Short Category Test (booklet format)(Wetzel and Boll, 1987), Controlled Oral Word Association Test (COWAT) (Benton and Hamsher, 1983), Paced Auditory Serial Addition Test (PASAT) (Gronwall, 1977), Block Design (WAIS-R) (Wechsler, 1981), Stroop Color and Word Test (Golden, 1978), Fregly Ataxia Battery (Fregly et al., 1973), and the Simulated Gambling Task (Bechara et al., 1994).
After a 15 minute break, the subject completed the MicroCog (MC) Assessment of Cognitive Functioning (standard version) (Powell et al., 1993). The MicroCog is a computer-administered and -scored test that assesses important neurocognitive function in adults. MicroCog was designed to be sensitive to detecting cognitive impairment across a wide range, and takes into account levels of premorbid intellectual functioning by providing age- and education-level adjusted norms.
Normative scores derived from a nationally representative sample of adults are available for each test, either from the creators or distributors of the tests. Z-scores for the neuropsychological domains and measures were computed based on standardized norms adjusted for age [Stroop (Golden, 1978), Short Categories (Wetzel and Boll, 1987), PASAT (Stuss et al., 1988), Block Design (Wechsler, 1997), and Rey (Denman, 1987)], years of education [AMNART (Schwartz and Saffran, 1987)], age and years of education [Symbol Digit Modalities (Smith, 1982), MicroCog (Powell et al., 1993)], and age, gender, and years of education [(Trails A and B (Heaton et al., 1991), COWAT (Ruff et al., 1996)]. The Stroop, Symbol Digit Modalities, and the MicroCog test norms are not specific to gender, since gender did not significantly affect scores in the normative samples (Golden, 1978; Powell et al., 1993; Smith, 1982). The AMNART is used as a measure of premorbid intelligence (Grober and Sliwinski, 1991). The AMNART did not have age norms because the test was designed to be resistant to the effects of normal aging and most neurodegenerative diseases. Additionally, Grober et al (1991) have reported that gender does not influence AMNART scores.
The final NP battery consisted of the following 10 domains, and their component tests: (1) Attention (Stroop Color, MC Numbers Forward, MC Numbers Reversed, MC Alphabet, MC Word List 1) (2) Verbal Fluency (COWAT, AMNART), (3) Abstraction/Cognitive Flexibility (Short Categories, Stroop interference score, Trail Making Test B, MC Analogies, MC Object Match A), (4) Psychomotor (Trails A, Symbol Digit), (5) Immediate Memory (MC Story immediate recall, Rey immediate recall, MC Word List 2), (6) Delayed Memory (MC Story delayed recall, Rey delayed recall), (7) Reaction Time (MC Timers simple and cued), (8) Spatial Processing (MC Tic Tac, MC Clocks, Block Design), and (9) Auditory Working Memory (PASAT at delays of 2.4, 2.0, 1.6, and 1.2 seconds). Table 2 presents raw scores as well as group, gender, and group by gender effect sizes for all of the neuropsychological measures.
Table 2.
Neuropsychological Domains and Individual Tests (Raw Scores)
| Abstinent Alcoholic | Control | Effect Size (%) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Male (n=25) | Female (n=23) | Male (n=25) | Female (n=23) | Group | Gender | Group by Gender |
| Neuropsychological Domains (Raw scores) | |||||||
|
| |||||||
| Abstraction/Cognitive Flexibility | |||||||
| MicroCog Analogies | 10.92 ± 3.45 | 13.65 ± 2.42 | 12.44 ± 3.43 | 13.61 ± 2.42 | 1.2 | 7.7** | 1.3 |
| MicroCog Object Match A | 8.60 ± 2.89 | 11.04 ± 1.62 | 10.10 ± 2.80 | 10.67 ± 1.97 | 1.4 | 9.3** | 3.8.06 |
| Short Categories | 28.84 ± 14.39 | 29.61 ± 16.08 | 28.44 ± 15.31 | 27.83 ± 14.23 | 0.1 | 0.0 | 0.1 |
| Stroop-Interference | 40.39 ± 9.94 | 45.17 ± 9.37 | 42.40 ± 8.66 | 45.65 ± 8.95 | 0.5 | 4.7* | 0.2 |
| Trails B | 61.48 ± 21.15 | 64.00 ± 21.26 | 57.60 ± 16.69 | 62.13 ± 18.93 | 0.6 | 0.8 | 0.1 |
| Attention | |||||||
| MicroCog Alphabet | 10.83 ± 0.48 | 11.00 ± 0.00 | 10.92 ± 0.40 | 11.04 ± 0.21 | 1.0 | 4.7* | 0.1 |
| MicroCog Numbers Forward | 9.92 ± 4.21 | 10.26 ± 2.67 | 10.72 ± 3.41 | 10.87 ± 3.03 | 1.1 | 0.1 | 0.0 |
| MicroCog Numbers Reversed | 8.88 ± 3.65 | 9.48 ± 2.84 | 10.36 ± 3.93 | 10.00 ± 3.33 | 2.1 | 0.0 | 0.5 |
| MicroCog Wordlist 1 | 9.71 ± 2.97 | 10.83 ± 2.25 | 10.04 ± 2.79 | 11.00 ± 1.35 | 0.3 | 4.5* | 0.0 |
| Stroop-Color | 71.43 ± 11.92 | 77.50 ± 11.92 | 72.00 ± 10.39 | 76.26 ± 13.20 | 0.0 | 4.7* | 0.1 |
| Auditory Working Memory | |||||||
| PASAT 2.4 (seconds delay) | 40.80 ± 11.40 | 39.76 ± 13.83 | 45.96 ± 9.85 | 42.50 ± 11.46 | 2.9 | 1.0 | 0.3 |
| PASAT 2.0 (seconds delay) | 37.44 ± 9.15 | 41.00 ± 7.34 | 40.12 ± 9.68 | 38.76 ± 9.55 | 0.0 | 0.4 | 1.9 |
| PASAT 1.6 (seconds delay) | 32.96 ± 8.80 | 34.00 ± 6.38 | 37.08 ± 10.62 | 35.76 ± 9.02 | 2.7 | 0.0 | 0.4 |
| PASAT 1.2 (seconds delay) | 25.79 ± 6.62 | 24.39 ± 5.07 | 27.22 ± 8.85 | 27.24 ± 7.13 | 2.3 | 0.2 | 0.3 |
| Immediate Memory | |||||||
| MicroCog Story Recall | 9.00 ± 1.59 | 9.48 ± 0.95 | 9.68 ± 1.18 | 9.52 ± 1.47 | 1.9 | 0.4 | 1.5 |
| MicroCog Wordlist 2 | 14.25 ± 3.87 | 14.96 ± 1.43 | 14.36 ± 3.28 | 15.39 ± 1.08 | 0.3 | 2.6 | 0.1 |
| Rey-Immediate Recall | 43.48 ± 11.86 | 43.78 ± 10.82 | 45.00 ± 11.22 | 46.22 ± 12.84 | 0.7 | 0.1 | 0.0 |
| Delayed Memory | |||||||
| MicroCog Story-Delayed Recall | 8.96 ± 2.97 | 10.83 ± 2.71 | 9.64 ± 3.16 | 11.22 ± 3.62 | 0.8 | 7.3** | 0.1 |
| Rey-Delayed Recall | 43.36 ± 11.24 | 42.91 ± 11.57 | 44.12 ± 12.26 | 46.22 ± 12.87 | 0.7 | 0.1 | 0.3 |
| Psychomotor | |||||||
| Symbol Digit Modalities | 49.88 ± 6.95 | 54.70 ± 7.19 | 54.68 ± 10.22 | 57.05 ± 10.84 | 4.0.06 | 4.0* | 0.5 |
| Trails A | 30.24 ± 9.02 | 27.57 ± 6.65 | 31.04 ± 12.18 | 30.43 ± 7.83 | 1.0 | 0.8 | 0.3 |
| Reaction Time | |||||||
| MicroCog Cued Timers | 10.96 ± 1.49 | 10.87 ± 1.58 | 11.08 ± 1.50 | 10.57 ± 2.04 | 0.1 | 0.9 | 0.4 |
| MicroCog Simple Timers | 10.82 ± 1.53 | 10.60 ± 1.73 | 10.83 ± 1.43 | 11.03 ± 1.50 | 0.5 | 0.0 | 0.5 |
| Spatial Processing | |||||||
| Block Design | 45.76 ± 8.18 | 42.83 ± 11.99 | 50.40 ± 10.14 | 45.35 ± 9.59 | 3.2 | 4.0* | 0.3 |
| MicroCog Clocks | 10.38 ± 2.39 | 11.43 ± 1.08 | 11.08 ± 1.47 | 11.35 ± 1.61 | 0.8 | 3.8.06 | 1.4 |
| MicroCog Tic Tac | 8.46 ± 2.40 | 8.09 ± 1.78 | 9.24 ± 2.67 | 8.91 ± 2.64 | 2.8 | 0.5 | 0.0 |
| Verbal | |||||||
| AMNART | 33.80 ± 6.98 | 37.00 ± 5.35 | 33.12 ± 7.50 | 37.83 ± 6.43 | 0.0 | 8.4** | 0.3 |
| COWAT | 43.32 ± 8.49 | 47.30 ± 11.21 | 43.48 ± 14.64 | 45.35 ± 11.39 | 0.2 | 1.6 | 0.2 |
Measures are reported mean ± standard deviation.
Effect is significant:
= p≤0.05,
= p≤0.01,
= p≤0.001
Global Clinical Impairment Score (GCIS)
Each domain’s average Z-score was converted to a global clinical impairment score (GCIS). A clinical impairment score of 0, or no impairment, was assigned to domain Z-scores falling above the 15th percentile, a score of 1, or moderately impaired, was assigned to domain Z-scores falling at or below the 15th and above the 5th percentile, and a score of 2, or severely impaired, was assigned to domain Z-scores falling at or below the 5th percentile. The cutoff points for the clinical impairment scores were designed to make the GCIS sensitive to clinically relevant impairment. The domain clinical impairment scores (0, 1, or 2) were then summed across domains to yield the GCIS, with greater GCIS scores indicating more severe impairment.
Statistical Analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS Inc., 2004). Analysis of Variance (ANOVA) for unbalanced designs was carried out using the General Linear Models procedure. We also used Analysis of Covariance (ANCOVA) to assess the effect of age on the performance of the LTAA group versus the NC group. We performed the ANCOVA with age as the covariate on both the domain scale scores and on the ranks (across all subjects) of each domain scale score. The analysis of the ranks was equivalent to examining Spearman correlations of age with domain scores within each group. (Spearman correlations are robust with respect to underlying distribution assumptions and resistant to the effects of outliers.) Following the use of ANCOVA on the ranks of each domain scale score, we tested for differences in the correlations between groups. Spearman correlations were used to examine the association of cognitive measures with alcohol use, demographic, and family history measures. Associations of cognitive performance measures with abstinence duration in the LTAA group were analyzed using partial correlation analysis (partialling out the effect of age).
Results
Demographic and Subject Variables
Table 1 presents demographic and alcohol use variables for all participants. The groups were similar in years of education, but did differ significantly in family drinking history, with the abstinent alcoholics having a greater proportion of first-degree relatives who were problem drinkers (F1,92 = 32.08 p < 0.001). On average, the LTAA men drank 6 drinks per day and the LTAA women drank 4 drinks per day. NC drank 0.2 drinks per day for men and women. LTAA had a peak alcohol dose that was almost twenty times the peak dose for NC.
NP performance
The NP performance results are presented in Table 2 and 3 (Table 2 presents the raw scores, and Table 3 presents the z-scores). ANOVA of the average z-score across domains and of the GCIS showed essentially equivalent performance in the LTAA and NC groups. The only domain score in which there was a difference between groups was in spatial processing, where LTAA performed worse than NC (F1, 92= 3.86, p = 0.05; uncorrected for multiple comparisons). In addition, none of the individual tests evidenced group differences. Figure 1 shows the essentially identical profile across cognitive domains of performance of LTAA and NC. There were also gender differences, with women performing better than men across a number of domains (see Table 3, multivariate test Wilks λ9,81 = 0.735, p = 0.002), but no group by gender interactions. There were no group, age, or group by age interactions for the average z-score across domains, or for the GCIS, or for the multivariate comparison across all domains Wilks λ9,81 = 0.892, p = 0.396. There were no correlations between any neuropsychological variable and any alcohol use measure.
Table 3.
Neuropsychological Domains and Individual Tests (Z-scores)
| Abstinent Alcoholic | Control | Effect Size (%) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Male (n=25) | Female (n=23) | Male (n=25) | Female (n=23) | Group | Gender | Group by Gender |
| Neuropsychological Domains (z-scores) | |||||||
|
| |||||||
| Abstraction/Cognitive Flexibility | 0.00 ± 0.64 | 0.40 ± 0.43 | 0.19 ± 0.56 | 0.37 ± 0.56 | 0.5 | 6.5** | 1.1 |
| MicroCog Analogies | 0.31 ± 1.15 | 1.22 ± 0.81 | 0.81 ± 1.14 | 1.20 ± 1.43 | 1.2 | 7.7** | 1.3 |
| MicroCog Object Match A | −0.47 ± 0.96 | 0.35 ± 0.54 | 0.03 ± 0.93 | 0.22 ± 0.66 | 1.4 | 9.3** | 3.8.06 |
| Short Categories | −0.14 ± 0.99 | −0.08 ± 1.33 | −0.18 ± 1.19 | 0.13 ± 1.21 | 0.1 | 0.6 | 0.3 |
| Stroop-Interference | 0.06 ± 0.75 | 0.38 ± 0.99 | 0.11 ± 0.67 | 0.30 ± 0.64 | 0.00 | 2.8 | 0.2 |
| Trails B | 0.27 ± 0.92 | 0.15 ± 1.31 | 0.18 ± 1.01 | −0.03 ± 1.07 | 0.4 | 0.6 | 0.0 |
| Attention | −0.15 ± 0.58 | 0.10 ± 0.39 | 0.05 ± 0.59 | 0.18 ± 0.52 | 1.8 | 3.3.08 | 0.4 |
| MicroCog Alphabet | 0.28 ± 0.16 | 0.33 ± 0.00 | 0.31 ± 0.13 | 0.35 ± 0.07 | 1.0 | 4.7* | 0.1 |
| MicroCog Numbers Forward | −0.03 ± 1.40 | 0.09 ± 0.89 | 0.24 ± 1.14 | 0.29 ± 1.01 | 1.1 | 0.1 | 0.0 |
| MicroCog Numbers Reversed | −0.38 ± 1.22 | −0.17 ± 0.95 | 0.12 ± 1.31 | 0.00 ± 1.11 | 2.1 | 0.0 | 0.5 |
| MicroCog Wordlist 1 | −0.10 ± 0.99 | 0.28 ± 0.75 | 0.01 ± 0.93 | 0.33 ± 0.45 | 0.3 | 4.5* | 0.0 |
| Stroop-Color | −0.41 ± 0.76 | 0.03 ± 0.79 | −0.42 ± 0.70 | −0.06 ± 0.86 | 0.1 | 6.2* | 0.1 |
| Auditory Working Memory | −0.07 ± 0.71 | −0.29 ± 1.15 | 0.14 ± 0.87 | −0.20 ± 1.27 | 0.6 | 2.0 | 0.1 |
| PASAT (2.4 seconds delay) | −0.23 ± 1.06 | −0.29 ± 1.23 | 0.25 ± 0.96 | −0.29 ± 1.33 | 1.1 | 1.8 | 1.2 |
| PASAT (2.0 seconds delay) | −0.21 ± 0.80 | 0.08 ± 0.72 | −0.13 ± 0.95 | −0.08 ± 0.92 | 0.1 | 1.0 | 0.5 |
| PASAT (1.6 seconds delay) | 0.06 ± 0.66 | 0.13 ± 0.49 | 0.34 ± 0.87 | 0.24 ± 0.67 | 2.0 | 0.0 | 0.4 |
| PASAT (1.2 seconds delay) | 0.20 ± 0.56 | 0.06 ± 0.47 | 0.28 ± 0.82 | 0.29 ± 0.66 | 1.4 | 0.3 | 0.3 |
| Immediate Memory | 0.48 ± 0.63 | 0.60 ± 0.40 | 0.54 ± 0.57 | 0.71 ± 0.46 | 0.6 | 1.9 | 0.1 |
| MicroCog Story Recall | −0.33 ± 0.53 | −0.17 ± 0.32 | −0.11 ± 0.39 | −0.16 ± 0.49 | 1.9 | 0.4 | 1.5 |
| MicroCog Wordlist 2 | 1.42 ± 1.29 | 1.65 ± 0.48 | 1.45 ± 1.09 | 1.80 ± 0.36 | 0.3 | 2.6 | 0.1 |
| Rey-Immediate Recall | 0.25 ± 0.95 | 0.33 ± 0.88 | 0.28 ± 0.82 | 0.49 ± 1.03 | 0.3 | 0.6 | 0.1 |
| Delayed Memory | 0.01 ± 0.85 | 0.27 ± 0.72 | 0.06 ± 0.81 | 0.45 ± 0.98 | 0.5 | 3.7.07 | 0.2 |
| MicroCog Story-Delayed Recall | −0.35 ± 0.99 | 0.28 ± 0.90 | −0.12 ± 1.05 | 0.41 ± 1.21 | 0.8 | 7.3** | 0.1 |
| Rey-Delayed Recall | 0.27 ± 0.94 | 0.26 ± 0.94 | 0.24 ± 0.96 | 0.49 ± 1.08 | 0.3 | 0.4 | 0.4 |
| Psychomotor | −0.31 ± 0.59 | 0.13 ± 0.81 | −0.21 ± 0.98 | 0.02 ± 0.90 | 0.0 | 4.1* | 0.4 |
| Symbol Digit Modalities | −0.27 ± 0.76 | 0.33 ± 0.84 | 0.14 ± 1.02 | 0.57 ± 1.21 | 2.9.10 | 6.9** | 0.2 |
| Trails A | −0.36 ± 0.89 | −0.07 ± 1.07 | −0.56 ± 1.14 | −0.45 ± 0.88 | 2.2 | 1.0 | 0.2 |
| Reaction Time | 0.30 ± 0.43 | 0.24 ± 0.42 | 0.32 ± 0.43 | 0.27 ± 0.51 | 0.1 | 0.3 | 0.0 |
| MicroCog Cued Timers | 0.32 ± 0.50 | 0.29 ± 0.53 | 0.36 ± 0.50 | 0.19 ± 0.68 | 0.1 | 0.9 | 0.4 |
| MicroCog Simple Timers | 0.27 ± 0.51 | 0.20 ± 0.58 | 0.28 ± 0.48 | 0.34 ± 0.50 | 0.5 | 0.0 | 0.5 |
| Spatial Processing | 0.14 ± 0.53 | 0.14 ± 0.50 | 0.42 ± 0.56 | 0.29 ± 0.50 | 4.0* | 0.4 | 0.4 |
| Block Design | 0.72 ± 0.75 | 0.59 ± 1.03 | 1.15 ± 0.92 | 0.78 ± 0.87 | 3.0.09 | 1.9 | 0.5 |
| MicroCog Clocks | 0.13 ± 0.80 | 0.48 ± 0.36 | 0.36 ± 0.49 | 0.45 ± 0.54 | 0.8 | 3.8.06 | 1.4 |
| MicroCog Tic Tac | −0.51 ± 0.80 | −0.64 ± 0.59 | −0.25 ± 0.89 | −0.36 ± 0.88 | 2.8 | 0.5 | 0.0 |
| Verbal | 0.97 ± 0.48 | 1.20 ± 0.50 | 0.87 ± 0.74 | 1.13 ± 0.55 | 0.6 | 4.3* | 0.0 |
| AMNART | 1.20 ± 0.45 | 1.40 ± 0.36 | 1.20 ± 0.46 | 1.47 ± 0.42 | 0.2 | 7.2** | 0.2 |
| COWAT | 0.75 ± 0.72 | 1.00 ± 0.96 | 0.54 ± 1.26 | 0.78 ± 0.85 | 1.2 | 1.7 | 0.0 |
| Average Domain z-Score | 0.16 ± 0.36 | 0.32 ± 0.34 | 0.26 ± 0.46 | 0.36 ± 0.52 | 0.8 | 2.3 | 0.2 |
| Global Clinical Impairment Score (GCIS) | 0.56 ± 1.00 | 0.30 ± 0.64 | 0.62 ± 1.05 | 0.52 ± 1.04 | 0.8 | 1.2 | 0.1 |
Measures are reported mean ± standard deviation.
Effect is significant:
= p≤0.05,
= p≤0.01,
= p≤0.001
Figure 1.
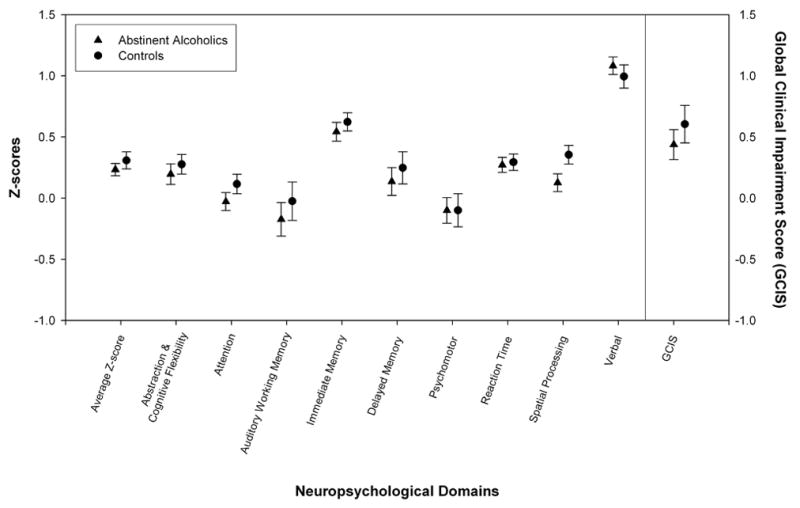
The mean ± standard error for each cognitive domain is presented for the LTAA and NC groups, illustrating the similarity of cognitive profiles across domains for the two groups.
Discussion
We examined cognitive function in middle-aged alcoholic men and women abstinent an average of 6.7 years. Generally, LTAA performed as well as NC; except in the spatial processing domain, where LTAA performed more poorly than NC. The cognitive performance of LTAA was not associated with duration of abstinence or age (consistent with the majority of cognitive recovery occurring during the first years of abstinence) (Crews et al., 2005).
We offer the finding of spatial processing deficits in LTAA versus NC with caution. First, there were no statistically significant group differences on any of the three tests within the spatial processing domain; only when the three tests were averaged together was there a statistically significant difference between groups. Second, spatial processing was one of nine neuropsychological domains tested, without correction for multiple comparisons. Therefore, there is not strong statistical evidence for the spatial processing deficit. Nevertheless, spatial processing deficits are among the impairments most often reported in abstinent alcoholics, and other investigators also report that this specific deficit may not resolve, even with long-term sobriety (Crews et al., 2005; Munro et al., 2000; Oscar-Berman et al., 1997; Sullivan, 2005; Sullivan et al., 2002).
We also found better cognitive performance across many domains in women (both normal and alcoholic) compared to men. No such effect should have been observed since we used gender specific norms for the majority of tests, and on the other tests, the normative data do not suggest gender differences. We hypothesize that the heightened performance of our San Francisco Bay Area participants compared to the normative sample (the average z-score across domains averaged about 0.33 in NC) was greater for women than for men. The fact that the highest domain score in the NC (and in the LTAA) was the verbal domain is consistent with the very high SES and incomes in the areas from which we recruited our subjects.
This study reinforces the generally held view that spatial processing function may be particularly vulnerable to impairment in alcoholics, and especially resistant to full recovery with long-term abstinence. Spatial information processing ability, which is dependent on right hemisphere and frontal cortex function declines with normal aging (Mattay et al., 2006; Meguro et al., 2003; Rajah and D’Esposito, 2005; Schretlen et al., 2000). These particular results lead one to wonder whether impairments in spatial processing (in abstinence) will become even more pronounced as brain functional reserve capacity declines in old age (Di Sclafani et al., 1998; Fein and Di Sclafani, 2004).
Footnotes
Citation
Fein, G., Torres, J., Price, L.J., and Di Sclafani, V. 2006. Cognitive performance in long-term abstinent alcoholic individuals, Alcohol Clin Exp Res, 30(9), p. 1538–44.
This work was supported by Grants AA11311 (GF) and AA13659 (GF), both from the National Institute of Alcoholism and Alcohol Abuse.
References
- American Psychiatric Association. DSM-IV-R: Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Publishing, Inc.; Washington, DC: 2000. [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-Term Neuropsychological Recovery in Clients with Substance Use Disorders. Alcoholism: Clinical and Experimental Research. 2005;29(3):367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. AJA Associates; Iowa City: 1983. [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162(8):1483–93. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh el W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29(8):1504–13. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Denman SB. Denman Neuropsychology Memory Scale. SB Denman; Charleston, SC: 1987. [Google Scholar]
- Di Sclafani V, Clark HW, Tolou-Shams M, Bloomer CW, Salas GA, Norman D, Fein G. Premorbid brain size is a determinant of functional reserve in abstinent crack-cocaine and crack-cocaine-alcohol-dependent adults. J Int Neuropsychol Soc. 1998;4(6):559–65. doi: 10.1017/s1355617798466049. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152(5):531–7. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V. Cerebral reserve capacity: implications for alcohol and drug abuse. Alcohol. 2004;32(1):63–7. doi: 10.1016/j.alcohol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol. 2005;35(1):19–26. doi: 10.1016/j.alcohol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Fisher JT. Mental defects following the use of alcohol. S Calif Practitioner. 1910;25:569–572. [Google Scholar]
- Fitzhugh LC, Fitzhugh KB, Reitan RM. Adaptive abilities and intellectual functioning in hospitalized alcoholics. Q J Stud Alcohol. 1960;21:414–423. [PubMed] [Google Scholar]
- Fitzhugh LC, Fitzhugh KB, Reitan RM. Adaptive abilities and intellectual functioning in hospitalized alcoholics: Further considerations. Q J Stud Alcohol. 1965;26:402–411. [PubMed] [Google Scholar]
- Fregly AR, Smith MJ, Graybiel A. Revised normative standards of performance of men on a quantitative ataxia test battery. Acta Otolaryngol. 1973;75(1):10–6. doi: 10.3109/00016487309139631. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Western Psychological Services; Los Angeles, CA: 1978. [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933–49. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44(2):367–73. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hamilton CL. Alcohol and the mind. Illinois Med J. 1906;9:39–46. [Google Scholar]
- Heaton RK, Grant I, Maththews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources, Inc.; Odessa, FL: 1991. [Google Scholar]
- Korsakoff S. Disturbance of psychic activity in alcoholic paralysis. Vestn Klin Psichiat Neurol. 1887;4:1–102. [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15(1–2):61–7. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392(1–2):32–7. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Meguro K, Constans JM, Shimada M, Yamaguchi S, Ishizaki J, Ishii H, Yamadori A, Sekita Y. Corpus callosum atrophy, white matter lesions, and frontal executive dysfunction in normal aging and Alzheimer’s disease. A community-based study: the Tajiri Project. Int Psychogeriatr. 2003;15(1):9–25. doi: 10.1017/s104161020300872x. [DOI] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res. 2000;24(10):1510–6. [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. NIAAA Publications; Bethesda: 2000. pp. 437–471. vol NIAAA Research Monograph No. 34. [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World. 1997;21(1):65–75. [PMC free article] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complex. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of alcoholism: Implications for diagnosis and treatment. Guilford Press; New York: 1987. [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog Assessment of Cognitive Functioning. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128(Pt 9):1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychology Press; Tucson: 1985. [Google Scholar]
- Robins LN, Cottler L, Buckholz K, Compton W. The Diagnostic Interview Schedule for DSM-IV. Washington University School of Medicine; St. Louis, MO: 1998. [Google Scholar]
- Rosenbloom MJ, O’Reilly A, Sassoon SA, Sullivan EV, Pfefferbaum A. Persistent cognitive deficits in community-treated alcoholic men and women volunteering for research: limited contribution from psychiatric comorbidity. J Stud Alcohol. 2005;66(2):254–65. doi: 10.15288/jsa.2005.66.254. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18(3):589–97. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–38. [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. J Int Neuropsychol Soc. 2000;6(1):52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Saffran E. The American-NART: Replication and extension of the British findings on the persistence of the word pronunciation skills in patients with dementia. Philadelphia, PA: 1987. [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43(11):1157–70. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith A. The symbol digit modalities test: A neuropsychological test of learning and other cerebral disorders. In: Helmuth J, editor. Learning Disorders. Special Child Publications; Seattle, WA: 1968. pp. 83–91. [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Western Psychological Services; Los Angeles, CA: 1982. [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: State of the art and future directions. Behaviorial Assessment. 1990;12:77–90. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49(3):225–32. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS 13.0 for Windows. 13. SPSS Inc; Chicago IL: 2004. [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93(10):1511–20. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Pelchat G. Three tests of attention and rapid information processing: an extension. The Clinical Neuropsychologist. 1988;2:246–250. [Google Scholar]
- Sullivan EV. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180(4):583–94. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16(1):74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000a;14(2):178–88. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000b;24(5):611–21. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale -- Third Edition: Administration and Scoring Manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wernicke C. Lehrbuch der Gehirnkrankheiten fur Aerzte und Studirende. Vol. 2. Theodor Fisher, Kassel U; Berlin: 1881. pp. 229–242. [Google Scholar]
- Wetzel L, Boll T. Short Category Test, Booklet Format. Western Psychological Services; Los Angeles, CA: 1987. [Google Scholar]


