Abstract
We recently demonstrated impairment on the Simulated Gambling Task (SGT) in long-term abstinent alcoholics (AbsAlc). Brain regions that have been shown to be necessary for intact SGT performance are the ventromedial prefrontal cortex (VMPFC) and the amygdala; patients with VMPFC or amygdalar damage demonstrate SGT impairments similar to those of substance abusing populations. We examined these brain regions, using T1-weighted MRIs, in the 101 participants from our previous study using voxel-based morphometry (VBM). VBM was performed using a modification we developed (Fein et al., 2006) of Baron’s procedure, (Baron et al., 2001), in which we use skull-stripped images as input. We also restricted the analysis to a ROI consisting of the amygdala and VMPFC as defined by the Talairach Daemon resource. Compared to the controls, the AbsAlc participants had significant foci of reduced gray matter density within the amygdala. Thus, SGT decision-making deficits are associated with reduced gray matter in the amygdala, a brain region previously implicated in similar decision-making impairments in neurological samples. This structurally based abnormality may be the result of long-term alcohol abuse or dependence, or it may reflect a pre-existing factor that predisposes one to severe alcoholism. From an image analysis perspective, this work demonstrates the increased sensitivity that results from using skull-stripped inputs and from restricting the analysis to a ROI. Without both of these methodological advances, no statistically significant finding would have been forthcoming from this work.
Keywords: Simulated Gambling Task, Alcohol Abuse, Long-Term Abstinence, MRI, Amygdala, Ventromedial Prefrontal Cortex, Decision-Making
Introduction
Alcoholism and drug abuse are disorders in which people continue their use of harmful substances despite major long-term negative consequences (e.g. in the areas of employment, family, education, and health). A number of studies (Bechara, 2001; Bechara et al., 2001; Grant et al., 2000) have examined the mechanisms underlying this aspect of substance dependence using the simulated gambling task (SGT) developed by Bechara and colleagues (1994). The SGT simulates real-life decision-making that requires an individual to weigh long and short-term rewards and punishments in an atmosphere of uncertain outcomes. A hallmark of drug and alcohol abuse is that users persist in behaviors that have short-term benefits (e.g., intoxication) despite long-term major negative consequences.
The gambling task was initially developed to study patients with acquired sociopathy due to damage to the ventromedial prefrontal cortex (VMPFC) (Bechara et al., 1994; Bechara et al., 1997). Such patients often take part in risky behaviors that are immediately gratifying while ignoring negative future outcomes. It is thought that they cannot see beyond short-term rewards to potential long-term consequences (Bechara et al., 1994). Compared with controls, when engaged in the SGT, patients with VMPFC lesions consistently choose to draw more cards from decks with larger immediate rewards and long-term net losses, than from decks with a smaller immediate reward, smaller delayed punishments and long-term net gains (Bechara et al., 1994; Bechara et al., 1997). Dysfunction of the VMPFC may predispose an individual to make disadvantageous personal choices possibly leading to socially inappropriate, or socially deviant behavior (Bechara et al., 1994; Bechara et al., 1997), or to drink excessively even when it leads to significant problems.
There is also a growing body of literature implicating the amygdala in decision-making and learning (Baxter and Murray, 2002; Baxter et al., 2000; Kahn et al., 2002; Rogers et al., 2004; Tabert et al., 2001; Winstanley et al., 2004). Furthermore, Bechara and others have shown that patients suffering from damage to the amygdala also show impairments on the SGT (Bar-On et al., 2003; Bechara et al., 2003; Bechara et al., 1999; Ernst et al., 2002). Research indicates that the amygdala and VMPFC are part of a ‘circuit’ that is activated in decision-making (Baxter et al., 2000; Bechara et al., 2003; Bechara et al., 1999; Ernst et al., 2002; Winstanley et al., 2004). Findings by Bechara and colleagues indicate that the roles played by the amygdala and VMPFC in decision-making are different (Bechara et al., 2003; Bechara et al., 1999). In an SGT study measuring skin conductance responses, Bechara and colleagues found that patients with either amygdalar or VMPFC damage were impaired on the SGT, but only those with amygdalar damage did not generate skin conductance responses when they received either the positive (winning money) or negative (losing money) feedback. This finding suggests that the amygdala is involved in the attachment of emotional valence to events (i.e., rewards and punishments). In contrast, the VMPFC is involved in integrating associations between events and their outcomes (including the emotional aspects of their outcomes) (Bechara et al., 1999).
Studies show that currently active or recently detoxified alcoholics (Bechara and Damasio, 2002; Bechara et al., 2001; Mazas et al., 2000) and drug abusers (Grant et al., 2000; Petry et al., 1998) exhibit impaired performance on the SGT. Their performance is characterized by favoring larger immediate rewards while disregarding long-term negative consequences. This pattern of impaired decision-making resembles the typical decisions made by an alcoholic to drink excessively to experience the immediate pleasure of intoxication in spite of the many longer-term consequences of intoxication (Clark and Robbins, 2002). Recent research suggests that substance abusers perform poorly on the SGT because they tend to be hyper-responsive to reward and under-responsive to punishment, (Stout et al., 2004), which might reflect differences in amygdalar function. Using mathematical models of SGT performance, Stout et al (2005) found that cocaine abusers differed significantly from controls on the valence model parameter, reflecting an increased attention/response to gains and decreased attention to losses.
In a recent paper we demonstrated that long-term abstinent alcoholics (AbsAlc) (abstinence duration ranging from 6 months to 13 years, with a mean abstinence duration of 6.7 years) were impaired on the SGT compared to age and gender comparable controls (Fein et al., 2004b). In the current manuscript we used Statical Parametric Mapping (SPM2) voxel based morphometry (VBM) to examine whether the long-term abstinent alcoholics in whom we found SGT impairments also have reduced volumes of their VMPFC or amygdala. SPM2, in its standard form implements voxel-based morphometry using T1-weighted brain images that include the scalp, skull and meninges as inputs, and incorporates a morphological clean-up step to remove ‘non-brain’ tissue. In a recent manuscript (Fein et al., 2006), we found that: 1) SPM2 does a poor job removing the non-brain tissue, 2) poor alignment of individual brains with the brain in the MNI template, resulting in an incorrect delineation of cortical gray matter, and 3) that this incorrect delineation of cortical gray matter dramatically increases the error term in the SPM2 analyses, reducing the sensitivity of standard SPM2 to detect experimental effects. We modified the SPM2 processing pipeline to accept skull-stripped inputs and demonstrated that the modification reduced the error term by about one third, with concomitant increases in the sensitivity to detect experimental effects. In the current manuscript, we use skull-stripped inputs to take advantage of the increased sensitivity that doing so affords. We also corrected for multiple comparisons only in a region of interest (ROI) encompassing the VMPFC and amygdala. Restricting the analysis to a ROI enables testing of a priori hypotheses with increased power (since the analysis needs to be corrected for fewer multiple comparisons). While ROI analyses have been used extensively with fMRI data, we believe the work presented below is the first application of ROI analysis to VBM.
Materials and Methods
Participants
A total of 101 participants were recruited from the community at large by postings at AA meeting places, café postings, newspaper advertisements and a posting on a local Internet site. Two groups were recruited, controls (n = 58, 21 men and 37 women), and abstinent alcoholics (n = 43, 25 men and 18 women). The inclusion criteria for the control group was a lifetime drinking average of less than 30 drinks per month with no periods of more than 60 drinks per month. Abstinent alcoholic’s needed to meet the lifetime criteria for alcohol dependence, have a lifetime drinking average of at least 100 drinks per month for men (80 drinks per month for women), and be abstinent for at least six months. Table 1 presents demographic and alcohol use data. All participants were informed of the study’s procedures and aims, and signed a consent form prior to their participation. A detailed description of the exclusion criteria, assessments and procedures, including the administration of the computerized SGT are included in our previous paper (Fein et al., 2004b).
Table 1.
Demographic and Alcohol Use Variables for the Abstinent Alcoholic and Control Samples
| Abstinent Alcoholics | Controls | |||
|---|---|---|---|---|
| Males (n=24) | Females (n=19) | Males (n=21) | Females (n=37) | |
| Demographic Variables | ||||
| Age in years | 45.8 ± 7.0(35.1 – 55.3) | 47.3 ± 6.1(37.2 – 57.4) | 43.6 ± 6.2(35.1 – 55.3) | 45.2 ± 6.8(35.3 – 55.7) |
| Years of Education | 15.5 ± 2.1(12.0 – 20.0) | 15.8 ± 2.3(12.0 – 20.0) | 16.0 ± 1.8(12.0 – 20.0) | 16.4 ± 1.7(12.0 – 19.0) |
| Alcohol Use Variables | ||||
| Average Alcohol Dose (drinks per month) | 183.2 ± 156.2(13.0 – 582.6) | 125.7 ± 86.1(33.6 – 370.7) | 7.4 ± 8.7(0.0 – 25.6) | 6.6 ± 6.1(0.0 – 24.1) |
| Duration of Alcohol Use (years) | 21.8 ± 8.1(4.3 – 37.8) | 21.6 ± 7.7(7.0 – 39.3) | 18.5 ± 11.2(0.0 – 37.4) | 22.3 ± 10.6(0.0 – 38.2) |
| Peak Alcohol Dose (drinks per month) | 353.9 ± 270.1(36.0 – 1200.0) | 271.3 ± 222.8(70.0 – 960.0) | 14.7 ± 14.7(0.0 – 40.0) | 16.6 ± 19.9(0.0 – 90.0) |
| Duration of Peak Alcohol Use (years) | 5.2 ± 5.1(1.0 – 25.8) | 7.4 ± 7.2(0.5 – 28.2) | 6.6 ± 7.2(0.0 – 31.7) | 8.3 ± 8.1(0.0 – 28.2) |
| Abstinence Duration(years) | 7.1 ± 6.2(.5 – 18.8) | 6.2 ± 4.9(.7 – 16.9) | N/A | N/A |
Data are reported as mean ± standard deviation, and range (minimum – maximum).
Image Acquisition and Assessment
All MRIs were collected on a 1.5T GE Signa Infinity with the LX platform (GE Medical Systems, Waukesha, WI) located at the Pacific Campus of the California Pacific Medical Center. The imaging protocol included a transaxial T1-weighted Spoiled Gradient image (TR/TE/NEX = 35/5/1: 0.859 × 0.859 mm2 in-plane resolution; contiguous 1.3 mm thick slices). A neuroradiologist read all MRI scans. All scans were free from abnormalities other than white matter signal hyperintensities. Fifteen participants (9 controls and 5 abstinent alcoholics had white matter signal hyperintensities, and of those people only six (3 controls and 3 abstinent alcoholics) were advised to seek clinical correlation by the neuroradiologist. White matter signal hyperintensities are commonly associated with the aging process (Cook et al., 2002; Fazekas et al., 2005; Gunning-Dixon and Raz, 2000; Ketonen, 1998), and are seen in many individuals without clinical history or impairment, thus participants were not excluded for the presence of these findings.
Image and VBM Processing
Non-brain tissue was removed from each subject’s MRI using FSL’s Brain Extraction Tool (Smith, 2002) (with default settings) followed by manual editing to remove any additional non-brain tissue missed by BET using an in-house custom written plugin to Image J (Rasband, 2002). Optimized VBM was implemented in the framework of Statistical Parameter Mapping (SPM2) (Good et al., 2001), testing for gray matter differences between groups and included age, years of education, and cranium size as covariates. The difference in tissue volumes associated with normal variation in cranium size was removed using the inverse of the FSL v-scaling parameter (Smith et al., 2002), which we have shown previously is an excellent surrogate variable for the size of the intracranial vault (Fein et al., 2004a). The analysis included all voxels in the ROI that segmented as more than 15% gray matter.
Regions of Interest
Regions of Interest (ROIs) were defined prior to the analyses by extracting Brodmann regions 10, 11, 12, and 47 (subset of the prefrontal cortex) for the VMPFC and the amygdala from the Talairach daemon database. Since the VMPFC is only part of Brodmann regions 10, 11, 12, and 47, we then edited those areas within Talairach space to focus on the ventral medial aspects of the prefrontal cortex. Editing of the Broadman areas was accomplished using the in-house custom plug-in written to Image J described above. Slice by slice manual editing was performed in order to exclude the lateral and dorsal regions of Broadmann area’s 10, 11, 12, and 47. The resulting mask was then converted to MNI space using Matthew Brett’s nonlinear Talairach to MNI Transformation (Brett et al., 2003). All subjects were registered into the common MNI space that allowed for application of the ROI mask. We applied SPM2’s Full Width Half Maximum (FWHM) = 12mm smoothing with a Gaussian kernel to the resulting mask in order to dilate the ROI and account for the area of spatial uncertainty in the registration of each participant’s data to the MNI template. We note that there was a single mask created for the regions of interest (it was created in the Talairach Daemon space), which was then applied to all subjects’ images. Thus the definition of the ROI was applied consistently to the MRIs of all research participants.
Statistics
The data were analyzed using SPM2 (Ashburner and Friston, 2000), which performs family wise error correction over all resels (voxels after smoothing) in the analysis region. The SGT data were analyzed in our previous paper using the General Linear Model analysis in SPSS (SPSS Inc., 2004).
Results
Gambling Game Results
The SGT results are taken from an earlier paper (Fein et al., 2004b). Compared with controls, long-term abstinent alcohol-dependent individuals made more disadvantageous decisions on the SGT (F1,98 = 4.03, p < 0.05), and women made less disadvantageous decisions than men (F1,98 = 4.08, p < 0.05) (see Figure 1). No group by gender interaction was observed.
Figure 1.
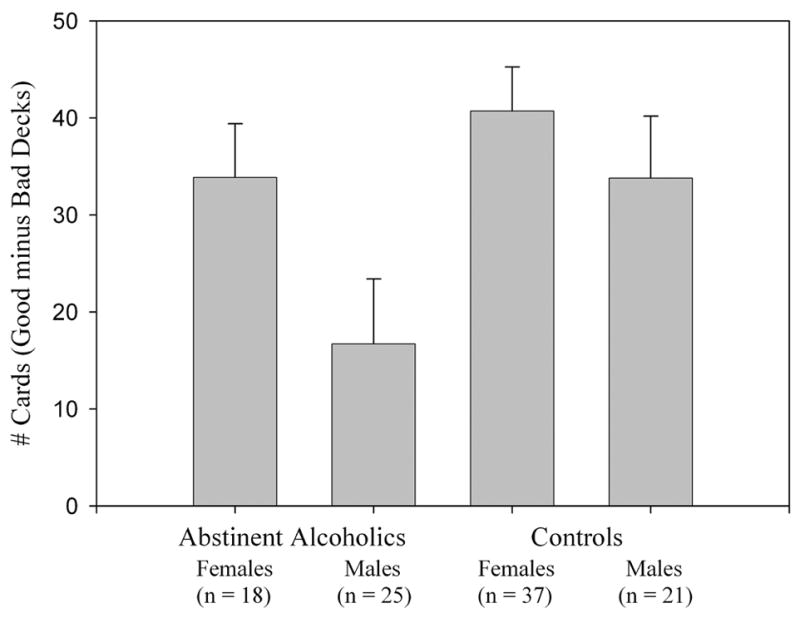
Figure 1 shows performance on the simulated gambling task (number of cards chosen from the good decks minus number of cards chosen from the bad decks) separately for males and females, and abstinent alcoholics and controls. Abstinent alcoholics performed worse on the gambling task when compared to controls (p < 0.05), and men performed poorer than women (p < 0.05). No gender by group interaction was observed.
VBM Results and Behavioral Correlations
Figure 2 depicts the results of the ROI SPM2 analysis on a glass brain. Abstinent alcoholics had significant areas of reduced gray matter bilaterally in the area of the amygdala compared to controls. On the left, there were 370 voxels of significantly reduced gray matter with a minimum p = 0.013 (family wise error corrected). On the right, there were 218 voxels of significantly reduced gray matter with a minimum p = 0.015 (family wise error corrected). There were no areas of difference between men and women, despite gender differences on the SGT. We also performed an SPM2 analysis on the ROI using SGT performance as the predictor variable, and an SPM2 analysis on the AbsAlc group only using abstinence duration as the predictor variable. There were no (family error wise error corrected) voxels in the ROIs that were associated with either predictor variable. Finally, these analyses were repeated on all participants using skulled (non-BET edited) brains, as well as entire brains without the ROI (both skulled and skull-stripped). No voxels of significance resulted from any of these analyses.
Figure 2.
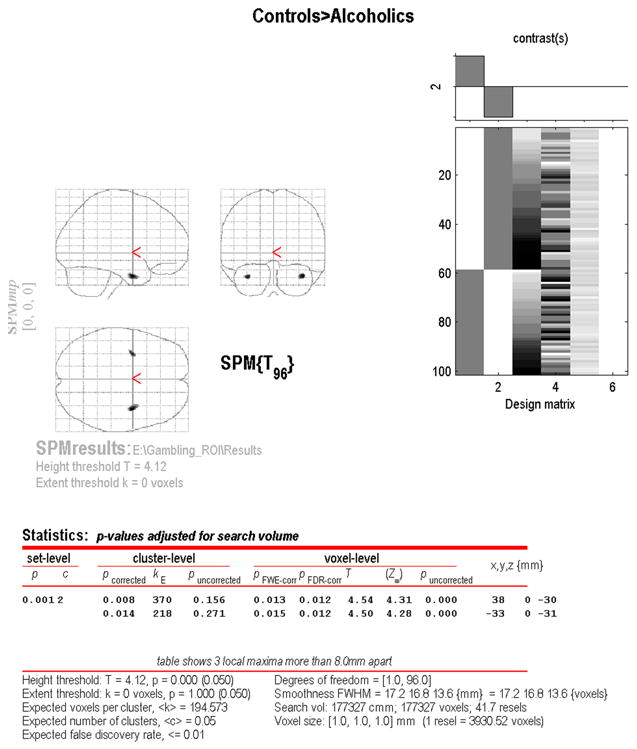
Figure 2 shows the output from the SPM2 analysis on the glass brain. The results use p(fwe) < 0.05, where p(fwe) is family wise error corrected in order to determine significance. On the left (glass brain right), the cluster of significance has 370 voxels having a minimum p(fwe) value of 0.013. On the right (glassbrain left), the cluster of significance has 218 voxels having a minimum p(fwe) value of 0.015. The glass brain coordinates are given in Talaraich space.
Figure 3 uses a gray MNI render brain with color overlay to show the areas of significantly reduced gray matter and areas of spatial uncertainty (on the left), and the masks of the VMPFC, amygdala, and ROI (on the right). Significant voxels were defined as those having SPM2 family wise error corrected p < 0.05. The spatial uncertainty is the projection of the areas of significant focus smoothed with a FWHM 12mm Gaussian kernel.
Figure 3.
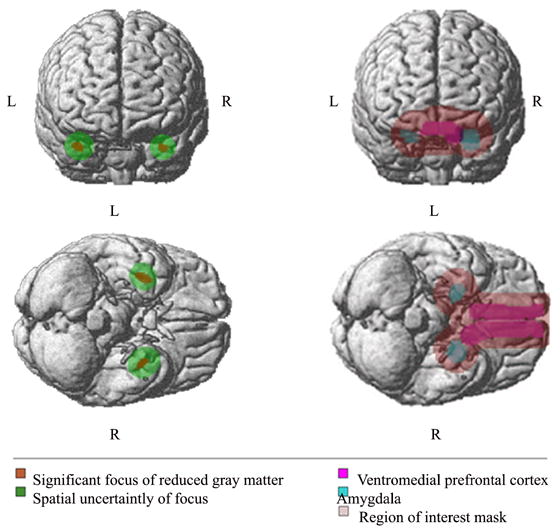
Figure 3 depicts the gray MNI template rendered brain with overlay in colors. The coronal and axial views on the right show the amygdala (blue), the ventromedial prefrontal cortex (magenta), and the ROI mask (light pink). The coronal and axial views on the left show the SPM2 areas of significant reduced gray matter (orange) (SPM2 family wise error corrected p < 0.05), and the spatial uncertainty of focus (green). The spatial uncertainty of focus is the projection of the areas of significant focus smoothed with a Full Width Half Maximum (FWHM) 12 mm Gaussian Kernel.
Discussion
The results from our VBM ROI analysis revealed that long-term abstinent alcoholics who show impairment on the SGT also show bilateral reduction of gray matter in the area of the amygdala when compared to controls. There was no evidence of differential gray matter reduction between abstinent alcoholics and controls within the VMPFC. These findings suggest that the decision-making deficits in these long-term abstinent alcoholics may reflect an abnormality in brain structure. This structurally based abnormality may be the result of long-term alcohol abuse or dependence. We have shown in a previous paper that active alcoholics have a reduction of gray matter in other areas of the brain (Fein et al., 2002). This paper goes one step further and demonstrates that there are specific areas of reduced gray matter that exist even in multi-year abstinent alcoholics when sensitive analytic methods (SPM2 with skull-stripping) are used and the analyses are restricted to the examination of specific structures. It is also possible that these brain structure abnormalities reflect a pre-existing condition that could predispose one to severe alcoholism. Individuals with a high genetic-loading for alcoholism may “start out” with a smaller amygdala, thus putting them at a disadvantage in the area of decision-making.
We did not find any association between gray matter in the ROIs and duration of abstinence. In our manuscript reporting gambling task impairments in the samples presented here (Fein et al., 2004b), and in a manuscript on cognitive function in these same subjects (Fein et al., in press), we did not find any associations between performance and duration of abstinence. In the cognition paper, we found that AbsAlc were essentially cognitively normal (except for impairments in spatial functioning which had to be interpreted gingerly because it was the only domain with impairment of 9 domains assessed – yet it was among the domains that are most frequently reported to be impaired in alcoholism and drug addiction (Crews et al., 2005; Munro et al., 2000; Oscar-Berman et al., 1997; Sullivan et al., 2002; Sullivan and Pfefferbaum, 2005). Nonetheless, we found no association between SGT performance and spatial processing ability. We interpreted the essentially intact cognitive performance of this sample (Fein et al., in press) and the lack of correlation between cognitive performance and abstinence duration as indicating that most of the cognitive recovery had already taken place by six months to one year of abstinence. As for the gambling task performance and for the reduced amygdalar gray matter finding reported here, we interpret the lack of association with duration of abstinence as being consistent with both of these phenomena reflecting predisposing factors that were present before active alcoholism occurred.
We observed gray matter reductions in the amygdala, but not in the VMPFC. Previous research indicates that disadvantageous decision-making on the SGT can be associated with gray matter reductions in either the VMPFC or the amygdala (Bechara et al., 1999). Bechara and colleagues observed decision-making deficits in patients with focal VMPFC lesions and patients with bilateral amygdalar lesions. While VMPFC and amygdalar lesions both were associated with similar overall impairments in SGT performance, the patterns of somatic activity associated performance differed between the two groups. Patients with amygdalar lesions did not generate skin conductance responses after rewards or losses, while VMPFC patients did generate such responses (Bechara et al., 1999). Bechara and colleagues (2003; 1999) propose that deficits in amygdalar function are associated with deficits in attaching emotional valence to motivationally significant events (i.e., a lack of anxiety – negative affect after experiencing loss), while VMPFC deficits are associated with problems effectively integrating somatic state information from the amygdala and other brain structures prior to executing a choice. This formulation is consistent with research that implicates the amygdala in the experience of emotion, or more specifically with the attachment of emotional valence to specific events (Aggleton and Mishkin, 1986; Boulis and Davis, 1989; LeDoux et al., 1990). Individuals with increased amygdalar activity are disposed to develop anxiety disorders (Schwartz et al., 2003), while those with amygdalar lesions fail to develop classically conditioned fear responses (Davis, 1989).
Abnormalities in the amygdala have also been related to anxiety disorders, such as social anxiety and PTSD, as well as a general behavioral inhibition in childhood (Pitman et al., 2001; Schwartz et al., 2003). However, anxiety disorders appear to be associated with increased amygdalar activation, while our data suggests that some cases of alcoholism are associated with decreased amygdala volumes. It is a reasonable hypothesis that this decreased volume is also associated with reduced amygdalar activation. Individuals with increased amygdalar activity are disposed to develop anxiety disorders (Schwartz et al., 2003), while those with lesions in the amygdala fail to develop classically conditioned fear responses (Davis, 1989) and may be more vulnerable to externalizing disorders (Patrick, 1994). The gray matter reductions in the amygdala observed in our sample of alcoholics is consistent with theory and research suggesting that the decision-making problems observed in alcoholics and other substance abusers are associated with differences in basic motivational processes affecting approach – avoidance behavior (Finn, 2002). Substance abusers prefer immediate over long-term rewards (Kirby et al., 1999; Kollins, 2003; Mitchell, 1999), have problems inhibiting behavior to avoid punishment when engaged in reward-seeking behavior (Finn et al., 2002), and show evidence of increased attention to rewards on the SGT task (Bechara et al., 2002; Finn, 2002; Stout et al., 2004). Further research should investigate the association between amygdala volume and function, SGT decision-making deficits, and decision-making problems in these populations in other areas of life, such as sexual behavior, finances, work-related behaviors, eating behaviors, interpersonal conflict, emotion-provoking situations, and other contexts that are motivationally relevant to the individual.
Poor decision-making also can be associated with hippocampal damage, however the specific mechanisms and patterns of poor decision-making associated with amygdalar and hippocampal lesions are different. While Bechara et al (2003) reported that amygdalar lesion patients made more disadvantageous decisions relative to controls, they note that those amygdalar patients that had intact hippocampal structures made more disadvantageous decisions than amygdalar patients who also had hippocampal damage. The decisions of patients with hippocampal damage, who had an amnestic syndrome, were more random, suggesting that memory for previous trials was not being accessed to guide the decision on the current trial. On the other hand memory appeared to guide the decisions of amygdalar patients with spared hippocampal areas, who were clearly biased to continue to draw from the disadvantageous decks, presumably because they were not responding with negative emotion to the large losses associated with disadvantageous deck choices.
We also feel that it is worthwhile to investigate possible differences in brain structure among individuals with and without a high family history for alcoholism, as well as those who go on to acquire the addiction and those who do not. Furthermore, we are currently involved in a project that hopes to investigate alcohol abuse/dependence in its earliest stages. Information from these studies could aid in deciphering whether or not structural brain abnormalities are a contributing factor to or a result of alcohol abuse.
From an image analysis perspective, this work demonstrates the increased sensitivity that results from using skull stripped inputs and from restricting the analysis to a ROI. Without both of these methodological advances, no statistically significant finding would have been forthcoming from this work
Acknowledgments
This work was supported by Grants AA11311 (GF) and AA13659 (GF), both from the National Institute of Alcoholism and Alcohol Abuse. We also express our appreciation to the NRI recruitment and assessment staff, and to each of our volunteer research participants.
References
- Aggleton J, Mishkin M. The amygdala: Sensory gateway to the emotions. In: Plutchik R, Kellerman H, editors. Emotional theory, research, and experience. Vol. 3. Academic Press; New York: 1986. pp. 281–299. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126(Pt 8):1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20(11):4311–9. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry. 2001;6(3):205–16. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675–89. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–69. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–89. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40(10):1690–705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Davis M. Footshock-induced sensitization of electrically elicited startle reflexes. Behav Neurosci. 1989;103(3):504–8. doi: 10.1037//0735-7044.103.3.504. [DOI] [PubMed] [Google Scholar]
- Brett M, Penny WD, Kiebel SJ. Introduction to random field theory. In: Frackowiak RS, Friston KJ, Frith CD, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human Brain Function. Academic Press; London: 2003. [Google Scholar]
- Clark L, Robbins T. Decision-making deficits in drug addiction. Trends Cogn Sci. 2002;6(9):361. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Morgan ML, Conlee EW, David S, Lufkin R, Babaie A, Dunkin JJ, O’Hara R, Simon S, Lightner A, Thomas S, Broumandi D, Badjatia N, Mickes L, Mody RK, Arora S, Zheng Z, Abrams M, Rosenberg-Thompson S. Cognitive and physiologic correlates of subclinical structural brain disease in elderly healthy control subjects. Arch Neurol. 2002;59(10):1612–20. doi: 10.1001/archneur.59.10.1612. [DOI] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh el W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29(8):1504–13. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. Ann N Y Acad Sci. 1989;563:165–83. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26(5):682–91. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Ropele S, Enzinger C, Gorani F, Seewann A, Petrovic K, Schmidt R. MTI of white matter hyperintensities. Brain. 2005;128(Pt 12):2926–32. doi: 10.1093/brain/awh567. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26(4):558–64. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Taylor C, Moon K, Barakos J, Tran H, Landman B, Shumway R. Controlling for premorbid brain size in imaging studies: T1-derived cranium scaling factor vs. T2-derived intracranial vault volume. Psychiatry Res. 2004a;131(2):169–76. doi: 10.1016/j.pscychresns.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004b;28(10):1487–91. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, Barakos J, Moon K, Di Sclafani V, Shumway R. Statistical parametric mapping of brain morphology: Sensitivity is dramatically increased by using brain-extracted images as inputs. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive Performance in Long-Term Abstinent Alcoholics. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2006.00185.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn P. Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Behav Cogn Neurosci Rev. 2002;1(3):183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res. 2002;26(2):186–206. [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180–7. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–32. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Kahn I, Yeshurun Y, Rotshtein P, Fried I, Ben-Bashat D, Hendler T. The role of the amygdala in signaling prospective outcome of choice. Neuron. 2002;33(6):983–94. doi: 10.1016/s0896-6273(02)00626-8. [DOI] [PubMed] [Google Scholar]
- Ketonen LM. Neuroimaging of the aging brain. Neurol Clin. 1998;16(3):581–98. doi: 10.1016/s0733-8619(05)70082-7. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict Behav. 2003;28(6):1167–73. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10(4):1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24(7):1036–40. [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146(4):455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res. 2000;24(10):1510–6. [PubMed] [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World. 1997;21(1):65–75. [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;31(4):319–30. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729–38. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- Rasband W. Image J [PC program], 1.30g ed. National Institute of Health; USA: 2002. [Google Scholar]
- Rogers RD, Lancaster M, Wakeley J, Bhagwagar Z. Effects of beta-adrenoceptor blockade on components of human decision-making. Psychopharmacology (Berl) 2004;172(2):157–64. doi: 10.1007/s00213-003-1641-5. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300(5627):1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS 13.0 for Windows. 13. SPSS Inc.; Chicago IL: 2004. [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11(4):742–7. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav. 2005;19(2):148–57. doi: 10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16(1):74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180(4):583–94. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Borod JC, Tang CY, Lange G, Wei TC, Johnson R, Nusbaum AO, Buchsbaum MS. Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia. 2001;39(6):556–73. doi: 10.1016/s0028-3932(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24(20):4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


