Abstract
Background
A number of studies have examined the amplitude of the mismatch negativity (MMN) evoked potential as a measure of a brain inhibitory deficit in alcoholics or those at risk for alcoholism. The current study examined MMN in alcoholics abstinent an average of 6.7 years (with a minimum of six months abstinence) compared to controls. This study examined the association of MMN with alcoholism family history density, with indices of the presence and severity of externalizing disorders (a risk-factor for alcoholism), and with alcohol use variables.
Methods
Electroencephalograms were gathered on 76 subjects (38 controls, 38 abstinent alcoholics) during a non-attending mismatch negativity experiment. Measures of alcoholism family history density, disinhibited personality traits, and antisocial symptoms served as measures of risk-factors known to be associated with a genetic liability to alcoholism. Alcohol use variables were used as measures of alcoholism severity.
Results
There were no differences in MMN amplitude or latency between controls and abstinent alcoholics. There also were no significant associations between MMN measures and the measures of risk for alcoholism or with the severity of alcohol use or duration of abstinence.
Conclusions
The results suggest that MMN is not affected in chronic alcoholics or associated with alcoholism vulnerability, and thus does not reflect a trait marker of alcoholism or alcoholism risk. The current results do not address effects on MMN of acute alcohol ingestion or withdrawal from alcohol.
Keywords: Mismatch negativity, alcoholism, EEG, family history density, externalizing symptoms
Introduction
There is accumulating evidence that the brains of alcoholics process stimuli differently than those of non-alcoholics. Research suggests the presence of information processing abnormalities in alcoholics on tasks that assess target detection (Polich et al., 1994), orienting (Fein et al., 1995), several aspects of inhibitory function (Ahveninen et al., 2000), behavioral disinhibition (LeMarquand et al., 1999), and response to reward and punishment (Bechara et al., 2001; Lejoyeux et al., 1998; Lejoyeux et al., 1999). These characteristics may be the result of chronic heavy alcohol use, or they may reflect biological vulnerabilities that predate alcohol consumption and increase risk for alcohol abuse (Begleiter et al., 1984). Research indicates that a positive family history of alcoholism, the presence of a conduct/antisocial personality disorder, and the presence of disinhibited personality traits, are all associated with a predisposition to alcoholism (Chassin et al., 1999a; Chassin et al., 1999b; Finn et al., 2002; Finn et al., 2000). In addition, all of these factors are also associated with patterns of information processing abnormalities similar to those seen in alcoholism (Finn et al., 2002; Iacono et al., 1999; Justus et al., 2001; Mazas et al., 2000). In fact, alcoholism is thought to belong to the class of disinhibitory disorders along with conduct disorder and antisocial personality disorder (Gorenstein and Newman, 1980). Recent evidence supporting this theory suggests that some forms of alcoholism share a common vulnerability with disinihbitory disorders, such as conduct disorder and antisocial personality disorder (Finn et al., 2002; Iacono et al., 1999).
There has been some excitement about the use of the mismatch negativity (MMN) event-related brain potential as a measure of the brain inhibitory deficit associated with alcoholism or the vulnerability to alcoholism (Kathmann et al., 1995; Zhang et al., 2001). Decreased inhibition is thought to be reflected by larger MMN amplitudes (Zhang et al., 2001). Larger MMN amplitudes have been reported in studies of recently detoxified alcoholics (Kathmann et al., 1995) and in alcohol high-risk samples (Zhang et al., 2001). If increased MMN amplitude is associated with the vulnerability to alcoholism, it should be present in abstinent alcoholics and it should covary with factors known to predispose to alcoholism, such as a positive family history of alcoholism, conduct disorder and antisocial personality symptoms, or disinhibited personality traits (Finn et al., 2002). MMN amplitude increases would be associated with the inverse of the abstinence duration in abstinent alcoholics to the degree that MMN amplitude increases result from acute alcohol consumption. If it is associated with a vulnerability to disinhibitory disorders, then MMN amplitude should covary with the severity of antisocial symptoms or disinhibited traits within both alcoholic and non-alcoholic samples.
Ethanol challenge studies in non-alcoholics have established that alcohol acutely attenuates and delays MMN (Hirvonen et al., 2000; Jaaskelainen et al., 1995a; Jaaskelainen et al., 1995b; Jaaskelainen et al., 1996). We are not aware of any studies on the acute effects of alcohol on MMN in alcoholics or in high risk samples. While there are no doubts that acute alcohol ingestion delays MMN latency and reduces MMN amplitude, the long-term effects of chronic alcohol exposure are not as clear. One group of findings has found that abstinent alcoholics display increased MMN amplitude in comparison to control subjects (Ahveninen et al., 2000; Kathmann et al., 1995); however, these studies examined subjects with relatively short-term (less than 2 mos) abstinence. This abstinence duration may have not been enough to get past the subtle post-withdrawal CNS hyperexcitablity associated with early cessation of alcohol, which have been shown to be detectable at 3–8 weeks after alcohol detoxification (Ahveninen et al., 2000)(Alling et al., 1982). Moreover, (Pekkonen et al., 1998) observed that as abstinence duration increased, MMN became smaller. This set of findings suggests that the MMN may be a state marker rather than a trait marker. Grau (Grau et al., 2001) studied alcoholic participants that had at least 28 days abstinence, with a mean abstinence duration of 70 days (SD = 42) and found there to be no difference in MMN amplitude or latency in comparison to controls.
Studies examining MMN in relation to the genetic predisposition to alcoholism also have reported contradictory results. Zhang et al. (Zhang et al., 2001) found that high-risk (HR) subjects (age 17–26, n=16) had larger MMNs than low risk (LR) controls (age 19–30, n=22). In contrast, Van der Stelt et al. found no difference in MMN between HR (age 9–18, n=20) and LR (age 9–18, n=20) groups (van der Stelt et al., 1997). Similarly, Holguin and colleagues (Holguin et al., 1998) found no MMN group differences between 19 HR children and 23 LR children between the ages of 8 and 15. These various results suggest that MMN abnormalities in high risk subjects are only evident in mature (i.e., adult) brains.
The current study assesses the effects of prior chronic alcohol abuse, abstinence duration, and alcoholism vulnerability on MMN amplitude. MMN amplitude was assessed in adult long-term abstinent alcoholics and non-alcoholic controls. The covariance with MMN amplitude was examined for the density of familial alcoholism, the extent of disinhibitory (antisocial) symptoms and traits, and indices of the magnitude and duration of alcohol abuse.
Methods
Participants
A total of 76 participants were recruited from the San Francisco Bay Area via café postings, mailings, newspaper ads, AA meeting postings, and an internet site. Two groups of participants were recruited: controls (C) and abstinent alcoholics (ABS). Table 1 presents the demographic, family history, alcohol use and externalizing disorder measures for the two subject groups. The abstinent alcoholic sample was on average 4.5 years older than the controls (t65.9 = 2.46, p < .05). Inclusion criteria for the control group was a lifetime drinking average of less than 30 alcohol-containing drinks per month, having never exceeded 60 drinks per month. The C group (n = 38) consisted of 19 females and 19 males between the ages of 24 and 55 (mean = 41.5, SD = 9.1). The inclusion criteria for the abstinent alcoholic group was that they have met lifetime DSM-IV (American Psychiatric Association, 1994) criteria for alcohol dependence, that their lifetime drinking average be at least 100 alcohol-containing drinks per month, and that they have been abstinent for a minimum of 6 months. Diagnoses of alcohol dependence were ascertained using a computerized version of the Diagnostic Interview Schedule (DIS: Robins et al., 1998) using DSM-IV criteria. The ABS group (n = 38) contained 19 females and 19 males varying between the ages of 35 and 55 (mean = 45.9, SD = 6.3). Exclusion criteria for all groups were: I) history or presence of an Axis I diagnosis on the DIS; ii) history of drug dependence other than nicotine; iii) significant history of head trauma or cranial surgery; iv) history of diabetes, stroke, or hypertension which required medical intervention, or of other significant neurological disease; v) clinical or laboratory evidence of active hepatic disease; vi) clinical evidence of Wernicke-Korsakoff syndrome; or vii) current substance abuse other than alcohol (aside from caffeine and nicotine).
Table 1.
Characteristics of Participant Groups
| Variable | Abstinent Alcoholics | Controls |
|---|---|---|
| N | 38 | 38 |
| Age (years) | 45.9 ± 6.3* | 41.5 ± 9.1 |
| Gender | 19 f, 19 m | 19 f, 19 m |
| FHD Score | .60 ± .42* | .40 ± .39 |
| High Risk N | 26 (68%) | 20 (53%) |
| CPI Socialization Scale | 27.7 ± 5.4** | 36.6 ± 3.7 |
| MMPI Pd Scale | 24.1 ± 7.6** | 18.4 ± 3.8 |
| ASP and CD Symptoms (#) | 13.7 ± 7.9** | 5.9 ± 5.0 |
| Duration of Active Drinking (mos) | 241.1 ± 84.1 | 223.4 ± 140.4 |
| Average Lifetime
Drinking Dose (std. drinks/mos) |
153.9 ± 118.7** | 5.9 ± 7.6 |
| Duration of Peak Drinking (mos) | 67.2 ± 64.3 | 109.9 ± 142.9 |
| Peak Drinking Dose (std. drinks/mos) | 319.2 ± 256.8** | 12.5 ± 13.9 |
| Duration of Abstinence (yrs) | 6.7 ± 6.2 | N/A |
Values are mean ± SD.
Group differences:
indicates p<0.05.
indicates p<0.001.
Procedures
All participants were informed of the study’s procedures and signed a consent form prior to their participation. There were a total of four sessions that lasted between an hour to two-and-a-half hours, involving clinical, neuropsychological, electrophysiological and neuroimaging assessments. The clinical assessment included a review of the subject’s medical history and was followed with a blood draw for liver function assessment. The electroencephalogram study took place on the third visit. For the purposes of this paper, we are only examining the data from the Mismatch Negativity experiment, along with measures of family history of alcoholism and disinhibitory symptoms and traits, and the magnitude and duration of alcohol abuse. All subjects were to abstain from drinking for 24 hours prior to their lab visits, and a breathalyzer was administered to all subjects preceding each session. Only one participant (a purported abstinent alcoholic) had a positive breathalyzer test at any session; that participant was excluded from the study. All subjects who completed the testing were paid for both their time and travel expenses. Subjects who completed all four aspects of the overall study received a completion bonus.
Measures
Alcohol Use Variables
Alcohol use variables were defined based on responses to the lifetime drinking history questionnaire that was designed to capture relevant features of consumption behavior. A standard drink was defined as 12 oz. beer, 1.5 oz. liquor, or 5 oz. wine. Alcohol lifetime duration was defined as the total number of months in which a subject was non-abstinent. Lifetime average dose was defined as the average number of standard drinks per month excluding periods of abstinence. The maximum consumption levels were captured by peak use variables. Peak dose was defined as the maximum monthly consumption of alcohol in standard drinks, and the peak duration refers to the total number of months (possibly discontinuous) that a subject engaged in this peak use. Subjects reported their abstinence period as of the date of last alcohol consumption. Separate lifetime use histories (using the lifetime drinking history questionnaire methodology) were gathered for all drugs used more than experimentally to determine whether subjects met inclusion/exclusion criteria.
Familial alcoholism-risk
The density of the participants’s family history of alcoholism was assessed with the Family Drinking History Questionnaire based on the Family Tree Questionnaire (Mann et al., 1985). The Family Drinking History Questionnaire was scored according to the methods presented by Stoltenburg (Stoltenberg et al., 1998) for Family History Density (FAD). Biological parents that were identified by the participant as problem drinkers were given a score of 0.50. Grandparents that were problem drinkers were given a score of 0.25; all other relatives were not included in the final score. Participants who were adopted and had no information about their birth parents (n=2) were counted as missing data. The highest FHD score possible is 2, and the lowest is 0. Participants who scored 0.50 and above were placed in the FH positive group, and those who scored below 0.50 were included in the FH negative group.
Disinhibitory symptoms and traits
Antisocial symptoms were measured by a total count of the symptoms for conduct disorder and antisocial personality derived from responses to questions on a computerized psychiatric Diagnostic Interview Schedule (DIS). Disinhibited personality traits were assessed with the Psychopathic Deviate (Pd) Scale of the MMPI-2 (Hathaway, 1989) and the Socialization Scale (So) of the California Psychological Inventory (Gough, 1969). These measures have been consistently associated with alcoholism-risk and behavioral disinhibition (Finn et al., 2002; Finn et al., 2000).
EEG/MMN measures
Upon arrival to the lab, the participant was seated in a small well-lit room containing a computer screen, a response box, an amplifier, and relevant lab supplies. The technician then put on a 40 channel electrode cap (QuickCap, Neuroscan, Inc.), and connected it to a 40-channel single-ended amplifier system (NuAmp, Neuroscan, Inc.). All electrodes were positioned using the 10-10 system, with AFZ, FZ, FCZ, CZ, CPZ, POZ, and OZ being on the midline. Ground was positioned at 4 cm above the naison and the right ear electrode (A2) was used as the reference for all recordings. Electrodes were placed above and below the participants left eye to monitor blinks and eye movements. The NuAmps amplifier had a fixed input range of ± 130 mV sampled with a 22 bit A/D converters where the least significant bit was .062 μV. Recordings did not begin until all impedances were less than 10 kOhms. Eleven EEG/ERP experiments were performed with mismatch negativity (MMN) being the eighth (approximately 45 minutes from the onset of the experiments).
Mismatch negativity
All stimuli were presented and responses were monitored using the E-Prime (Psychology Software Tools, Inc.) system on a Pentium 4 computer. EEG data were acquired using Scan 4.2 software (Neuroscan, Inc.) on a second computer. These two computer systems were connected in such a way that for each stimulus or response presented by the E-Prime computer, the E-Prime computer sent an appropriate signal (between 4–5 ms after the stimulus or response event) to the Scan computer, indicating the type of stimuli so that that information could be integrated into the continuous data recordings of the Scan system. The timing of the E-Prime computer/Scan computer interface was verified using an oscilliscope and 4 ms was subtracted from all Scan timings so that events were accurate to within 1 ms.
The Mismatch experiment involved the presentation of 400 stimuli: 350 standard tones, and 50 mismatch tones, with the mismatch tone presented pseudo-randomly on one-eighth of the trials. The stimuli had a duration of 100 ms, with a 600 ms delay between stimuli, and a stimulus intensity level of 60 dB. Subjects were randomized as to whether the mismatch tone was the low (500 Hz) or the high (2000 Hz) frequency tone. Prior to starting the experiment, the participant was asked to choose something to read from a collection of books and magazines provided. As the subject became focused on reading, the MMN stimuli were started. All data reduction (filtering and artifact rejection) was performed offline.
Data Analysis
As noted above, EEG data were amplified using the Nuamps (Neurosoft, Inc.) single-ended amplifier (with reference to A2) and Scan 4.2 Acquisition software (Neurosoft, Inc.). Raw data were processed offline using the Edit program in Scan 4.2 (Neurosoft, Inc.). Original continuous data were first processed to compute a vertical eye movement channel by computing the difference between the recordings above vs. below the left eye. The files were then epoched from 100 ms before to 496 ms after the stimulus events. Epochs with eye movement excursions exceeding +75 μV were excluded. Resulting data were then bandpass filtered from 0.5 to 15 Hz (at 48dB per octave, zero phase shift). Average mismatch and standard responses were computed, as was the difference between mismatch and standard. The area under the curve of the mismatch minus standard response was then integrated from 100 to 192 ms as the measure of MMN. The latency of the MMN was computed from the peak of the MMN integral. The MMN measurements follow the methods used by Zhang et al. (Zhang et al., 2001). The analysis presented below was restricted to FZ and CZ recordings. The data were analyzed using the Statistical Analysis System (SAS Institute, 1990). The amplitude and latency data were analyzed by Analysis of Covariance, which was carried out using the General Linear Models procedure implemented in SAS, with age, group, and gender effects and interactions included. The data were analyzed both without baseline correction and after correcting for the baseline recorded from the 100 ms pre-stimulus. The association of the MMN measures with alcoholism risk factors and alcohol use variables were analyzed using Spearman correlations within and across groups. Due to group difference in age, age was controlled for as a covariate.
Results
Abstinent alcoholics (ABS) significantly differed from controls (C) in alcoholism family drinking density and the presence and severity of externalizing disorder signs and symptoms. Group membership accounted for 6.5% of the alcoholism family history density variance (F1,75 = 5.00, p < .03). Group membership accounted for 19.6% of the variance on the MMPI Pd scale (F1,75 = 17.79, p < .0001), 24.0% of the variance of the number of antisocial personality and conduct disorder symptoms (F1,75 = 25.79, p ≪ .0001), and 45.4% of the variance of the CPI Socialization Scale score (F1,75 = 66.88, p ≪ .0001).
ANCOVA revealed no MMN integral main effects or interactions at either FZ or CZ, whether baseline corrected or not (each effect accounted for less than 1.1% of MMN integral variance, p’s > .38). Similarly, there were no significant MMN latency effects (each effect accounted for less than 0.3% of MMN latency variance, p’s > .67). There were no age or age by group interaction effects on MMN integrals or MMN latency. Figure 1 presents the group average mismatch and standard responses along with the MMN integrals for the data after baseline correction.
Figure 1.
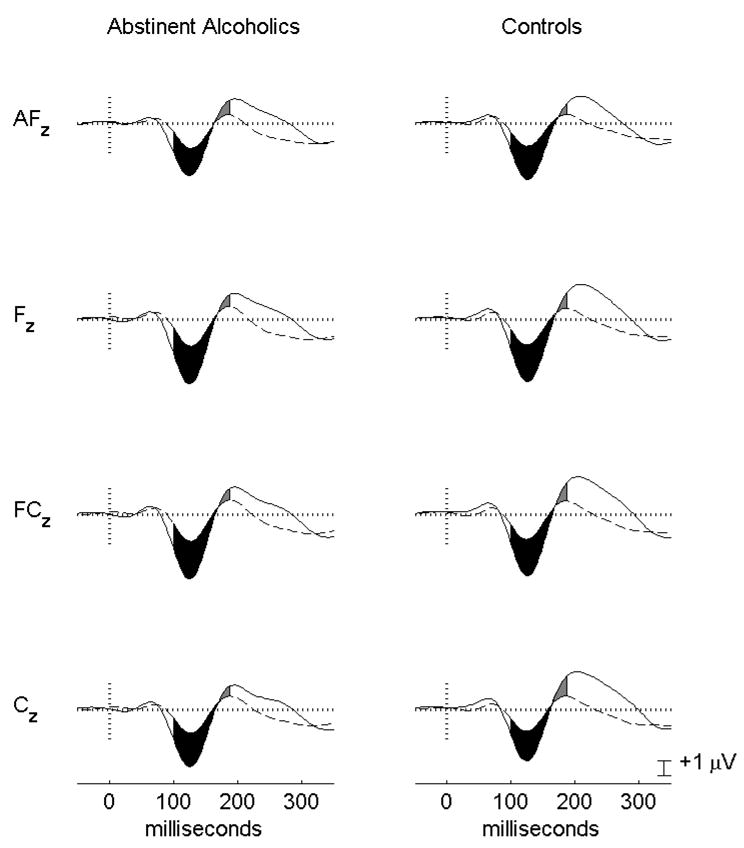
Group average ERP responses to the standard (dashed lines) and mismatch stimuli (solid lines). Data have been baseline corrected for activity from the 100 ms pre-stimulus interval. The group average MMN integrals from 100 – 190 ms are displayed as the filled in areas between the standard and mismatch curves.
The association of MMN with Family History Density scores was analyzed using Spearman Rank order correlations across all subjects and within each group. None of the correlations were significant (all |r|’s < 0.14, p’s > .24). Figure 2 presents the raw data showing no difference between groups in MMN amplitude and illustrates the lack of association between FH status and MMN amplitude. Data that had not been baseline corrected produced similar results. The two controls and seven abstinent alcoholic subjects with less than 40 trials in their mismatch average are indicated by hollow symbols. Note that 88% of subjects had at least 40 trials in the mismatch response average (the remaining ones had at least 26 trials).
Figure 2.
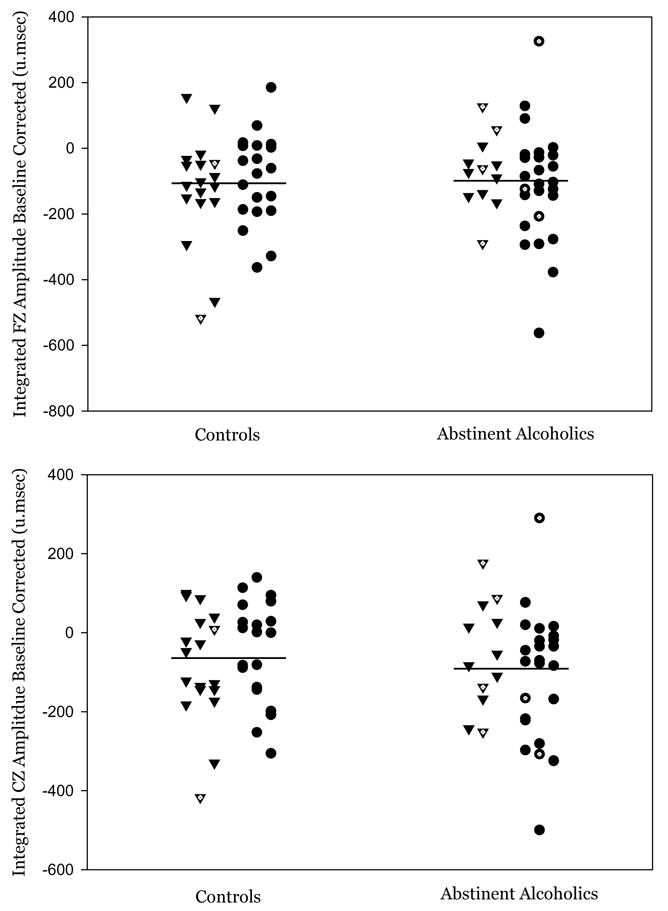
A scatter plot of the MMN integrals for the baseline corrected data is presented. Within each group, family history positive subjects are represented by triangles and family history negative subjects are represented by circles. Subjects whose MMN integral was computed from less than 40 artifact-free trials are indicated by hollow symbols.
Spearman Rank order correlations were used to analyze the association between MMN and the scores from the MMPI Pd, the CPI Socialization Scale, and the number of antisocial personality and conduct disorder symptoms from the DIS. There were no significant associations between MMN and any of the externalizing symptom measures, either across groups (all |r|’s < .13, p’s > .24), or within the abstinent alcoholic group (all |r|’s < .18, p’s > .27). Within the controls, there was a trend toward a negative association between the MMN integral and the CPI Socialization Scale score (r = −.29 at Fz, and r = −.31 at Cz, both p’s < .08). Spearman p-values for family history density and for externalizing disorder measures were not Bonferroni corrected (n=4).
Spearman Rank order correlations were also used to analyze the association between MMN and alcohol use variables within the abstinent alcoholic sample. No significant correlations were found (all |r|’s < .27, p’s > .10).
In order to assess the probability that our data are true null findings, we performed a power analysis vs. Zhang’s study. Using the methods of Cohen (Cohen, 1988), we estimated the size of Zhang’s reported effect as d=0.6 (from Figure 3 of (Zhang et al., 2001)). The currtne study had power of 0.79 to detect a difference of this size between the abstinent alcoholics and controls, both at alpha = .05. It is likely that the current study would have detected an effect size similar to ones previously reported had such effects been evident in the sample populations.
Discussion
The results indicate that chronic alcoholism has no effect on MMN amplitude or latency. The data do not address the issues of acute effects of alcohol or post-alcohol withdrawal hyperexcitability on MMN. Although our abstinent alcoholic group was older than the controls, we do not believe this could have accounted for our lack of MMN findings as neither intra-group or inter-group age correlations with MMN were observed.
We found no association between MMN amplitude or latency and family history of alcoholism (presence or density) or measures of externalizing disorder signs and symptoms. We also examined the association of MMN with the presence and severity of externalizing disorder signs and symptoms and did not find any associations. However, we found much larger alcoholism associated differences between groups in the externalizing disorder variables than in alcoholism family history, supporting the hypothesis that externalizing disorder signs and symptoms are highly associated with alcoholism. There is a controversy in the P300 high-risk literature as to whether the presence of externalizing disorders is an important mediating factor underlying observed P300 reductions in alcoholic samples. Thus, our failure to find an association between MMN measures and measures of externalizing disorders is an important addition to the literature.
The study of MMN in high-risk subjects by Zhang and colleagues (Zhang et al., 2001) suggested that increased MMN is a trait marker associated with the vulnerability to alcoholism. The existing literature with positive MMN results of Zhang and colleagues (in subjects 17–26 years), and the negative MMN results of Van der Stelt et al. (in subjects 9–18 years) and Holguin and colleagues (in subjects 8–15 years) left open the hypothesis that MMN abnormalities in high risk subjects are only evident in mature (i.e., adult) brains. Our results clearly argue against that hypothesis. The power analysis shows that this study was adequately equipped to detect such a finding if it existed. Thus, we can conclude that increased MMN is not a trait marker associated with vulnerability to alcoholism as manifest in adult samples of abstinent alcoholics. We do not have a hypothesis as to why our results differ from Zhang’s, other than possible differences in exclusionary criteria; however, we do believe it is an important enough issue for further research to be conducted.
Footnotes
Citation
Fein, G., Whitlow, B., and Finn, P. 2004. Mismatch negativity: no difference between controls and abstinent alcoholics, Alcohol Clin Exp Res, 28(1), p. 137–42.
This work was supported by Grants AA11311 (GF) and AA13659 (GF), both from the National Institute of Alcoholism and Alcohol Abuse.
References
- Ahveninen J, Escera C, Polo MD, Grau C, Jaaskelainen IP. Acute and chronic effects of alcohol on preattentive auditory processing as reflected by mismatch negativity. Audiol Neurootol. 2000;5(6):303–11. doi: 10.1159/000013896. [DOI] [PubMed] [Google Scholar]
- Alling C, Balldin J, Bokstrom K, Gottfries CG, Karlsson I, Langstrom G. Studies on duration of a late recovery period after chronic abuse of ethanol. A cross-sectional study of biochemical and psychiatric indicators. Acta Psychiatr Scand. 1982;66(5):384–97. doi: 10.1111/j.1600-0447.1982.tb06720.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–89. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, DeLucia C. The relation of adolescent substance use to young adult autonomy, positive activity involvement, and perceived competence. Dev Psychopathol. 1999a;11(4):915–32. doi: 10.1017/s0954579499002382. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, DeLucia C, Todd M. A longitudinal study of children of alcoholics: predicting young adult substance use disorders, anxiety, and depression. J Abnorm Psychol. 1999b;108(1):106–19. doi: 10.1037//0021-843x.108.1.106. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Fein G, Biggins CA, MacKay S. Delayed latency of the event-related brain potential P3A component in HIV disease. Progressive effects with increasing cognitive impairment. Arch Neurol. 1995;52(11):1109–18. doi: 10.1001/archneur.1995.00540350103022. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res. 2002;26(2):186–206. [PubMed] [Google Scholar]
- Finn PR, Sharkansky EJ, Brandt KM, Turcotte N. The effects of familial risk, personality, and expectancies on alcohol use and abuse. J Abnorm Psychol. 2000;109(1):122–33. doi: 10.1037//0021-843x.109.1.122. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87(3):301–15. [PubMed] [Google Scholar]
- Gough HGPD. Manual for the California Psychological Inventory (So Scale) Consulting Psychological Press; Palo Alto, CA: 1969. [Google Scholar]
- Grau C, Polo MD, Yago E, Gual A, Escera C. Auditory sensory memory as indicated by mismatch negativity in chronic alcoholism. Clin Neurophysiol. 2001;112(5):728–31. doi: 10.1016/s1388-2457(01)00490-4. [DOI] [PubMed] [Google Scholar]
- Hathaway JMS. MMPI-2: Minnesota Multiphasic Personality Inventory. The University of Minnesota Press; Minneapolis: 1989. [Google Scholar]
- Hirvonen J, Jaaskelainen IP, Naatanen R, Sillanaukee P. Adenosine A1/A2a receptors mediate suppression of mismatch negativity by ethanol in humans. Neurosci Lett. 2000;278(1–2):57–60. doi: 10.1016/s0304-3940(99)00897-6. [DOI] [PubMed] [Google Scholar]
- Holguin RS, Corral M, Cadaveira F. Mismatch negativity in young children of alcoholics from high-density families. Alcohol Clin Exp Res. 1998;22(6):1363–8. doi: 10.1111/j.1530-0277.1998.tb03920.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11(4):869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen IP, Lehtokoski A, Alho K, Kujala T, Pekkonen E, Sinclair JD, Naatanen R, Sillanaukee P. Low dose of ethanol suppresses mismatch negativity of auditory event-related potentials. Alcohol Clin Exp Res. 1995a;19(3):607–10. doi: 10.1111/j.1530-0277.1995.tb01555.x. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen IP, Pekkonen E, Alho K, Sinclair JD, Sillanaukee P, Naatanen R. Dose-related effect of alcohol on mismatch negativity and reaction time performance. Alcohol. 1995b;12(6):491–5. doi: 10.1016/0741-8329(95)00009-7. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen IP, Pekkonen E, Hirvonen J, Sillanaukee P, Naatanen R. Mismatch negativity subcomponents and ethyl alcohol. Biol Psychol. 1996;43(1):13–25. doi: 10.1016/0301-0511(95)05174-0. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early-onset alcohol problems. Alcohol Clin Exp Res. 2001;25(10):1457–66. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Wagner M, Rendtorff N, Engel RR. Delayed peak latency of the mismatch negativity in schizophrenics and alcoholics. Biol Psychiatry. 1995;37(10):754–7. doi: 10.1016/0006-3223(94)00309-Q. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M, Feuche N, Loi S, Solomon J, Ades J. Impulse-control disorders in alcoholics are related to sensation seeking and not to impulsivity. Psychiatry Res. 1998;81(2):149–55. doi: 10.1016/s0165-1781(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M, Feuche N, Loi S, Solomon J, Ades J. Study of impulse-control disorders among alcohol-dependent patients. J Clin Psychiatry. 1999;60(5):302–5. doi: 10.4088/jcp.v60n0506. [DOI] [PubMed] [Google Scholar]
- LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. Am J Psychiatry. 1999;156(11):1771–9. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15(1–2):61–7. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24(7):1036–40. [PubMed] [Google Scholar]
- Pekkonen E, Ahveninen J, Jaaskelainen IP, Seppa K, Naatanen R, Sillanaukee P. Selective acceleration of auditory processing in chronic alcoholics during abstinence. Alcohol Clin Exp Res. 1998;22(3):605–9. doi: 10.1111/j.1530-0277.1998.tb04299.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock V, Bloom F. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115(1):55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS procedures guide : version 6. 3. SAS Institute; Cary, NC: 1990. [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93(10):1511–20. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Gunning WB, Snel J, Kok A. No electrocortical evidence of automatic mismatch dysfunction in children of alcoholics. Alcohol Clin Exp Res. 1997;21(4):569–75. doi: 10.1097/00000374-199706000-00001. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Cohen HL, Porjesz B, Begleiter H. Mismatch negativity in subjects at high risk for alcoholism. Alcohol Clin Exp Res. 2001;25(3):330–7. doi: 10.1097/00000374-200103000-00003. [DOI] [PubMed] [Google Scholar]


