Abstract
Objective
To determine the association between body mass index (BMI) and hospital mortality for critically ill adults.
Design
Retrospective cohort study.
Setting
One-hundred six intensive care units (ICUs) in 84 hospitals.
Patients
Mechanically ventilated adults (n = 1,488) with acute lung injury (ALI) included in the Project IMPACT database between December 1995 and September 2001.
Interventions
None.
Measurements and Main Results
Over half of the cohort had a BMI above the normal range. Unadjusted analyses showed that BMI was higher among subjects who survived to hospital discharge vs. those who did not (p < .0001). ICU and hospital mortality rates were lower in higher BMI categories. After risk-adjustment, BMI was independently associated with hospital mortality (p < .0001) when modeled as a continuous variable. The adjusted odds were highest at the lowest BMIs and then declined to a minimum between 35 and 40 kg/m2. Odds increased after the nadir but remained below those seen at low BMIs. With use of a categorical designation, BMI was also independently associated with hospital mortality (p = .0055). The adjusted odds were highest for the underweight BMI group (adjusted odds ratio [OR], 1.94; 95% confidence interval [CI], 1.05–3.60) relative to the normal BMI group. As in the analysis using the continuous BMI variable, the odds of hospital mortality were decreased for the groups with higher BMIs (overweight adjusted OR, 0.72; 95% CI, 0.51–1.02; obese adjusted OR, 0.67; 95% CI, 0.46 – 0.97; severely obese adjusted OR, 0.78; 95% CI, 0.44–1.38). Differences in the use of heparin prophylaxis mediated some of the protective effect of severe obesity.
Conclusions
BMI was associated with risk-adjusted hospital mortality among mechanically ventilated adults with ALI. Lower BMIs were associated with higher odds of death, whereas overweight and obese BMIs were associated with lower odds.
Keywords: obesity, adult respiratory distress syndrome, outcome assessment (health care), artificial respiration, critical care, body mass index
Almost two-thirds of U.S. adults are overweight or obese, and this trend is accelerating (1, 2). Whereas the effect on all-cause mortality is well-described (3), the association between excess weight and outcomes among critically ill patients remains ambiguous. Obese patients undergo physiologic changes that may impair their ability to adapt to stresses of critical illness (4, 5) and have a greater prevalence of comorbid conditions that may affect outcome (3). While conventional wisdom holds that obesity increases mortality and morbidity for intensive care unit (ICU) patients, published reports show increased (6–8) and decreased mortality (9–11) for obese subjects, as well as no association with excess weight (12–14). If obesity is a risk factor for the critically ill, investigators should determine the cause and target interventions to this group. If, instead, obesity is protective for ICU patients, then the mechanism underlying such an effect might lead to treatments to reduce the risk for nonobese patients.
Because of the disparity in reported results, we completed a retrospective cohort study of an observational database to describe the influence of admission body mass index (BMI) on outcome of critical illness. We examined standard outcomes, such as hospital mortality, as well as selected processes of care and comorbidities (e.g., diabetes) that could mediate an association between BMI and outcome. To minimize possible selection bias due to differences in the ICU admitting diagnosis, we limited the study sample to a population with a clear indication for ICU admission (mechanical ventilation) and the presence of a defined admission diagnosis (acute lung injury, or ALI). ALI is an inflammatory pulmonary condition associated with a number of precipitating insults and a frequent cause of respiratory failure requiring mechanical ventilation, with a reported mortality rate of 40% to 60% (15).
MATERIALS AND METHODS
Study Sample and Subjects
Project IMPACT (v2.3; PI), a subscription database, collected data from 106 ICUs in 84 U.S. hospitals from December 1995 to September 2001. We obtained records for adult patients (≥18 yrs old) with an admission diagnosis consistent with ALI (“pulmonary edema, acute respiratory distress syndrome” or “pulmonary edema, noncardiogenic fluid overload without congestive heart failure”) and mechanical ventilation within 24 hrs of ICU admission (as indicated by the Mortality Probability Model coding at admission and/or at 24 hrs). PI data collectors coded diagnoses on the basis of documentation in the patient’s medical record.
Body Mass Index
The admission weight and height was used to calculate the BMI (BMI = weight in kg/height in m2) for each subject. (One subject had a height recorded as 60 cm, producing a BMI of 258 kg/m2. This record was recoded as 60 inches.) PI data abstractors received the following instructions (L. Manganaro, personal communication): “Use the ICU admission weight and do not modify the weight to account for changes which may have resulted from fluid resuscitation or diuresis. If the actual weight is not available, use the best clinical estimate from patient care providers. If the actual admission height is not available, use the best clinical estimate from patient careproviders.”
Statistical Analyses
Hospital mortality was the primary outcome. We compared demographics, clinical characteristics, and BMI between those surviving to hospital discharge and those dying. Secondary outcomes, including ICU mortality, length of stay, and discharge location, were examined. We used the Kruskal-Wallis test for continuous variables and the Pearson chi-square test (or Fisher’s exact test for variables with sparse cell counts) for categorical variables in unadjusted analyses.
We explored this relationship between BMI and outcome in a number of ways. When BMI was included as a continuous variable in a logistic regression model of hospital mortality, we discovered that BMI was not linear in the logit. The best fit of the association was a quadratic transformation of BMI, as determined by the method of fractional polynomials. For the categorical analysis, an exploration of deciles of BMI showed no compelling statistical reason to use an alternate classification (data not shown). Therefore, we employed the categorization endorsed by the National Institutes of Health (underweight BMI, <18.5 kg/m2; normal BMI, 18.5 kg/m2 to <25 kg/m2; overweight BMI, 25 kg/m2 to <30 kg/m2; obese BMI, 30 kg/m2 to <40 kg/m2; severely obese BMI, ≥40 kg/m2) (3).
To adjust for risk, we first estimated the unadjusted relationship between BMI and hospital mortality, using BMI either as the transformed continuous variable or as the five-level categorical variable. With the categorical BMI, we treated the normal BMI group as the referent. We considered demographic data, pre-existing diagnosis, admission diagnoses, and processes of care for inclusion in the risk-adjusting model. The Simplified Acute Physiology Score II (SAPS II) (16) provided a physiology-based measure of severity of illness. For the 7% of records (n = 109) lacking SAPS II data, we employed multivariate imputation to complete these records. We performed analyses both with and without the imputed data.
After estimating the unadjusted (or “crude”) association between BMI and hospital mortality, we sequentially added covariates to the logistic regression model if they substantially altered the coefficient for BMI. We a priori defined the level of significance for this process at 15% for the categorical analysis and at 10% for the continuous BMI model. The sequence of considered covariates was based upon the strength of the univariable association. We checked continuous variables in the model for linearity in the logit, using the method of fractional polynomials. Only SAPS-II required transformation. After estimation of the model, we constructed second-order effect modifiers (interaction terms) between BMI and the other included covariates. None was statistically significant, so the final models included no interaction terms. We also explored the role of selected processes of care in the association between BMI and outcome. If they were not initially selected in the risk-adjusting process, we forced these covariates into the models and explored their role as effect modifiers with BMI.
We were concerned that the highest BMIs found in the cohort did not represent realistic measures. Because these records (n = 14; mean, 76.4 kg/m2; range, 60.8–141.1 kg/m2) resulted in relatively unstable coefficients for the association between BMI and hospital mortality, we excluded them from the final model. We also explored the impact of removing the five most influential records (as determined by Pregibon’s delta beta) from the risk-adjusting model. The adjusted odds ratios for the BMI categories did not appreciably change (data not shown), so these records were included in the final analysis.
We completed all analyses using Stata (8.2, Stata, College Station, TX).
Human Subject Protection
PI provided data without identifying information and had no role in the preparation or approval of this manuscript. The Ohio State University Institutional Review Board approved the conduct of the study without additional informed consent.
RESULTS
The initial cohort included 1,673 mechanically ventilated adults with ALI. However, 185 records (11.1%) did not include enough information to calculate a BMI. Hospital mortality was not significantly different between the records with a calculable BMI and those without (37.0% vs. 35.9%; p = .764). The remaining 1,488 subjects were included in the analysis (Fig. 1).
Figure 1.
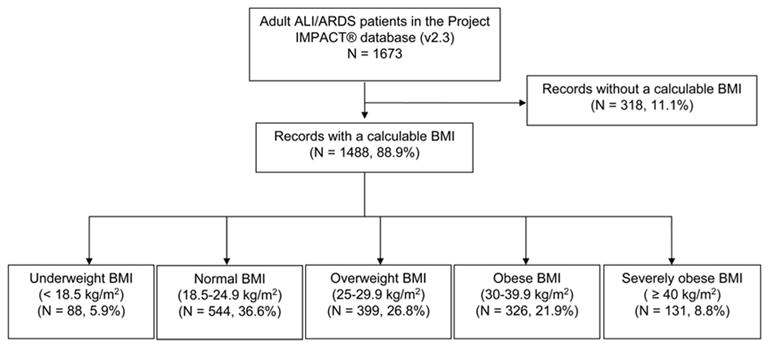
Included and excluded subjects in study cohort (ALI, acute lung injury; ARDS, adult respiratory distress syndrome; BMI, body mass index).
Table 1 shows the unadjusted differences between patients surviving to hospital discharge and those dying during hospitalization. Nonsurvivors had a significantly lower mean BMI than survivors. Hospital survivors and nonsurvivors were similar with respect to race, ethnicity, prior ICU admissions, and medical system characteristics, including type of hospital, presence of a critical care training program, ICU medical team model, and number of hospital beds (data not shown).
Table 1.
Differences in unadjusted analysis between patients surviving to hospital discharge and those dying
| Alive | Dead | p Valuea | |
|---|---|---|---|
| BMI (n = 1,488) | 28.8 (9.39) | 26.8 (8.57) | <.0001 |
| BMI category, kg/m2 (n = 1,488) | <.0001 | ||
| Underweight: <18.5 | 40 (4.3%) | 48 (8.7%) | |
| Normal: 18.5–24.9 | 321 (34.2%) | 223 (40.6%) | |
| Overweight: 25–29.9 | 257 (27.4%) | 142 (25.8%) | |
| Obese: 30–39.9 | 227 (24.2%) | 99 (18.0%) | |
| Severely obese: ≥40 | 93 (9.9%) | 38 (6.9%) | |
| SAPS II probability of survival (n = 1,379) | 0.68 (0.24) | 0.45 (0.29) | <.0001 |
| Age, yrs (n = 1,487) | 56.8 (16.9) | 63.7 (16.1) | .0001 |
| Male (n = 1,488) | 464 (49.5%) | 303 (55.1%) | .036 |
| Type of ICU patient (n = 1,487) | .076 | ||
| Scheduled postoperative | 142 (15.2%) | 70 (12.7%) | |
| Unscheduled postoperative | 128 (13.7%) | 59 (10.7%) | |
| Nonoperative | 667 (71.2%) | 421 (76.6%) | |
| Origin (n = 1,488) | <.0001 | ||
| Emergency department | 239 (25.5%) | 100 (18.2%) | |
| Hospital | 426 (45.4%) | 336 (61.1%) | |
| Operating room | 143 (15.3%) | 46 (8.4%) | |
| External to hospital | 130 (13.9%) | 68 (12.4%) | |
| Do-not-resuscitate or limited resuscitation status (n = 1,488) | 27 (2.9%) | 44 (8.0%) | <.0001 |
BMI, body mass index; SAPS, Simplified Acute Physiology Score; ICU, intensive care unit.
Kruskal-Wallis test for continuous variables; Pearson’s chi-square test for categorical variables. Continuous variables are presented as mean (SD); categorical variables are presented as frequency (%).
Unadjusted associations between BMI categories and outcomes are shown in Table 2. Patients with the lowest BMIs had the highest rates of hospital and ICU mortality. There were no differences in the number of ICU complications, lengths of stay, or discharge location. Table 3 illustrates the significant differences in severity of illness, demographic data, and preexisting diagnoses between the BMI groups.
Table 2.
Differences in unadjusted outcomes among BMI categories
| Variable | Underweight (n = 88) | Normal (n = 544) | Overweight (n = 399) | Obese (n = 326) | Severely Obese (n = 131) | p Valuea |
|---|---|---|---|---|---|---|
| Hospital mortality | 48 (54.6%) | 223 (41.0%) | 142 (35.6%) | 99 (30.4%) | 38 (29.0%) | <.001 |
| ICU mortality | 34 (38.6%) | 173 (31.8%) | 122 (30.6%) | 75 (23.0%) | 29 (22.1%) | .0004 |
| Hospital length of stay | 24.6 (20.4) | 24.7 (23.6) | 24.7 (29.3) | 26.9 (23.2) | 26.8 (27.1) | .095 |
| ICU length of stay | 12.0 (10.4) | 11.6 (12.7) | 11.2 (11.7) | 11.9 (11.0) | 14.1 (15.6) | .180 |
| Discharge destination for survivors | .065 | |||||
| Home | 19 (47.5%) | 193 (60.1%) | 150 (58.6%) | 114 (50.2%) | 52 (55.9%) | |
| Extended care facility | 19 (47.5%) | 106 (33.0%) | 81 (31.6%) | 99 (43.6%) | 37 (39.8%) | |
| Other | 2 (5.0%) | 22 (6.85%) | 25 (9.8%) | 14 (6.2%) | 4 (4.3%) |
BMI, body mass index; ICU, intensive care unit.
Kruskal-Wallis test for continuous variables; Pearson’s chi-square test for categorical variables. Body mass categories are defined as follows: Underweight BMI, <18.5 kg/m2; Normal BMI, 18.5–24.9 kg/m2; Overweight BMI, 25–29.9 kg/m2; Obese BMI, 30–39.9 kg/m2; Severely Obese BMI, ≥40 kg/m2. Continuous variables are presented as mean (SD). Categorical variables are presented as frequency (%).
Table 3.
Differences in potential confounders among BMI categories
| Variable | Underweight (n = 88) | Normal (n = 544) | Overweight (n = 399) | Obese (n = 326) | Severely Obese (n = 131) | p Valuea |
|---|---|---|---|---|---|---|
| SAPS II probability of survival | 0.53 (0.29) | 0.58 (0.28) | 0.59 (0.29) | 0.59 (0.28) | 0.68 (0.29) | <.001 |
| Age, yrs | 62.4 (16.2) | 61.0 (17.8) | 59.4 (16.7) | 58.0 (16.3) | 53.6 (14.9) | <.001 |
| Male | 41 (46.6%) | 307 (56.4%) | 223 (55.9%) | 152 (46.6%) | 44 (33.6%) | <.001 |
| Diabetes diagnosis | 10 (11.4%) | 87 (16.0%) | 98 (24.6%) | 113 (34.7%) | 52 (39.7%) | <.001 |
| Hypertension diagnosis | 23 (26.1%) | 207 (38.0%) | 172 (43.1%) | 166 (50.9%) | 66 (50.4%) | <.001 |
| Pulmonary disease diagnosis | 38 (43.2%) | 215 (39.5%) | 129 (32.3%) | 109 (33.4%) | 60 (45.8%) | .012 |
| Cancer diagnosis | 14 (15.9%) | 110 (20.2%) | 67 (16.8%) | 49 (15.0%) | 14 (10.7%) | .071 |
| Cardiovascular disease diagnosis | 42 (47.7%) | 325 (59.7%) | 239 (59.9%) | 209 (64.1%) | 83 (63.4%) | .078 |
BMI, body mass index; SAPS, Simplified Acute Physiology Score.
Kruskal-Wallis test for continuous variables; Pearson’s chi-square test for categorical variables. Body mass categories are defined as follows: Underweight BMI, <18.5 kg/m2; Normal BMI, 18.5–24.9 kg/m2; Overweight BMI, 25–29.9 kg/m2; Obese BMI, 30–39.9 kg/m2; Severely Obese BMI, ≥40 kg/m2. Continuous variables are presented as mean (SD). Categorical variables are presented as frequency (%).
In a simple logistic regression, the transformed BMI modeled as a continuous variable was associated with hospital mortality (p < .0001). This association remained after adjustment for multiple covariates (p < .0001). Figure 2 shows the shape of the association between BMI and mortality. The odds of mortality are highest at the lowest BMI. These odds then decrease to a nadir between 35 and 40 kg/m2. After this nadir, the odds then begin to increase but remain lower than those seen among the underweight subjects. We refit the model with imputed data for SAPS II scores and found a similar association (data not shown).
Figure 2.
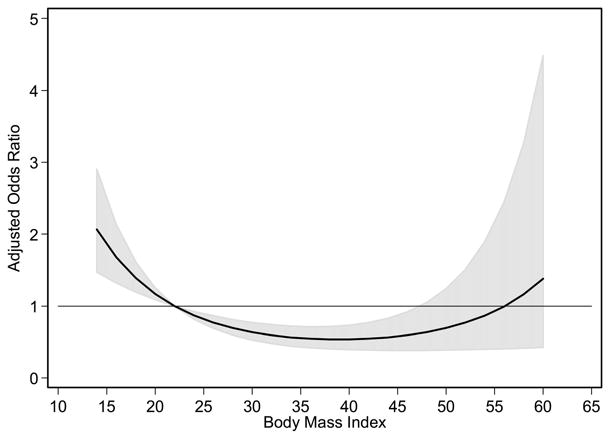
Risk-adjusted odds ratio for continuous body mass index (BMI) model and hospital mortality. The solid line indicates the point estimates for the adjusted odds ratios for the BMI shown on the x-axis. The gray area represents the 95% confidence interval for the adjusted odds ratio at each BMI. The referent BMI is the rounded midpoint (BMI = 22 kg/m2) of the normal BMI category. Above the horizontal line indicates increased hospital mortality relative to those individuals with a BMI of 22 kg/m2 and below the line indicates decreased hospital mortality. The risk-adjusting model includes age, gender, race, Simplified Acute Physiology Score II probability of survival, diagnosis of renal or genitourinary disease, and an intensive care unit–acquired renal or genitourinary complication. Hosmer-Lemeshow goodness of fit test, p = .227.
The unadjusted analysis using the five BMI categories demonstrated a similar association between BMI and hospital mortality (p < .0001). After adjustment for confounders, there remains an independent association between BMI and hospital mortality (p = .0055, Table 4). As with the model using a continuous BMI variable, the odds of mortality are higher at lower BMIs and lower at higher BMIs. Inclusion of records with imputed SAPS II data did not appreciably change the results (data not shown). Addition of diabetes to the model had minimal effect on the coefficients for the underweight (increasing by 0.5%) and overweight BMI categories (decreasing by 3.3%). For the obese and severely obese categories, the effect was larger (decreasing by 6.5% and by 8.3%, respectively) but failed to alter the coefficients for BMI at the a priori defined level for inclusion.
Table 4.
Risk-adjusted association between body mass index category and hospital mortality
| Body Mass Index (kg/m2) | Adjusted Odds Ratioa (95% Confidence Interval) | p Value |
|---|---|---|
| Underweight (<18.5) | 1.94 (1.05–3.60) | .035 |
| Normal (18.5–24.9) | Reference | |
| Overweight (25–29.9) | 0.72 (0.51–1.02) | .067 |
| Obese (30–39.9) | 0.67 (0.46–0.97) | .033 |
| Severe obesity (≥40) | 0.78 (0.44–1.38) | .385 |
Adjusted for age, gender, race, SAPS II (Simplified Acute Physiology Score; probability of survival), intensive care unit medical team model, admission condition, patient origin, hospital diagnosis of skin or subcutaneous tissue disease, pre-existing diseases (liver disease; alcohol or drug use), use of vasopressors, use of heparin for thromboembolism prophylaxis, tube thoracostomy, intensive care unit complications (musculoskeletal, renal/genitourinary), and number of pre-existing diseases. Hosmer-Lemeshow goodness of fit test, p = .5570.
Table 5 illustrates unadjusted differences in selected processes of care among the BMI categories. For several processes of care (e.g., central venous catheters, pulmonary artery catheters, hemodialysis), there were no significant differences in the unadjusted rates (data not shown). Patients with higher BMIs more commonly received heparin prophylaxis for thromboembolic disease. The inclusion of this covariate in the categorical BMI model increased the odds of mortality for the severely obese by 10%, suggesting mediation of the “benefit” for the severely obese. There was no significant effect modification of heparin prophylaxis based upon BMI category (data not shown). Tracheostomy did not significantly change the association between hospital mortality and BMI in either the continuous or the categorical analyses, and there was no significant effect modification.
Table 5.
Unadjusted differences in selected processes of care among body mass index categories
| Process of carea | Underweight (<18.5 kg/m2) n = 88 | Normal (18.5–24.9 kg/m2) n = 544 | Overweight (25–29.9 kg/m2) n = 399 | Obese (30–39.9 kg/m2) n = 326 | Severely obese (≥40 kg/m2) n = 131 | p Valueb |
|---|---|---|---|---|---|---|
| Heparin prophylaxis for thromboembolic disease | 36 (40.9%) | 238 (43.8%) | 165 (41.4%) | 150 (46.0%) | 75 (57.2%) | .026 |
| Tracheostomy | 20 (22.7%) | 94 (17.3%) | 77 (19.3%) | 80 (24.5%) | 34 (26.0%) | .045 |
| Tracheostomy among hospital survivors | 14 (35.0%) | 56 (17.4%) | 52 (20.2%) | 55 (24.2%) | 23 (24.2%) | .054 |
| Use of specialty bed | 18 (20.4%) | 80 (14.7%) | 79 (18.9%) | 57 (17.5%) | 38 (29.0%) | .003 |
Categorical variables presented as frequency (%);
Pearson’s chi-square test.
DISCUSSION
In an observational multicenter database, BMI was independently associated with hospital mortality for mechanically ventilated patients with ALI. Patients with excess weight accounted for the majority of the cohort. Patients with very low BMIs had the highest risk-adjusted odds of hospital mortality. The lowest odds were among those with obese BMIs, although the highest BMIs attenuated this protective association.
We feel that this analysis provides additional information to the existing studies using PI data (9, 11). Earlier studies included all ICU admissions. As a result, obese patients might be admitted to the ICU at a different severity of illness or for admission reasons dissimilar to those for nonobese patients. This could be due to a perceived difference in risk for the obese subjects or due to real difficulties in providing adequate care in the ward setting. To reduce such selection bias, we included a more homogeneous population of subjects (ALI patients) with a clear indication for ICU admission (mechanical ventilation). We also provide information on possible mediators of the association between BMI and outcome (e.g., diabetes, processes of care). Although there is some overlap of subjects included in the three studies, our analysis used a dataset with 60% more records than the previous studies (M. Stark, personal communication). Due to more in-depth analysis, we found a nonlinear association that mirrors but is not identical to the usual National Institutes of Health categories of BMI. We employed extensive risk-adjusting techniques that produced well-fit estimates of mortality odds. Prior study reports did not provide similar detail (11) and presented data that suggest poorly calibrated risk-adjusting estimates (9). Such lack of detail in risk-adjustment techniques is common in observational studies of critically ill subjects (17).
Comparison with Prior Studies
Previous studies have shown harm (6–8), protection (9–11), and no association (12–14) between excess weight and outcomes. The prior studies using PI data (9, 11) confirm our finding of protection from mortality for heavier ICU patients. We did not find increased lengths of stay for the obese subjects as observed in one of the these studies (9). One explanation for this difference is that BMI may not affect the length of stay of ALI patients but has an influence on duration of ICU stay for other types of critically ill patients. All three studies using PI records showed a higher mortality for those with underweight BMIs. This association may be from underlying disease that leads to thinness and inadequate nutritional reserve to compensate for the stresses of critical illness. We also cannot exclude that this is due to a true increased risk for the healthy thin when they require ICU care. Investigators have reported a similar association between lower BMIs and higher mortality in other populations (18).
Like the current analysis, two other studies limited enrollment to subjects requiring mechanical ventilation (6, 13). One of these is an analysis of ALI patients enrolled in a clinical trial of ventilator management (ARDS Network) (13). After risk-adjustment, there was no association between higher BMIs and any of the measured outcomes. The benefit of a lower tidal volume strategy (19) extended to ALI patients of all BMIs. Differences in the study sample are a possible explanation for the discrepancy in results between the ARDS Network study and the current report. PI is an observational dataset including all patients admitted to participating hospitals. The ARDS Network study was a therapeutic trial with strict inclusion and exclusion criteria. A higher overall mortality in the current cohort (40.0%) than in the ARDS Network study (35.4%) supports the effect of such restriction in subject selection. Neither study showed an increased risk-adjusted mortality associated with excess weight, a finding contrary to conventional wisdom.
The second study including only mechanically ventilated patients enrolled those admitted to a medical/surgical ICU in France (6). The investigators matched obese subjects (BMI >30 kg/m2) with nonobese controls (BMI 18.5–24.9 kg/m2). Patients with underweight and overweight BMIs were not included in the analysis. The authors reported a two-fold increase in risk-adjusted odds of ICU mortality among the obese (adjusted OR, 2.1 [95% confidence interval, 1.2–3.6]; p = .007). The ARDS Network analysis suggests a confounder that might bias these results. In that analysis, there was a wide variation of fluid balance in the 24 hrs preceding study enrollment (from 9,526 mL [9.5 kg] lost to 28,472 mL [28.4 kg] gained), with 14% of the sample changing BMI category after adjustment for fluid balance (13). In the French study, patients with normal BMIs who required large volume resuscitation before weight assessment might move into higher BMI categories. Subsequently, investigators would either classify such patients as overweight and exclude them from analysis or consider them “obese” and include them. Patients with normal BMIs who did not require volume resuscitation would remain in the study in the control group. Ultimately, the obese group could include “pseudo-obese” subjects (those with normal and overweight BMIs who required significant volume resuscitation) and obese subjects who did or did not require resuscitation. Meanwhile, the comparative, “normal” BMI group would include only those with a normal BMI who did not require sufficient resuscitation to move them to a higher BMI class. As a result, the “obese” subjects could be at higher risk because they required greater volume resuscitation than normal BMI subjects, rather than because of an inherent risk associated with excess body weight.
Possible Mechanisms of the Association Between BMI and Outcome
We considered differences in preexisting diabetes and disparities of provided care as possible mediators of the difference in outcomes among BMI groups. Despite a higher prevalence of diabetes, those with higher BMIs had lower odds of death. Adjustment for the presence of diabetes appeared to augment this “protection” for the obese. This suggests that although diabetics had a poorer prognosis, diabetes had a weaker association with mortality than BMI. However, these findings were not statistically significant and require confirmation in future studies.
There were differences in some processes of care (e.g., tracheostomy, use of heparin prophylaxis) but not in others (e.g., central venous catheters, pulmonary artery catheters). After adjusting for the use of heparin prophylaxis, the adjusted odds ratio for the severely obese rose from 0.71 to 0.78. Thus, the increased use of heparin prophylaxis among higher BMIs mediated some of the protective effects of severe obesity. There was no significant effect modification, arguing that the benefit of heparin prophylaxis was similar for all BMI categories. However, the power of the current study is too low to make definitive conclusions. Attention to the processes of care is integral to assess the full association between excess weight and outcome. In the analysis using ARDS Network data, obese subjects had significantly higher tidal volumes per predicted body weight than did those with normal BMIs at study enrollment (13). This resulted in higher airway pressures and may have placed obese patients at higher risk of ventilator-associated lung injury (20) if tidal volumes were not standardized by study protocol. Unfortunately, limitations in the dataset prevented us from examining ventilator management in the current study.
Differences in processes of care among the obese could have influenced the results of prior studies. In the previously cited French study, there was no difference in the baseline prevalence of coronary heart disease, but obese subjects were more likely to die of myocardial infarction (6). This could be the result of undetected or more severe cardiovascular disease among the obese, arguing for inadequate matching. Alternatively, it could be due to differences in provided care. Obese subjects might be less likely to receive potentially life-saving therapies, such as percutaneous coronary interventions or bypass grafting surgery, because of concerns about their body habitus. There is documented bias against obese individuals in healthcare settings (21–24) that can affect provided care (25, 26). By incorporating an assessment of provided care, subsequent studies can explore if such a disparity is the mechanism of a mortality difference for obese ICU patients.
Limitations
In a retrospective cohort study, we must rely on the validity, accuracy, and comprehensiveness of the collected data. During the time of data collection for this study, training for PI abstractors was optional (M. Stark, personal communication). We cannot strictly confirm the diagnosis of ALI in this cohort. It is possible that differences in the interpretation of chest radiographs, baseline increased venous admixture associated with obesity (4), or difficulties in detecting signs of cardiogenic pulmonary edema in obese patients could have led to a detection bias for the presence of ALI. In addition, weight and height data were not collected in the most stringent fashion and the PI dataset did not contain sufficient information to adjust for fluid balance. However, we doubt there was a systematic variation of weight and height determinations or of volume resuscitation based on BMI that might confound our results.
We reduced selection bias by restricting the study population. This approach also limits the validity of our findings for other populations of critically ill patients. In addition, if decisions about ICU admission were associated with BMI, only the “fittest” of obese ALI subjects might receive ICU care, whereas the “less fit” might have a limitation in the aggressiveness of care, not be admitted to an ICU, and not appear in the cohort. We cannot completely exclude such a selection bias. However, there was no difference in limitation of care orders across BMI groups for those included in the study, arguing against a systematic difference in aggressiveness of care based on BMI.
The odds of death were highest among those with the lowest body mass indexes (BMIs); patients with obese BMIs had the lowest odds of hospital mortality.
While trying to explore a variety of outcome measures, we were limited by the content of the dataset. We were not able to explore more durable outcomes (e.g., 6-month survival) or patient-centered outcomes (e.g., health-related quality of life). Some processes of care known to be associated with outcome (such as head-of-bed elevation) were not available. Finally, we could not determine the burden the obese critically ill might have for care providers, such as nurses and aides. As the critically ill population grows heavier, attention to the caregivers will be an important component of designing ICUs that are safe for patients and staff.
CONCLUSIONS
In this retrospective cohort study of mechanically ventilated adults with ALI, BMI was independently associated with hospital mortality. The odds of death were highest among those with the lowest BMIs; patients with obese BMIs had the lowest odds of hospital mortality. We also found differences in the rate of certain processes of care. Further study with attention to the provided care should allow for a greater appreciation of any true association between excess body weight and outcomes among critically ill patients.
Footnotes
See also p. 915.
The authors have no financial interests to disclose.
Dr. O’Brien is supported by a Career Development Award (HL075076) from the NHLBI and a Davis/Bremer Award from the Ohio State University College of Medicine. Dr. Marsh is supported by HL63800 and HL70294.
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, et al. Overweight and obesity in the United States: Prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. The Evidence Report. National Institutes of Health Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 4.Vaughan RW, Conahan TJ., 3rd Part I: Cardiopulmonary consequences of morbid obesity. Life Sci. 1980;26:2119–2127. doi: 10.1016/0024-3205(80)90598-6. [DOI] [PubMed] [Google Scholar]
- 5.Alpert MA, Hashimi MW. Obesity and the heart. Am J Med Sci. 1993;306:117–123. doi: 10.1097/00000441-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Bercault N, Boulain T, Kuteifan K, et al. Obesity-related excess mortality rate in an adult intensive care unit: A risk-adjusted matched cohort study. Crit Care Med. 2004;32:998–1003. doi: 10.1097/01.ccm.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 7.Goulenok C, Monchi M, Chiche JD, et al. Influence of overweight on ICU mortality: A prospective study. Chest. 2004;125:1441–1445. doi: 10.1378/chest.125.4.1441. [DOI] [PubMed] [Google Scholar]
- 8.El-Solh A, Sikka P, Bozkanat E, et al. Morbid obesity in the medical ICU. Chest. 2001;120:1989–1997. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123:1202–1207. doi: 10.1378/chest.123.4.1202. [DOI] [PubMed] [Google Scholar]
- 10.Garrouste-Orgeas M, Troche G, Azoulay E, et al. Body mass index: An additional prognostic factor in ICU patients. Intensive Care Med. 2004;30:437–443. doi: 10.1007/s00134-003-2095-2. [DOI] [PubMed] [Google Scholar]
- 11.Marik PE, Doyle H, Varon J. Is obesity protective during critical illness? An analysis of a national ICU database. Crit Care Shock. 2003;6:127–133. [Google Scholar]
- 12.Ray DE, Matchett SC, Baker K, et al. The effect of body mass index on patient outcomes in a medical ICU. Chest. 2005;127:2125–2131. doi: 10.1378/chest.127.6.2125. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien JM, Jr, Welsh CH, Fish RH, et al. Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med. 2004;140:338–345. doi: 10.7326/0003-4819-140-5-200403020-00009. [DOI] [PubMed] [Google Scholar]
- 14.Galanos AN, Pieper CF, Kussin PS, et al. Relationship of body mass index to subsequent mortality among seriously ill hospitalized patients: SUPPORT Investigators: The Study to Understand Prognoses and Preferences for Outcome and Risks of Treatments. Crit Care Med. 1997;25:1962–1968. doi: 10.1097/00003246-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 16.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multi-center study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 17.Moss M, Wellman DA, Cotsonis GA. An appraisal of multivariable logistic models in the pulmonary and critical care literature. Chest. 2003;123:923–928. doi: 10.1378/chest.123.3.923. [DOI] [PubMed] [Google Scholar]
- 18.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 19.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 20.Matthay MA, Bhattacharya S, Gaver D, et al. Ventilator-induced lung injury: In vivo and in vitro mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L678–L682. doi: 10.1152/ajplung.00154.2002. [DOI] [PubMed] [Google Scholar]
- 21.Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: Is anyone immune? Int J Obes Relat Metab Disord. 2001;25:1525–1531. doi: 10.1038/sj.ijo.0801745. [DOI] [PubMed] [Google Scholar]
- 22.Maddox GL, Liederman V. Overweight as a social disability with medical implications. J Med Educ. 1969;44:214–220. doi: 10.1097/00001888-196903000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Blumberg P, Mellis L. Medical students’ attitudes toward the obese and morbidly obese. Int J Eat Disord. 1985;4:169–175. [Google Scholar]
- 24.Rand CS, Macgregor AM. Morbidly obese patients’ perceptions of social discrimination before and after surgery for obesity. South Med J. 1990;83:1390–1395. doi: 10.1097/00007611-199012000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Hebl MR, Xu J. Weighing the care: Physicians’ reactions to the size of a patient. Int J Obes Relat Metab Disord. 2001;25:1246–1252. doi: 10.1038/sj.ijo.0801681. [DOI] [PubMed] [Google Scholar]
- 26.Adams CH, Smith NJ, Wilbur DC, et al. The relationship of obesity to the frequency of pelvic examinations: Do physician and patient attitudes make a difference? Women Health. 1993;20:45–57. doi: 10.1300/J013v20n02_04. [DOI] [PubMed] [Google Scholar]


