Abstract
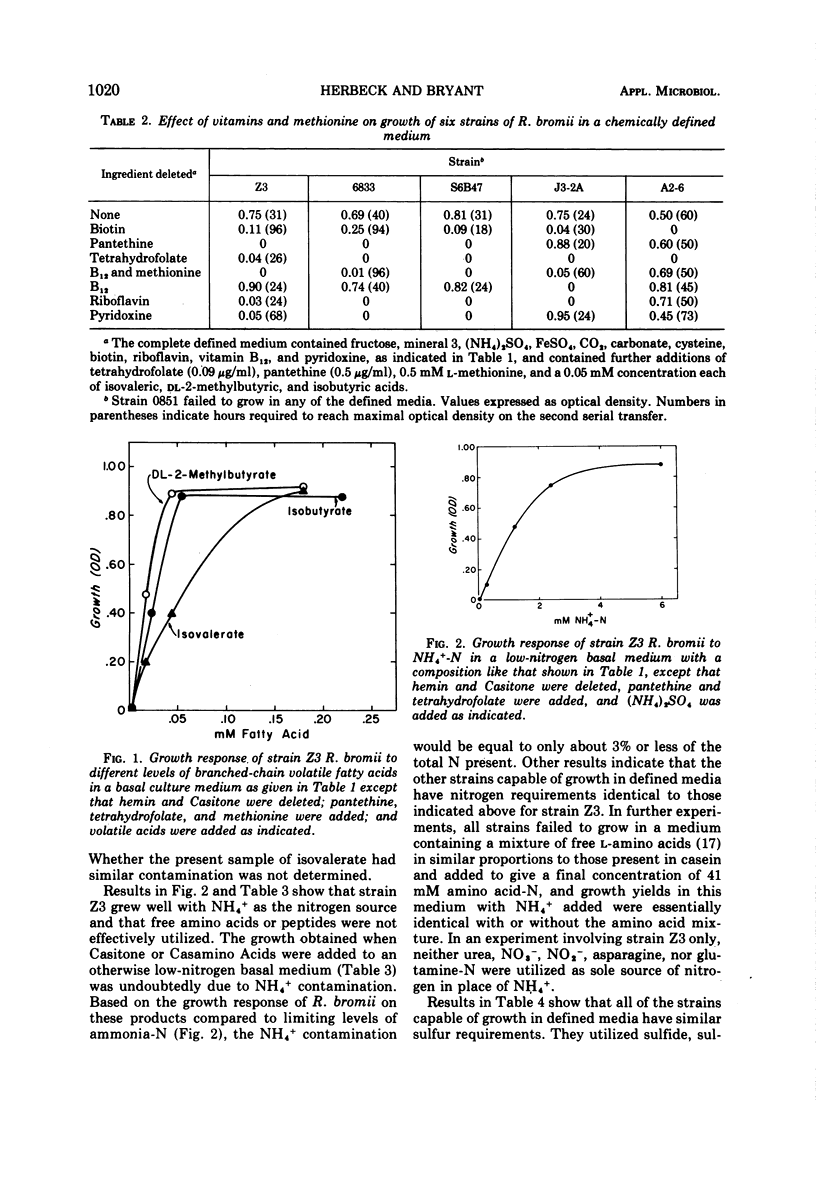
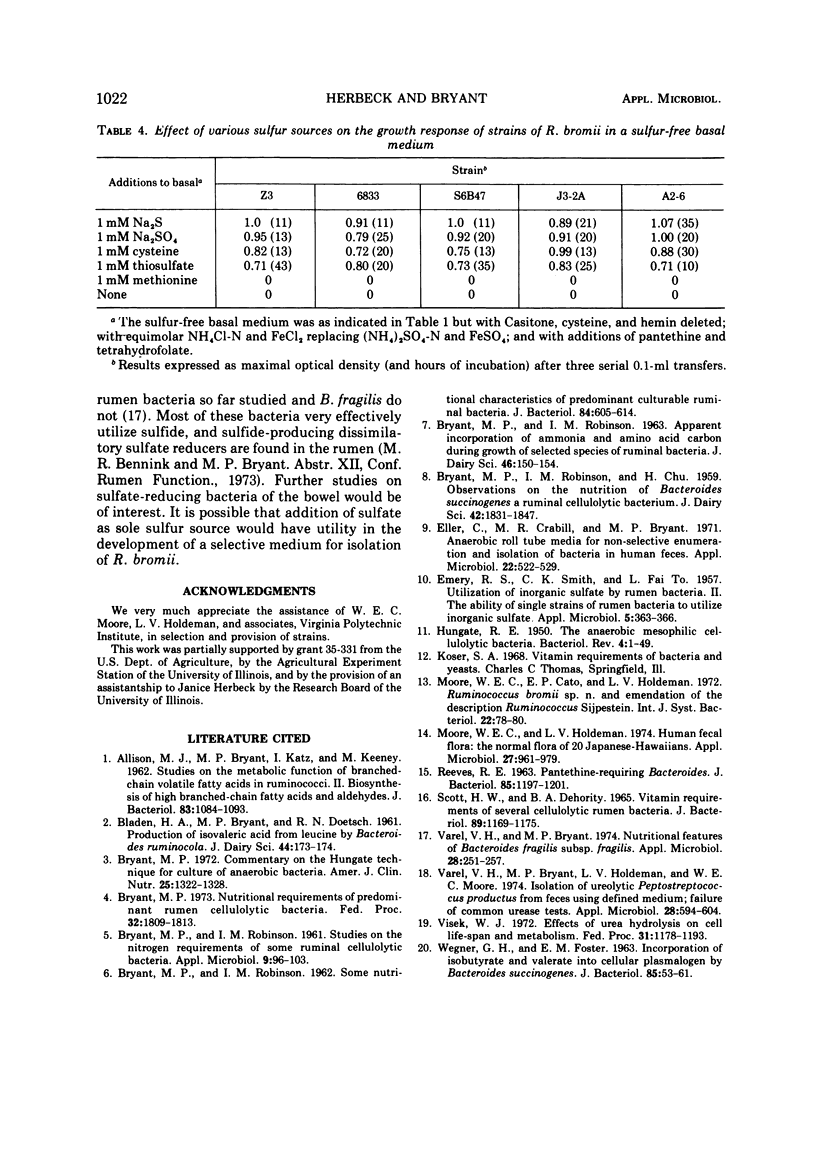
Of six strains of Ruminococcus bromii studied, five grew in a minimal chemically defined medium containing minerals, NH4+ as nitrogen source, sulfide or sulfate as sulfur source, fructose as energy and carbon source, isobutyrate or 2-methylbutyrate and carbonic acid-bicarbonate as additional carbon sources, and the vitamins biotin, riboflavin, pyridoxine, vitamin B12 (replaced by L-methionine), pantethine, and tetrahydrofolate. The strains also could utilize cysteine or thiosulfate but not methionine; and strain Z3 failed to use dithiothreitol, thioglycolate, sulfite, or β-mercaptoethanol as sole sources of sulfur. Mixtures of amino acids, peptides (Casitone), urea, nitrate, asparagine, or glutamine failed to replace NH4+ as N source. Three strains isolated from Americans were identical in nutritional features, whereas one from a Japanese and one from a South African native differed slightly in having requirements for fewer vitamins. One strain from the cecum of a sow grew well in a rumen fluid-supplemented medium but not in the various chemically defined media plus Casitone. The nutritional features suggest that the environment which selects R. bromii contains relatively little amino acid nitrogen and a relatively large amount of NH4+-N and indicate that these bacteria must depend upon other bacteria such as those that produce NH4+ from urea or protein and those that produce branched-chain volatile acids to grow.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Studies on the Nitrogen Requirements of Some Ruminal Cellulolytic Bacteria. Appl Microbiol. 1961 Mar;9(2):96–103. doi: 10.1128/am.9.2.96-103.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERY R. S., SMITH C. K., FAI TO L. Utilization of inorganic sulfate by rumen microorganisms. II. The ability of single strains of rumen bacteria to utilize inorganic sulfate. Appl Microbiol. 1957 Nov;5(6):363–366. doi: 10.1128/am.5.6.363-366.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller C., Crabill M. R., Bryant M. P. Anaerobic roll tube media for nonselective enumeration and isolation of bacteria in human feces. Appl Microbiol. 1971 Oct;22(4):522–529. doi: 10.1128/am.22.4.522-529.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES R. E. PANTETHINE-REQUIRING BACTEROIDES. J Bacteriol. 1963 Jun;85:1197–1201. doi: 10.1128/jb.85.6.1197-1201.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P., Holdeman L. V., Moore W. E. Isolation of ureolytic Peptostreptococcus productus from feces using defined medium; failure of common urease tests. Appl Microbiol. 1974 Oct;28(4):594–599. doi: 10.1128/am.28.4.594-599.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visek W. J. Effects of urea hydrolysis on cell life-span and metabolism. Fed Proc. 1972 May-Jun;31(3):1178–1193. [PubMed] [Google Scholar]
- WEGNER G. H., FOSTER E. M. Incorporation of isobutyrate and valerate into cellular plasmalogen by Bacteroides succinogenes. J Bacteriol. 1963 Jan;85:53–61. doi: 10.1128/jb.85.1.53-61.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



