Abstract
The mycotoxin CTN (citrinin), a natural contaminant in foodstuffs and animal feeds, has cytotoxic and genotoxic effects on various mammalian cells. CTN is known to cause cell injury, including apoptosis, but the precise regulatory mechanisms of CTN action, particularly in stem cells and embryos, are currently unclear. In the present paper, I report that CTN has cytotoxic effects on mouse embryonic stem cells and blastocysts, and is associated with defects in their subsequent development, both in vitro and in vivo. Experiments in embryonic stem cells (ESC-B5) showed that CTN induces apoptosis via ROS (reactive oxygen species) generation, increased Bax/Bcl-2 ratio, loss of MMP (mitochondrial membrane potential), induction of cytochrome c release, and activation of caspase 3. In this model, CTN triggers cell death via inactivation of the HSP90 [a 90 kDa isoform of the HSP (heat-shock protein) family proteins]/multichaperone complex and subsequent degradation of Ras and Raf-1, further inhibiting anti-apoptotic processes, such as the Ras→ERK (extracellular-signal-regulated kinase) signal transduction pathway. In addition, CTN causes early developmental injury in mouse ESCs and blastocysts in vitro. Lastly, using an in vivo mouse model, I show that consumption of drinking water containing 10 μM CTN results in blastocyst apoptosis and early embryonic developmental injury. Collectively, these findings show for the first time that CTN induces ROS and mitochondria-dependent apoptotic processes, inhibits Ras→ERK survival signalling via inactivation of the HSP90/multichaperone complex, and causes developmental injury in vivo.
Keywords: apoptosis, blastocyst, citrinin, embryonic development, heat-shock protein, mycotoxin
Abbreviations: CTN, citrinin; DCFH-DA, 2′,7′-dichlorofluorescein diacetate; DHR 123, dihydrorhodamine 123; DiOC6(3), 3,3′-dihexyloxacarbocyanine iodide; DMEM, Dulbecco's modified Eagle's medium; EB, embryoid body; ERK, extracellular-signal-regulated kinase; FBS, fetal bovine serum; hCG, human chorionic gonadotropin; HSP, heat-shock protein; ICM, inner cell mass; JNK, c-Jun N-terminal kinase; LIF, leukaemia inhibitory factor; MAP-2, microtubule-associated protein-2; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; MMP, mitochondrial membrane potential; NAC, N-acetylcysteine; NGF, nerve growth factor; PKB, protein kinase B; PMSG, pregnant mare serum gonadotropin; ROS, reactive oxygen species; TE, trophectoderm; TMRE, tetramethylrhodamine ethyl ester; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; Z, benzyloxycarbonyl
INTRODUCTION
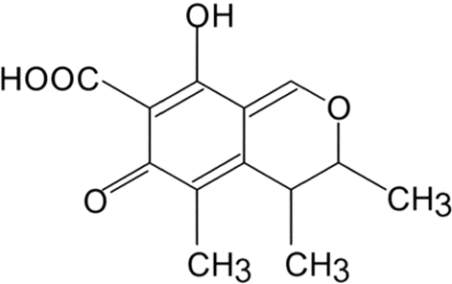
Several fungal species belonging to the genera Penicillium and Monascus produce secondary metabolites, including the mycotoxin, CTN (citrinin; Figure 1). CTN has been identified as a contaminant in several types of food, including corn, wheat, rice, barley and nuts, and may cause environmental and human health injury [1,2]. Experiments in cell lines and animals models have shown that CTN triggers nephropathy and hepatotoxicity [3,4], as well as renal adenoma formation [5]. However, although CTN appears to have multiple biological functions and cytotoxicities, the precise molecular mechanisms underlying these actions remain unknown.
Figure 1. Chemical structure of CTN.
During normal embryogenesis, apoptosis (a unique morphological pattern of cell death) functions to clear abnormal or redundant cells in pre-implantation embryos [6,7]. However, apoptotic processes do not occur prior to the blastocyst stage during normal mouse embryonic development [8], and induction of apoptosis during the early stages of embryogenesis (i.e. by exposure to a teratogen) has been shown to cause embryonic developmental injury [9,10]. Many of the chemical and physical treatments that induce apoptosis stimulate oxidative stress via generation of ROS (reactive oxygen species) [9,11–13], suggesting a close relationship between oxidative stress and apoptosis. While the precise molecular mechanisms underlying apoptosis have not yet been clearly defined, studies have shown that caspase activation, MMP (mitochondrial membrane potential) changes and JNK (c-Jun N-terminal kinase) activation are critical for the mitochondria-dependent apoptotic pathway [9,14]. In addition, previous studies have shown that some apoptotic stimuli trigger heat-shock responses, such as activation of the HSPs (heat-shock proteins), which are molecular chaperones that rescue damaged proteins by facilitating their refolding [15,16].
HSP90, a 90 kDa isoform of the HSP family proteins, acts in concert with other chaperones and partners to facilitate the maturation and folding of client proteins via formation of a HSP90/multichaperone complex [17]. Significantly, two HSP90 client proteins, Raf-1 and MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase], are components of the Ras/ERK-dependent signal transduction pathway that regulates cell apoptosis and proliferation [18]. In addition, the Ras-regulated ERK activation signal transduction pathway, which includes Raf-1 and MEK, is involved in both proliferation and anti-apoptosis [18]. Ras→ERK-mediated survival signalling has been shown to protect human epidermoid cancer KB cells from various apoptotic inducers [19–21]. Together, these previous results suggest that HSP90/multichaperone complex-mediated regulation of apoptotic or survival signal pathway kinases is an efficient mechanism for regulating cell death and survival at the protein level. A previous report has showed that inhibition of Ras→ERK-mediated survival signalling can effectively induce cell apoptosis in electromagnetic fields-treated human epidermoid cancer cells [22]. However, no previous study has tested the effect of CTN on Ras→ERK-mediated survival signalling in ESCs (embryonic stem cells) or embryos.
In the present study, I investigated whether CTN has a hazardous effect on embryonic development in both cell culture and animal assay models, and tested several possible mechanisms of CTN action. Our results show that CTN triggers apoptosis via ROS generation, increases in the Bax/Bcl-2 protein ratio, loss of MMP, activation of caspase 3, and down-regulation of anti-apoptotic signalling via the Ras→ERK pathway. Furthermore, CTN significantly inhibits embryonic development in blastocysts, and induces apoptosis in vitro and in vivo.
MATERIALS AND METHODS
Chemicals and reagents
DMEM (Dulbecco's modified Eagle's medium), CTN, DCFH-DA (2′,7′-dichlorofluorescein diacetate), DHR 123 (dihydrorhodamine 123), PMSG (pregnant mare serum gonadotropin), NAC (N-acetylcysteine), and synthetic α-tocopherol (DL-all-rac-α-tocopherol) were obtained from Sigma. hCG (human chorionic gonadotropin) was purchased from Serono. Z (benzyloxycarbonyl)-DEVD-AFC was obtained from Calbiochem. Anti-JNK1 (C17), anti-p-JNK (G-7), anti-ERK-1/2, anti-p-ERK-1/2, anti-p38K, anti-HSP, anti-Raf-1, anti-β-actin, and alkaline phosphatase-conjugated goat anti-rabbit and anti-mouse IgG antibodies were purchased from Santa Cruz Biotechnology. U0126 and anti-Ras monoclonal antibodies were obtained from Calbiochem. TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) in situ cell death detection kits were acquired from Roche, and the CMRL-1066 medium was from Gibco Life Technologies.
Cell culture, CTN treatment and immunoblotting
Mouse embryonic stem cells (ESC-B5) were cultured in DMEM supplemented with 20% (v/v) heat-inactivated FBS (fetal bovine serum), 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were incubated in a medium containing various concentrations of CTN at 37 °C in a CO2 incubator for 24 h, washed twice with ice-cold PBS, and lysed. Immunoblotting was performed using anti-JNK1 (C17), p-JNK, ERK, p-ERK, Ras, Raf-1 and p38K antibodies.
Apoptosis assay
Cells (1×105) were treated with or without various concentrations of CTN at 37 °C for 24 h. Oligonucleosomal DNA fragmentation (a hallmark of apoptosis) was measured using the Cell Death Detection ELISAplus kit, according to the manufacturer's protocol (Roche Molecular Biochemicals). Spectrophotometric data were obtained at 405 nm using an ELISA reader.
ROS assay
ROS were measured in arbitrary units using the DCFH-DA or DHR 123 fluorescent dyes. Briefly, cells (1.0×106) were incubated in 50 μl of PBS containing 20 μM DCFH-DA or DHR 123 for 1 h at 37 °C, and relative ROS units were determined with a fluorescence ELISA reader (λex=485 nm and λem=530 nm). An aliquot of each cell suspension was lysed, and the protein concentrations were determined using a BCA (bicinchoninic acid) protein assay kit (Pierce). The results are expressed as arbitrary absorbance units/mg of protein.
Detection of MMP
ESC-B5 cells were plated and grown on 96-well plates for 24 h, and then incubated with various concentrations of CTN for 24 h. The cells were then separately exposed to the fluorescent dyes, DiOC6(3) (3,3′-dihexyloxacarbocyanine iodide) (40 nM/well) or TMRE (tetramethylrhodamine ethyl ester) (1 μM/well), for 15 min, and fluorescence was measured with a plate spectrofluorimeter {λex=485 nm [DiOC6(3)], 535 nm (TMRE); λem=535 nm [DiOC6(3)], 590 nm (TMRE)}.
Cytochrome c release and caspase activity assays
Mitochondrial cytochrome c release from CTN-treated cells was assayed as described previously [23]. Caspase 3 activity was measured using the Z-DEVD-AFC fluorogenic substrate [23,24].
Transient expression of exogenous hHSP90β
For transient transfection, cells grown in 60 mm culture dishes were incubated at 37 °C in 1 ml of Opti-MEM I medium (modified Eagle's minimum essential medium buffered with Hepes and sodium bicarbonate) containing 12 μg of Lipofectamine™ 4 (Life Technologies) and pMT2/hHsp90β DNA (4 μg) for 72 h. The transfected cells were treated and analysed as described above.
Antisense knockdown of HSP90
Antisense oligonucleotides targeting HSP90 were used to reduce its protein expression. Briefly, 70 μM sense (5′-CAGTTGCTTCAGCGTCC-3′) or antisense (5′-GGACGCTGAAGCAACTG-3′) oligonucleotides [25] were transfected to ESC-B5 cells, and the cells were incubated for 48 h prior to CTN treatment and analysis as described above.
EB (embryoid body) formation
EBs were generated as previously described [10]. Briefly, ESC-B5 cells were dissociated with trypsin–EDTA (0.25%) and cultured in LIF (leukaemia inhibitory factor)-free stem cell medium to induce differentiation. Cell suspension liquid cultures (<103 cells/ml) were dispensed into 10 cm Petri dishes (10 ml per dish). EB formation was initiated in hanging drop cultures prepared with 10-μl droplets, each containing an appropriate number of ESC-B5 cells. The ESC-B5 cells were allowed to aggregate in the hanging drops for 2 days, and were then transferred to liquid suspension culture.
Collection of mouse blastocysts
ICR virgin albino mice (6–8 weeks old) were induced to superovulate by injection of 5 IU of PMSG followed 48 h later by injection of 5 IU of hCG, and mated overnight with a single fertile male of the same strain. Female mice with vaginal plugs were separated for experiments. All mice were maintained on breeder chow under conditions of 12 h day/12 h night, with food and water available ad libitum. Animals received humane care, as outlined in the Guidelines for Care and Use of Experimental Animals (Canadian Council on Animal Care, Ottawa, 1984). Detection of a vaginal plug was defined as ‘day 0’ of pregnancy. Blastocysts were obtained by flushing the uterine horn on day 4. The flushing solution consisted of CMRL-1066 culture medium containing 1 mM glutamine and 1 mM sodium pyruvate. Expanded blastocysts from different females were pooled and randomly selected for experiments.
CTN treatment and TUNEL assay of blastocysts
Blastocysts were incubated in a medium containing various concentrations of CTN for 24 h. For TUNEL staining, embryos were washed in CTN-free medium, fixed, permeabilized and subjected to labelling using an in situ cell death detection kit (Roche Molecular Biochemicals), according to the manufacturer's protocol. Photographic images were obtained using a fluorescence microscope under bright-field illumination.
Morphological analysis of mouse embryos in vitro
Blastocysts from ICR mice were collected on day 4 of pregnancy and incubated in the presence or absence of 10–30 μM CTN. After 24 h, treated blastocysts were individually transferred to fibronectin-coated culture wells and grown in the absence of CTN for 72 h in CMRL-1066 medium supplemented with 20% FBS (CMRL-FBS) [26]. Under these culture conditions, each hatched blastocyst attached to the fibronectin and grew to form a cluster of ICM (inner cell mass) cells over the trophoblastic layer, in a process called TE (trophectoderm) outgrowth. After a total incubation period of 96 h, morphological scores for outgrowth were estimated. Growing embryos were classified as either ‘attached’ or ‘outgrowth’, with the latter defined by the presence of a cluster of ICM cells over the trophoblastic layer. ICM clusters were scored according to shape, ranging from compact and rounded ICM (+++) to a few scattered cells (+) over the trophoblastic layer, as described previously [27].
Effects of maternal CTN intake on embryonic development in an animal model
The effects of CTN on embryonic development were analysed in ICR virgin albino mice 6–8 weeks of age. Female mice were mated overnight with a single fertile male of the same strain, and those displaying vaginal plugs were separated for experiments. Detection of a vaginal plug was defined as ‘day 0’ of pregnancy. Mice were randomly divided into two diet groups of 20 animals each and given a standard diet with or without supplementation with 10 μM CTN in the drinking water from days 0 to 4. Embryos were obtained by flushing the uterine horn on day 4. Embryonic development was assessed by morphological analysis under a light microscope, and apoptosis was determined by TUNEL staining.
Statistics
Data were analysed using one-way ANOVA and t tests, and are presented as means±S.D. Data were considered statistically significant at P<0.05.
RESULTS
The effects of CTN on ESC-B5 cells
While CTN clearly induces apoptosis in mammalian cells [28,29], its effects on embryonic development and the precise regulatory mechanisms governing these effects are currently unclear. To identify the apoptotic signalling pathway(s) involved in CTN-induced cell death in embryonic cells, I initially analysed the effects of the toxin on embryonic stem cells (ESC-B5) in vitro. When ESC-B5 cells were treated with 10–30 μM CTN for 24 h and cell viability and apoptosis were measured, I found that CTN dose-dependently reduced ESC-B5 cell viability (Figure 2A). Next, I determined whether this CTN-induced cell death was due to apoptosis or necrosis. ELISA-based quantitative measurement of histone-associated oligonucleosomal DNA fragmentation revealed that CTN treatment induced a 2.1–3.1-fold increase in DNA fragmentation in ESC-B5 cells (Figure 2B), whereas a lactase dehydrogenase assay for necrosis showed that the necrotic indices were similar in CTN-treated and untreated control ESC-B5 cells (results not shown). These results indicate that CTN induces ESC-B5 cell death via apoptosis, not necrosis (Figure 2).
Figure 2. Effects of CTN on ESC-B5 cells.
ESC-B5 cells (1×106) were incubated with or without the indicated concentrations of CTN for 24 h. Cell viability was determined by the MTT assay (A), and apoptosis was evaluated using the Cell Death Detection ELISA kit (TUNEL assay kit) (B), as described in the Materials and methods section. Results are means±S.D. of five determinations. *P<0.05, **P<0.01 and ***P<0.001 compared with the CTN-free group.
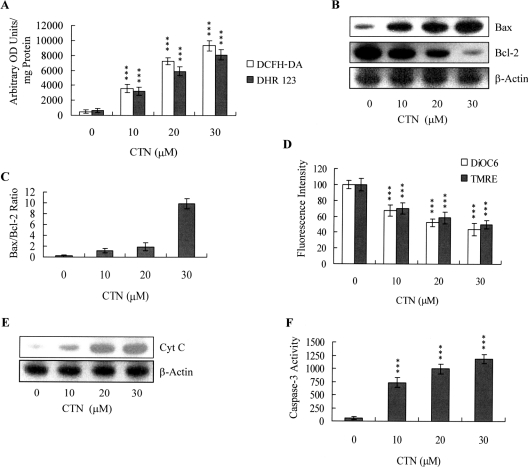
In view of the previous finding that ROS effectively induces stem cell apoptosis [10], I next used the fluorescent dyes, DCFH-DA and rhodamine 123, to examine whether the ROS content increased in CTN-treated ESC-B5 cells. As shown in Figure 3(A), 10–30 μM CTN directly induced a ∼7–18-fold increase in the intracellular ROS content of ESC-B5 cells, compared with untreated control cells. Since the protein expression ratio of Bax versus Bcl-2 is relevant to the mitochondria-dependent apoptotic pathway [30,31], with high and low Bax/Bcl-2 ratios associated with lower and higher apoptotic thresholds respectively, I determined whether CTN induced apoptosis via modulation of the Bax/Bcl-2 ratio. Immunoblotting revealed that CTN treatment of ESC-B5 cells caused the Bax and Bcl-2 expression levels to increase and decrease respectively (Figure 3B), and densitometric analysis confirmed that CTN-treated ESC-B5 cells had a higher Bax/Bcl-2 ratio, which is indicative of apoptosis (Figure 3C). We further investigated the effects of CTN on MMP changes in ESC-B5 cells, and found that CTN dose-dependently decreased the uptake of DiOC6(3) and TMRE into the mitochondria of ESC-B5 cells, indicating a significant loss of MMP (Figure 3D). We then analysed the effect of CTN on cytochrome c release from mitochondria to the cytosol. Immunoblotting revealed that a significant amount of cytochrome c was released into the cytosol of CTN-treated ESC-B5 cells (Figure 3E). Moreover, CTN treatment significantly stimulated caspase 3 activity, which is an important indicator of apoptosis (Figure 3F). To clarify further the role of ROS in CTN-induced apoptosis, I assessed the effects of two commonly used ROS scavengers, α-tocopherol and NAC, on CTN-treated ESC-B5 cells. Pre-treatment of cells with α-tocopherol (300 μM; dissolved in DMSO) or NAC (500 μM) attenuated CTN-induced intracellular ROS generation, apoptosis, and caspase 3 activation (Figures 4A–4C). Collectively, these results suggest that CTN treatment triggers ROS generation, which in turn activates mitochondria-dependent apoptotic processes in ESC-B5 cells.
Figure 3. CTN induces apoptotic biochemical changes in ESC-B5 cells.
ESC-B5 cells (1×106) were incubated with or without the indicated concentrations of CTN for 24 h. (A) ROS generation was assayed using 20 μM DCFH-DA and DHR 123, and was expressed as absorbance (‘arbitrary OD units’)/mg of protein. (B, C) Bax and Bcl-2 levels were analysed by immunoblotting (B) and densitometry (C). (D) MMP changes were analysed by 40 nM DiOC6(3) or 1 μM TMRE. (E) To examine cytochrome c release from mitochondria to the cytosol, cytosolic and mitochondrial fractions were separated, and cytosolic fractions were immunoblotted with an anti-cytochrome c antibody. (F) Cell extracts (60 μg) were analysed for caspase 3 activity, using Z-DEVD-AFC as the substrate. Results are means±S.D. of five determinations. ***P<0.001 compared with the CTN-free group.
Figure 4. Effects of ROS scavengers on CTN-treated ESC-B5 cells.
ESC-B5 cells were treated with or without the indicated concentrations of α-tocopherol (Toc; 300 μM) or NAC (500 μM) for 30 min, followed by incubation with CTN (30 μM) for another 24 h. ROS generation (A), cell apoptosis (B), and caspase 3 activity (C) were measured. Results are means±S.D. of five determinations. **P<0.01, ***P<0.001 compared with the ‘CTN-only’ treatment group. OD units, absorbance units
Effects of CTN on components of survival signalling
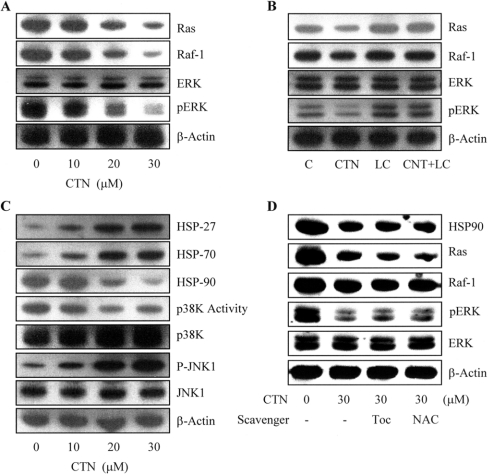
Survival signalling processes protect against apoptosis induced by specific stimuli [19,21]. Our group and others have demonstrated that some apoptotic stimuli inhibit the Ras→ERK survival signal pathway by decreasing the protein levels of various survival components [21,32]. Based on these results, I evaluated the effects of CTN on the expression levels and activities of components critical to the Ras→ERK-dependent survival pathway. Our results revealed that CTN treatment triggered dose-dependent decreases in the protein levels of Ras and Raf-1 (Figure 5A). Although ERK-1/2 protein expression was not affected, immunoblotting revealed that ERK activity was dose-dependently decreased in CTN-treated ESC-B5 cells (Figure 5A). These results suggest that CTN-induced apoptosis is mediated by decreased expression and activity of Ras and Raf-1, leading to inhibition of ERK-1/2 activity.
Figure 5. Effects of CTN on components of survival signalling and the multichaperone complex.
ESC-B5 cells were incubated with or without various concentrations of CTN for 24 h, and the levels of survival signalling proteins and HSPs were determined. (A) Immunoblotting was used to examine the expression levels of Ras, Raf-1, and ERK-1 and -2 and the phosphorylation levels of ERK-1 and -2. (B) Cells were incubated with or without lactacystin (LC; 10 μM) for 12 h and treated with CTN (30 μM) for another 24 h. The protein expression levels of Ras, Raf-1, and ERK-1 and -2 and the phosphorylation levels of ERK-1 and -2 were evaluated. (C) Immunoblotting was used to examine the expression levels of HSP27, HSP70, HSP90, p38 kinase and JNK-1, as well as the phosphorylation levels of p38 kinase and JNK-1. (D) ESC-B5 cells were treated with or without α-tocopherol (Toc; 300 μM) or NAC (500 μM) for 30 min and then incubated with CTN (30 μM) for another 24 h. The protein expression levels of Ras, Raf-1, and ERK-1 and -2 and the phosphorylation levels of ERK-1 and -2 were evaluated. β-Actin was used as the loading control. Results are representative of five independent experiments.
During normal cellular processes, misfolded and/or unfolded proteins are degraded by a ubiquitin-dependent pathway [33,34]. To examine whether the reduced expression of Ras and Raf-1 in CTN-treated cells was due to increased degradation via the proteasome-dependent pathway, I treated ESC-B5 cells with CTN and/or the specific proteasome inhibitor, lactacystin, and examined Ras and Raf-1 expression. Our results revealed that pre-treatment with 10 μM lactacystin for 12 h effectively counteracted CTN-induced suppression of Ras and Raf-1 protein levels (Figure 5B). Evaluation of the ERK-1/2 expression and activity in cells co-treated with lactacystin and CTN revealed that lactacystin did not affect ERK-1/2 expression, but was able to rescue the CTN-induced decrease in ERK activity (Figure 5B). These results imply that Ras and Raf-1 are degraded by a proteasome-dependent pathway in CTN-treated ESC-B5 cells, subsequently decreasing ERK activity without changing its protein levels.
Since I found that CTN activated proteasome-dependent degradation of Ras and Raf-1 (Figure 5B), and previous studies showed that the HSP90/multichaperone complex could prevent proteasome-mediated degradation of several signalling molecules, including Raf-1 [17,35], I analysed the effects of CTN on the expression of multichaperone complex components, including HSP90, HSP27 and HSP70. Immunoblotting revealed that CTN-treatment of ESC-B5 cells dose-dependently up-regulated the expression levels of HSP27 and HSP70 (Figure 5C), while simultaneously down-regulating HSP90 expression (Figure 5C). We then examined the effect of CTN on the activities of JNK-1 and p38, two kinases that play key roles in the apoptotic pathway. Our results revealed that CTN treatment dose-dependently decreased p38 kinase activity (Figure 5C) while stimulating JNK-1 activity, but did not affect the expression levels of total p38 kinase or JNK-1 (Figure 5C). In an effort to further elucidate the regulatory mechanisms underlying CTN-induced ROS generation and survival signalling changes, I pre-treated ESC-B5 cells with the ROS scavengers, α-tocopherol and NAC, which could prevent CTN-triggered ROS generation (Figure 4A), and analysed the protein levels and activities of various cell survival signalling components. Our results showed that pre-treatment with α-tocopherol and NAC had no significant effect on the CTN-induced decreases in HSP-90, Ras and Raf-1 protein levels, or the kinase activity of ERK-1/2 (Figure 5D). Taken together, these observations suggest that CTN suppresses HSP90 expression, leading to inactivation of the HSP90/multichaperone complex, which subsequently inhibits ERK and activates JNK in ESC-B5 cells. Notably, the above results also indicate that ROS generation is not involved in CTN-induced suppression of survival signal protein levels.
CTN induces apoptosis and suppresses ERK-1/2 activity in HSP90-overexpressing ESC-B5 cells
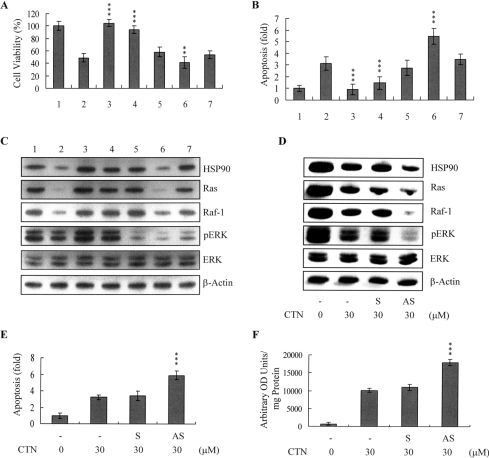
To determine whether CTN-induced inactivation of the HSP90/multichaperone complex is involved in the degradation of Ras→ERK survival signalling components in CTN-treated cells, I used transient transfection to generate ESC-B5 cells overexpressing HSP90. After transfection, the cells were treated with or without 10 μM U0126, a specific inhibitor of MEK-1 (the upstream activator of ERK-1/2). After 24 h, the cells were incubated with CTN, and viability and apoptosis were analysed. Our results revealed that CTN treatment of control cells decreased cell viability and promoted apoptosis, whereas overexpression of HSP90 almost completely inhibited CTN-induced apoptosis (Figures 6A and 6B). Moreover, treatment of control cells with U0126 alone induced apoptosis, and co-treatment of these cells with CTN and U0126 led to enhanced cell death (Figures 6A and 6B). Notably, U0126 treatment of HSP90-overexpressing cells completely suppressed the counteracting effects of HSP90 on CTN-induced apoptosis (Figures 6A and 6B). Under similar experimental conditions, CTN blocked the expression of HSP90, Ras and Raf-1, and decreased the activity of ERK-1/2 (Figure 6C, lane 2). A 3–3.5-fold increase in HSP90 protein levels was detected in transfected cells (Figure 6C, lane 3), and CTN blocked the expression of Ras and Raf-1, and the decreased activity of ERK-1/2 was restored in CTN-treated HSP90-transfected cells (Figure 6C, lane 4). In contrast, although U0126 treatment had no significant effect on the expression levels of HSP90, Ras or Raf-1 (Figure 6C, lane 5), it prevented HSP90-induced restoration of ERK activity in CTN-treated cells (Figure 6C, lane 7). To investigate further the role of HSP90 in CTN-induced apoptosis, I pre-incubated ESC-B5 cells with antisense oligonucleotides targeting HSP90. Antisense treatment significantly decreased HSP90 expression by ∼70% compared with that seen in sense oligonucleotide-treated or untreated control cells (Figure 6D). Notably, HSP90 knockdown significantly promoted CTN-induced suppression of Ras and Raf-1 protein expression, inhibition of ERK-1/2 activity (Figure 6D), and cell apoptosis (Figure 6E) and ROS generation (Figure 6F). These findings suggest that CTN appears to trigger apoptosis via inactivation of the HSP90/multichaperone complex and subsequent degradation of Ras and Raf-1, thus further inactivating anti-apoptotic processes, including the Ras→ERK signal transduction pathway.
Figure 6. HSP90 overexpression antagonizes CTN-induced ERK-1/2 activity and apoptosis in ESC-B5 cells.
Parental or HSP90-transfected ESC-B5 cells were exposed to 30 μM CTN for 24 h and/or 10 μM U0126 for 24 h. Lane 1, untreated parental cells; lane 2, untreated parental cells exposed to CTN; lane 3, untreated HSP90-transfected cells; lane 4, HSP90-transfected cells exposed to CTN; lane 5, parental cells treated with U0126; lane 6, parental cells treated with U0126 and CTN; lane 7, HSP90-transfected cells treated with U0126 and CTN. Cell viability was determined with the MTT assay (A), and apoptosis was evaluated using the Cell Death Detection ELISA kit (B). The expression levels of HSP90, p-ERK-1/2, ERK-1/2, Ras and Raf-1 were analysed by immunoblotting (C). (D–F) Cells were incubated with 70 μM HSP90 sense (S) or antisense (AS) oligonucleotides in the presence of Lipofectamine™ for 72 h and then incubated with CTN (30 μM) for another 24 h. The expression levels of HSP90, p-ERK-1/2, ERK-1/2, Ras and Raf-1 were measured (D), and cell apoptosis (E) and ROS generation (by DCFH-DA) (F) were analysed. Results are means±S.D. of five determinations. **P<0.01, ***P<0.001 compared with the ‘CTN-only’ treatment group. OD units, absorbance units.
Effects of CTN on embryonic development in vitro
To determine further the effects of CTN on early embryonic development in a stem cell assay model, I incubated cells with or without CTN and examined their ability to form EBs in vitro. In this system, withdrawal of the anti-differentiation agent allows ESCs to differentiate spontaneously and develop into typical three-dimensional aggregates (EBs) that consist of ectodermal, mesodermal and endodermal tissues, thus resembling the egg cylinder stage of an embryo. Our results revealed that EB formation was significantly and dose-dependently decreased in CTN-pre-treated cells (Figure 7A). Treatment of control EB cells with 50 ng/ml NGF (nerve growth factor) for 14 days induced their differentiation into nerve cells, as shown by immunoblotting for MAP-2 (microtubule-associated protein-2), a major nerve cell biomarker. Significantly, pre-treatment with 20 or 30 μM CTN effectively inhibited the NGF-induced expression of MAP-2 (Figure 7B). To determine whether CTN induces embryonic stem cell development injury and impairs subsequent growth, blastocysts were incubated with or without 10–30 μM CTN and then incubated in CTN-free medium on fibronectin-coated dishes for 72 h. In control dishes (no CTN pre-treatment), most blastocysts (250/260; 96.15%) attached and grew, whereas fewer CTN-treated blastocysts (87.6–90.1%) attached and displayed outgrowth. As outgrowth continues in vitro, attached blastocysts develop a compact and structured ICM at the centre of the TE layer. This TE eventually expands to surround a compact and rounded ICM (denoted ICM+++). Significantly fewer outgrowths derived from CTN-pre-treated blastocysts reached the ICM+++ stage compared with control blastocysts, particularly in the presence of 30 μM CTN (Figure 7C). Most embryos stopped developing at the ICM+ stage in the CTN-treated group.
Figure 7. Effects of CTN on embryonic development.
ESC-B5 cells were incubated with or without the indicated concentrations of CTN for 24 h. (A) Cells were dissociated with trypsin–EDTA, and cultured in a medium without LIF to induce differentiation. EBs were formed with the hanging drop method, as described in the Materials and methods section. (B) ESC-B5 cells were incubated with or without the indicated concentrations of CTN for 24 h, and then treated further with 50 ng/ml NGF for 14 days. Cell extracts (60 μg) were immunoblotted with anti-MAP-2 antibodies. (C) Mouse blastocysts were treated with or without CTN (10–30 μM) for 24 h, and observed in culture for 72 h. Morphological assessment was used to identify blastocysts as hatched, ICM(+), ICM(++) and ICM(+++). The total numbers of blastocysts examined in each group were 260 (control), 272 (10 μM), 265 (20 μM) and 290 (30 μM). Results are means±S.D. **P<0.01, ***P<0.001 compared with the CTN-free group.
Effects of CTN on mouse blastocyst development in vivo
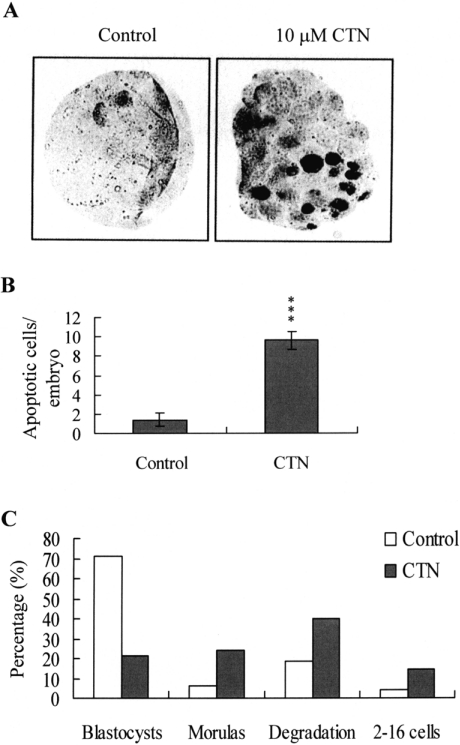
Since CTN triggered apoptosis and developmental injury in vitro, I analysed whether it had a similar activity in vivo in an animal model. Female mice were fed a standard diet and their drinking water was supplemented with or without CTN (10 μM) for the duration of the experiment. After 24 h, mice were mated overnight with a single fertile male of the same strain. Blastocysts were obtained by flushing the uterine horn on day 4 after mating, and cell apoptosis and development were analysed. Our results revealed that dietary CTN induced significant apoptosis in mouse blastocysts (Figures 8A and 8B), and caused embryonic injuries that inhibited embryonic development to the blastocyst stage, and often terminated development at the 2–16 cells stage, morula stage, or leading to degradation (Figure 8C). In the control group, most of the embryos (356/500; 71.2%) developed to the blastocyst stage, enabling them to be implanted; among the embryos that did not develop to the implantation stage, 6.2% (percentage of total) stalled at the morula stage, 18.4% degraded, and 4.2% stalled at the 2–16 cell stage. Notably, only 21.6% (121/560) of the embryos from CTN-treated mice reached the blastocyst stage; the embryos that did not reach the implantation stage included those that underwent degradation (223/560; 39.82% of total), stalled at the morula stage (134/560; 23.92%) and stalled prior to the morula stage (82/560; 14.6%) (Figure 8C).
Figure 8. Effects of dietary CTN consumption on apoptosis and blastocyst development in an animal model.
Randomly chosen female mice were fed a standard diet and drinking water supplemented with or without CTN (10 μM) for 24 h, and then mated overnight with a single fertile male of the same strain. Various types of embryos were obtained by flushing the uterine horn on day 4 after mating. (A) Apoptosis of mouse blastocysts was examined by TUNEL staining followed by light microscopy, which displays the TUNEL-positive cells as black. (B) The mean number of apoptotic (TUNEL-positive) cells per blastocyst was calculated. Results are means±S.D. of five determinations. ***P<0.001 compared with the CTN-untreated control group. (C) The embryos obtained from the mouse uterine horns on day 4 were examined for comparison of developmental stage. Results are percentages of total obtained embryos.
Taken together, our findings conclusively show that CTN induces apoptosis via both a mitochondria-dependent pathway and suppression of the survival signalling pathway, and causes embryonic developmental injury, both in vitro and in vivo.
DISCUSSION
Foodstuffs and animal feeds are often contaminated with various fungal toxins, including the mycotoxin, CTN, which has been identified in cereal grains and fermented maize dough at levels of approx. 180–580 ng/g [36]. CTN contamination has additionally been reported in Monascus fermentation products [29,37] used as food colourants and flavour enhancers in the Orient, and as dietary supplements believed to prevent heart disease and decrease plasma triacylglycerol and cholesterol levels [38]. One recent study identified 0.28–6.29 μg/g CTN in lipid extracts from commercialized Monascus products [29], while another showed that treatment of human HEK-293 cells (human embryonic kidney cells) with lipid extracts of Monascus products (60 μM CTN) for 72 h induced over 50% cell death [29]. As these and other studies have shown that CTN can induce apoptosis, I examined the effects of 30 μM CTN (∼3.75 μg/g in culture medium) on blastocysts in vitro and in vivo. Based on these studies, the treatment dosage of CTN in the present study could reflect its concentration in contaminated foods.
Mechanistically, our study showed that CTN directly evokes intracellular oxidative stress (Figure 3A), leading to ROS-mediated apoptosis in ESC-B5 cells (Figure 4). These effects appeared to involve the mitochondria-dependent apoptotic pathway, as shown by CTN-induced changes in the intracellular levels of Bcl family members, loss of MMP, and release of cytochrome c from mitochondria to the cytosol. Our findings are consistent with a previous study showing that CTN induces apoptosis via activation of the mitochondrial pathway in HL-60 cells [28]. We expanded upon this by further elucidating the precise mechanisms of CTN-triggered apoptosis in embryonic stem cells (Figures 3B–3E). Since previous papers have shown that the addition of some compounds to commonly-utilized cell culture media can trigger generation of ROS such as H2O2 [39,40], I incubated CTN and culture medium together, and measured ROS by the ferrous iron oxidation–Xylenol Orange method [39]. The results showed no detectable artefactual ROS generation under these conditions (results not shown). In addition, I found that two well-known ROS scavengers, NAC and α-tocopherol, effectively prevented CTN-induced ROS generation and apoptosis in embryonic stem cells (Figure 4). Taken together, our results demonstrate that CTN triggers apoptosis in embryonic stem cells via ROS generation, which stimulates downstream processes through the mitochondria-dependent pathway.
We further showed that CTN treatment triggered dose-dependent decreases in the protein levels of Ras and Raf-1 (Figure 5A). Protein degradation is driven by the proteasome-dependent pathway through covalent binding of the target protein with ubiquitin, a 14 kDa small molecule [33,34]. HSPs act as molecular chaperones to prevent degradation of damaged proteins and remove them from the proteasome-dependent degrading pathway [15,16,41,42]. HSP90, the most abundant chaperone protein, is part of the multicomponent complex, and forms a super-chaperone machine to promote the proper folding of client proteins. Moreover, HSP90 is specifically involved in maintaining the correct conformation of several intracellular proteins (HSP90 clients), many of which are kinases involved in the control of cell proliferation and survival [43]. In the present study, I found that CTN-induced apoptosis was associated with reduced protein expression of survival components, including Ras and Raf-1, and inactivation of ERK-1 and ERK-2 (Figure 5A). The proteasome inhibitor, lactacystin, effectively blocked CTN-induced degradation of survival signal components (Figure 5B), prompting us to investigate the possible involvement of HSPs. HSP70 renders client proteins susceptible to ubiquitination and thus promotes delivery to the proteasome for degradation [44]. Recent studies have shown that HSP27 and HSP70 are involved in microwave-electromagnetic field-induced apoptotic processes [22]. In the present study, I found that CTN enhanced the expression levels of HSP27 and HSP70 and decreased that of HSP90, thus promoting the degradation of client proteins (Figure 5C). Moreover, the activities of p38 kinase and JNK were decreased and increased respectively in CTN-treated cells (Figure 5C). Accordingly, I hypothesized that CTN-induced reductions in HSP90 expression caused Ras and Raf-1 to be targeted for degradation by the proteasome-dependent machinery, leading to their down-regulation and related changes in the related signal pathways. We confirmed this hypothesis in HSP90-overexpressing cells. Notably, overexpression of HSP90 effectively protected ESC-B5 cells from CTN-induced apoptosis (Figures 6A and 6B), but this effect could be blocked by pre-treatment with U0126, a specific inhibitor of MEK-1 (Figures 6A and 6B). Furthermore, the HSP90-induced protection against apoptosis was correlated with rescue of Ras and Raf-1 expression and ERK-1/2 activity (Figure 6C). Consistent with these findings, CTN-induced apoptosis was enhanced in cells subjected to antisense oligonucleotide knockdown of HSP90 expression (Figures 6D and 6E). A previous study showed that the serine/threonine kinase, Akt/PKB (protein kinase B), could inhibit mitochondrial cytochrome c release, thereby effectively preventing apoptosis triggered by several different apoptotic stimuli [45]. Since Akt/PKB functions to maintain the association of hexokinase with mitochondria [45], the disruption of mitochondria–hexokinase interactions by apoptotic triggers could promote cytochrome c release and apoptosis. Thus, to elucidate whether Akt/PKB was involved in CTN-induced apoptosis of ESC-B5 cells, I measured the kinase activity of Akt/PKB signalling molecules. The results revealed that the expression levels and kinase activities of PI3K (phosphoinositide 3-kinase) and Akt/PKB were unchanged in CTN-treated ESC-B5 cells (results not shown), implying that Akt/PKB is not involved in CTN-induced apoptosis. These findings collectively indicate that CTN treatment decreases HSP90 expression, leading to increased proteasome-dependent degradation of Ras and Raf-1, with decreased Raf-1 levels resulting in down-regulation of ERK-1 and ERK-2 and subsequent cell apoptosis. Moreover, the results indicate that ROS generation is not involved in CTN-induced suppression of survival signal protein levels (Figure 5D), and overexpression of HSP90 almost completely inhibited CTN-induced apoptosis (Figures 6A and 6B). In addition, HSP90 knockdown significantly promoted CTN-induced apoptosis (Figure 6E) and ROS generation (Figure 6F). Accordingly, I hypothesized that inhibition of Ras→ERK survival signals were the upstream regulators of ROS-mediated apoptotic pathway in CTN-induced apoptosis. However, the precise regulatory mechanisms are unclear and need further investigation.
Based on the pro-apoptotic effects of CTN in mouse embryonic stem cells, I examined the possible effects of the toxin on embryonic development. Analysis of EB formation and MAP-2 expression in embryonic stem cells during NGF-induced cell differentiation confirmed that CTN caused early embryonic developmental injury (Figures 7A and 7B). Our previous studies demonstrated that apoptotic injury due to chemical treatment had significant negative effects on embryonic development [10,46]. In the present study, I have shown that CTN-induced apoptosis led to a high injury risk during early embryonic development. When the number of cells in the ICM of a blastocyst is reduced by ∼30% or more, there is a high risk of fetal loss or developmental injury, even in cases where the implantation rate and TE cell numbers are normal [47]. In the present study, I found that CTN induced embryonic developmental injury, as shown by the reduced ability of blastocysts to develop into outgrowths with structured ICM clusters, and the lower number of nuclei in outgrowths, both in a stem cell model and a blastocyst assay (Figures 7A–7C). These findings collectively suggest that CTN markedly suppresses proper mouse embryonic development, implantation, and embryogenesis.
After establishing that CTN negatively affects blastocyst development in vitro (Figure 7), I analysed the effects of dietary CTN on embryonic development in vivo. Using an animal model and TUNEL analysis, I have shown for the first time that dietary CTN induced cell apoptosis at the blastocyst stage (Figures 8A and 8B). A developmental comparison of 4-day-old embryos flushed from the uterine horns of mice with and without dietary supplementation of CTN showed decreased development to the blastocyst stage and increased embryonic degradation in the CTN-treated group (Figure 8C), suggesting that CTN-treated mice will have a much lower rate of successful embryonic implantation.
In summary, I herein show that CTN induces apoptosis through ROS- and mitochondria-dependent pathways in ESC-B5 cells, concomitant with down-regulation of survival signalling molecules, such as HSP90, Ras, Raf-1 and ERK-1/2. In addition, dietary contamination with CTN has an injurious effect on embryogenesis. To our knowledge, this is the first report showing that CTN induces apoptotic signalling cascades and affects survival signalling components. Although future studies will be required to elucidate the possible teratogenic actions of CTN on human embryogenesis, our present findings provide important new insights into potential CTN-induced safety risks for embryonic development.
Acknowledgments
This work was supported by grants (NSC 95-2311-B-033-001-MY3) from the National Science Council of Taiwan, Republic of China and the Center-of-Excellence Program on Membrane Technology, Ministry of Education, Taiwan, Republic of China.
References
- 1.Blanc P. J., Laussac J. P., Le Bars J., Le Bars P., Loret M. O., Pareilleux A., Prome D., Prome J. C., Santerre A. L., Goma G. Characterization of monascidin A from Monascus as citrinin. Int. J. Food Microbiol. 1995;27:201–213. doi: 10.1016/0168-1605(94)00167-5. [DOI] [PubMed] [Google Scholar]
- 2.CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems. CAST (Council of Agricultural Science and Technology) Task Force Report No. 139, CAST, Ames. 2003.
- 3.Aleo M. D., Wyatt R. D., Schnellmann R. G. The role of altered mitochondrial function in citrinin-induced toxicity to rat renal proximal tubule suspensions. Toxicol. Appl. Pharmacol. 1991;109:455–463. doi: 10.1016/0041-008x(91)90008-3. [DOI] [PubMed] [Google Scholar]
- 4.Kogika M. M., Hagiwara M. K., Mirandola R. M. Experimental citrinin nephrotoxicosis in dogs: renal function evaluation. Vet. Hum. Toxicol. 1993;35:136–140. [PubMed] [Google Scholar]
- 5.Arai M., Hibino T. Tumorigenicity of citrinin in male F344 rats. Cancer Lett. 1983;17:281–287. doi: 10.1016/0304-3835(83)90165-9. [DOI] [PubMed] [Google Scholar]
- 6.Hardy K. Cell death in the mammalian blastocyst. Mol. Hum. Reprod. 1997;3:919–925. doi: 10.1093/molehr/3.10.919. [DOI] [PubMed] [Google Scholar]
- 7.Hardy K., Stark J., Winston R. M. Maintenance of the inner cell mass in human blastocysts from fragmented embryos. Biol. Reprod. 2003;68:1165–1169. doi: 10.1095/biolreprod.102.010090. [DOI] [PubMed] [Google Scholar]
- 8.Byrne A. T., Southgate J., Brison D. R., Leese H. J. Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. J. Reprod. Fertil. 1999;117:97–105. doi: 10.1530/jrf.0.1170097. [DOI] [PubMed] [Google Scholar]
- 9.Hsuuw Y. D., Chang C. K., Chan W. H., Yu J. S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell. Physiol. 2005;205:379–386. doi: 10.1002/jcp.20408. [DOI] [PubMed] [Google Scholar]
- 10.Chan W. H. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum. Reprod. 2006;21:2985–2995. doi: 10.1093/humrep/del255. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 12.Pathak N., Khandelwal S. Oxidative stress and apoptotic changes in murine splenocytes exposed to cadmium. Toxicology. 2006;220:26–36. doi: 10.1016/j.tox.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Cai Y., Qi X.-M., Gong L.-K., Liu L.-L., Chen F.-P., Xiao Y., Wu X.-F., Li X.-H., Ren J. Tetrandrine-induced apoptosis in rat primary hepatocytes is initiated from mitochondria: caspases and endonuclease G (Endo G) pathway. Toxicology. 2006;218:1–12. doi: 10.1016/j.tox.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Chan W. H., Wu H. J. Anti-apoptotic effects of curcumin on photosensitized human epidermal carcinoma A431 cells. J. Cell. Biochem. 2004;92:200–212. doi: 10.1002/jcb.20059. [DOI] [PubMed] [Google Scholar]
- 15.Leszczynski D., Joenvaara S., Reivinen J., Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer- and blood–brain barrier-related effects. Differentiation. 2002;70:120–129. doi: 10.1046/j.1432-0436.2002.700207.x. [DOI] [PubMed] [Google Scholar]
- 16.French P. W., Penny R., Laurence J. A., McKenzie D. R. Mobile phones, heat shock proteins and cancer. Differentiation. 2001;67:93–97. doi: 10.1046/j.1432-0436.2001.670401.x. [DOI] [PubMed] [Google Scholar]
- 17.Blagosklonny M. V. Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia. 2002;16:455–462. doi: 10.1038/sj.leu.2402415. [DOI] [PubMed] [Google Scholar]
- 18.Caraglia M., Abbruzzese A., Leardi A., Pepe S., Budillon A., Baldassare G., Selleri C., Lorenzo S. D., Fabbrocini A., Giuberti G., et al. Interferon-α induces apoptosis in human KB cells through a stress-dependent mitogen activated protein kinase pathway that is antagonized by epidermal growth factor. Cell Death Differ. 1999;6:773–780. doi: 10.1038/sj.cdd.4400550. [DOI] [PubMed] [Google Scholar]
- 19.Caraglia M., Tagliaferri P., Marra M., Giuberti G., Budillon A., Gennaro E. D., Pepe S., Vitale G., Improta S., Tassone P., et al. EGF activates an inducible survival response via the RAS→Erk-1/2 pathway to counteract interferon-α-mediated apoptosis in epidermoid cancer cells. Cell Death Differ. 2003;10:218–229. doi: 10.1038/sj.cdd.4401131. [DOI] [PubMed] [Google Scholar]
- 20.Caraglia M., D'Alessandro A. M., Marra M., Giuberti G., Vitale G., Viscomi C., Colao A., Prete S. D., Tagliaferri P., Tassone P., et al. The farnesyl transferase inhibitor R115777 (Zarnestra) synergistically enhances growth inhibition and apoptosis induced on epidermoid cancer cells by Zoledronic acid (Zometa) and Pamidronate. Oncogene. 2004;23:6900–6913. doi: 10.1038/sj.onc.1207814. [DOI] [PubMed] [Google Scholar]
- 21.Caraglia M., Marra M., Pelaia G., Maselli R., Caputi M., Marsico S. A., Abbruzzese A. α-Interferon and its effects on signal transduction pathways. J. Cell. Physiol. 2005;202:323–335. doi: 10.1002/jcp.20137. [DOI] [PubMed] [Google Scholar]
- 22.Caraglia M., Marra M., Mancinelli F., D'Ambrosio G., Massa R., Giordano A., Budillon A., Abbruzzese A., Bismuto E. Electromagnetic fields at mobile phone frequency induce apoptosis and inactivation of the multi-chaperone complex in human epidermoid cancer cells. J. Cell. Physiol. 2005;204:539–548. doi: 10.1002/jcp.20327. [DOI] [PubMed] [Google Scholar]
- 23.Chan W. H., Wu C. C., Yu J. S. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J. Cell. Biochem. 2003;90:327–338. doi: 10.1002/jcb.10638. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh Y. J., Wu C. C., Chang C. J., Yu J. S. Subcellular localization of Photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: when plasma membranes are the main targets. J. Cell. Physiol. 2003;194:363–375. doi: 10.1002/jcp.10273. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M. H., Lee J. S., Kim H. J., Jin D. I., Kim J. I., Lee K. J., Seo J. S. HSP90 protects apoptotic cleavage of vimentin in geldanamycin-induced apoptosis. Mol. Cell. Biochem. 2006;281:111–121. doi: 10.1007/s11010-006-0638-x. [DOI] [PubMed] [Google Scholar]
- 26.Armant D. R., Kaplan H. A., Lennarz W. J. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev. Biol. 1986;116:519–523. doi: 10.1016/0012-1606(86)90152-1. [DOI] [PubMed] [Google Scholar]
- 27.Pampfer S., Wuu Y. D., Vanderheyden I., De Hertogh R. In vitro study of the carry-over effect associated with early diabetic embryopathy in the rat. Diabetologia. 1994;37:855–862. doi: 10.1007/BF00400939. [DOI] [PubMed] [Google Scholar]
- 28.Yu F. Y., Liao Y. C., Chang C. H., Liu B. H. Citrinin induces apoptosis in HL-60 cells via activation of the mitochondrial pathway. Toxicol. Lett. 2006;161:143–151. doi: 10.1016/j.toxlet.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Liu B. H., Wu T. S., Su M. C., Chung C. P., Yu F. Y. Evaluation of citrinin occurrence and cytotoxicity in Monascus fermentation products. J. Agric. Food Chem. 2005;53:170–175. doi: 10.1021/jf048878n. [DOI] [PubMed] [Google Scholar]
- 30.Yu F., Watts R. N., Zhang X. D., Borrow J. M., Hersey P. Involvement of BH3-only proapoptotic proteins in mitochondrial-dependent Phenoxodiol-induced apoptosis of human melanoma cells. Anticancer Drugs. 2006;17:1151–1161. doi: 10.1097/01.cad.0000231484.17063.9a. [DOI] [PubMed] [Google Scholar]
- 31.Criollo A., Galluzzi L., Chiara Maiuri M., Tasdemir E., Lavandero S., Kroemer G. Mitochondrial control of cell death induced by hyperosmotic stress. Apoptosis. 2007;12:3–18. doi: 10.1007/s10495-006-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan W. H., Shiao N. H., Lu P. Z. CdSe quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial-dependent pathways and inhibition of survival signals. Toxicol. Lett. 2006;167:191–200. doi: 10.1016/j.toxlet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr. Opin. Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 34.Hershko A., Ciechanover A., Varshavsky A. The ubiquitin system. Nat. Med. 2000;6:1073–1081. doi: 10.1038/80384. [DOI] [PubMed] [Google Scholar]
- 35.Pratt W. B., Toft D. O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 36.Vrabcheva T., Usleber E., Dietrich R., Martlbauer E. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J. Agric. Food Chem. 2000;48:2483–2488. doi: 10.1021/jf990891y. [DOI] [PubMed] [Google Scholar]
- 37.Sabater-Vilar M., Maas R. F., Fink-Gremmels J. Mutagenicity of commercial Monascus fermentation products and the role of citrinin contamination. Mutat. Res. 1999;444:7–16. doi: 10.1016/s1383-5718(99)00095-9. [DOI] [PubMed] [Google Scholar]
- 38.Wei W., Li C., Wang Y., Su H., Zhu J., Kritchevsky D. Hypolipidemic and anti-atherogenic effects of long-term Cholestin (Monascus purpureus-fermented rice, red yeast rice) in cholesterol fed rabbits. J. Nutr. Biochem. 2003;14:314–318. doi: 10.1016/s0955-2863(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 39.Long L. H., Clement M. V., Halliwell B. Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000;273:50–53. doi: 10.1006/bbrc.2000.2895. [DOI] [PubMed] [Google Scholar]
- 40.Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 2003;540:3–6. doi: 10.1016/s0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 41.de Pomerai D., Daniells C., David H., Allan J., Duce I., Mutwakil M., Thomas D., Sewell P., Tattersall J., Jones D., et al. Non-thermal heat-shock response to microwaves. Nature. 2000;405:417–418. doi: 10.1038/35013144. [DOI] [PubMed] [Google Scholar]
- 42.Hyland G. J. Physics and biology of mobile telephony. Lancet. 2000;356:1833–1836. doi: 10.1016/s0140-6736(00)03243-8. [DOI] [PubMed] [Google Scholar]
- 43.Mayer M. P., Bukau B. Molecular chaperones: the busy life of Hsp90. Curr. Biol. 1999;9:R322–R325. doi: 10.1016/s0960-9822(99)80203-6. [DOI] [PubMed] [Google Scholar]
- 44.Isaacs J. S., Xu W., Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 45.Majewski N., Nogueira V., Bhaskar P., Coy P. E., Skeen J. E., Gottlob K., Chandel N. S., Thompson C. B., Robey R. B., Hay N. Hexokinase–mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Chan W. H. Ginkgolides induce apoptosis and decrease cell numbers in mouse blastocysts. Biochem. Biophys. Res. Commun. 2005;338:1263–1267. doi: 10.1016/j.bbrc.2005.10.085. [DOI] [PubMed] [Google Scholar]
- 47.Tam P. P. Postimplantation development of mitomycin C-treated mouse blastocysts. Teratology. 1988;37:205–212. doi: 10.1002/tera.1420370305. [DOI] [PubMed] [Google Scholar]