Abstract
We have previously shown that ADP-induced thromboxane generation in platelets requires signalling events from the Gq-coupled P2Y1 receptor (platelet ADP receptor coupled to stimulation of phospholipase C) and the Gi-coupled P2Y12 receptor (platelet ADP receptor coupled to inhibition of adenylate cyclase) in addition to outside-in signalling. While it is also known that extracellular calcium negatively regulates ADP-induced thromboxane A2 generation, the underlying mechanism remains unclear. In the present study we sought to elucidate the signalling mechanisms and regulation by extracellular calcium of ADP-induced thromboxane A2 generation in platelets. ERK (extracllular-signal-regulated kinase) 2 activation occurred when outside-in signalling was blocked, indicating that it is a downstream event from the P2Y receptors. However, blockade of either P2Y1 or the P2Y12 receptors with corresponding antagonists completely abolished ERK phosphorylation, indicating that both P2Y receptors are required for ADP-induced ERK activation. Inhibitors of Src family kinases or the ERK upstream kinase MEK [MAPK (mitogen-activated protein kinase)/ERK kinase] abrogated ADP-induced ERK phosphorylation and thromboxane A2 generation. Finally ADP- or Gi+Gz-induced ERK phosphorylation was blocked in the presence of extracellular calcium. The present studies show that ERK2 is activated downstream of P2Y receptors through a complex mechanism involving Src kinases and this plays an important role in ADP-induced thromboxane A2 generation. We also conclude that extracellular calcium blocks ADP-induced thromboxane A2 generation through the inhibition of ERK activation.
Keywords: aggregation, calcium, mitogen-activated protein kinase (MAPK), protein kinase C, Src family kinase
Abbreviations: BAPTA, bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid; ERK, extracellular-signal-regulated kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; 2MeSADP, 2-methyl-thio-ADP; PAR, protease-activated receptor; PKC, protein kinase C; PLA2, phospholipase A2; PLC, phospholipase C; PP3, 4-amino-7-phenylpyrazol[3,4-d]pyrimidine; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; PRP, platelet-rich plasma; P2Y1, platelet ADP receptor coupled to stimulation of phospholipase C; P2Y12, platelet ADP receptor coupled to inhibition of adenylate cyclase; TBST, Tris-buffered saline-Tween 20
INTRODUCTION
Platelet activation is important for haemostasis and abnormal activation of platelets leading to thrombosis. Upon vascular injury circulating platelets adhere to the collagen-rich sub-endothelium and become activated. This activation leads to the release of the contents of the platelet granules, which contain other platelet agonists such as ADP, and generation of lipid-based agonists such as thromboxane A2 [1]. These secondary agonists amplify the signal resulting in the cascade of platelet activation events that results in the formation of a stable thrombus [1]. Secreted ADP is important for platelet activation, as patients with defects in dense granule storage or specific receptors have bleeding abnormalities [2]. It is now established that ADP acts on two receptors on platelets: the Gq-coupled P2Y1 receptor and Gi-coupled P2Y12 receptor [3]. Once released, ADP not only amplifies the primary responses of other agonists, but also causes generation of thromboxane A2 from platelets, leading to enhanced platelet aggregation and stability of the thrombus [3].
The P2Y1 receptor contributes to ADP-induced platelet shape change whereas the P2Y12-coupled Gi signalling is crucial for potentiating dense granule secretion, thromboxane A2 generation and irreversible aggregation [3,4]. However, concomitant signalling through Gq-coupled P2Y1 and Gi-coupled P2Y12 is necessary and sufficient for fibrinogen receptor activation [3]. Furthermore, selective Gi activation also potentiates other agonist-mediated responses [5,6]. The anti-thrombotic drugs such as clopidogrel and ticlopidine irreversibly block the ADP binding to the platelet P2Y12 receptor [7]. We have previously shown that ADP-induced generation of thromboxane A2 requires co-ordinated signalling events from both the P2Y1 and the P2Y12 receptors as well as the integrin αIIbβ3 [8]. However, the specific signalling events involved in this process have not been adequately delineated.
P2Y1 receptor stimulation results in the increase in intracellular calcium through the generation of IP3 (inositol 3,4,5-trisphosphate), and activation of PKC (protein kinase C) through formation of DAG (diacylglycerol), following PLC (phospholipase C) activation [1,9]. P2Y12 receptor stimulation results in Gαi-mediated inhibition of stimulated adenylate cyclase and Gβγ-mediated activation of PI3Kγ (phosphoinositide 3-kinase γ), Akt/PKB (protein kinase B), and Rap 1b [3]. However, the signalling events occurring downstream of these P2Y receptors mediating the thromboxane generation remain obscure. Multiple enzymes have been implicated in the regulation of PLA2 (phospholipase A2) including PKC [10], MAPKs (mitogen-activated protein kinases) [11] and calcium [12], though the candidates that phosphorylate and activate PLA2 remain the subject of controversy.
Platelet MAPKs, particularly ERK1/2 (extracellular-signal-regulated kinase 1/2), are shown to be activated in platelets by a number of strong agonists such as thrombin and convulxin [13–19], their activation by ADP has not been properly evaluated. PAR (protease-activated receptor)-mediated ERK2 phosphorylation was recently shown to be regulated by the P2Y12 receptor in platelets [20]. Two groups that have investigated ADP-induced ERK phosphorylation have reported that ADP causes little or no ERK activation in platelets [21,22]. Activation of ERK2 downstream of P2X1 receptor activation plays an important role in mediating the effects of P2X1 on collagen-induced platelet responses including aggregation and secretion [13].
Extracellular calcium is known to negatively regulate thromboxane generation in platelets [23]. Thus, when extracellular calcium levels are reduced, agonist-induced thromboxane generation is enhanced [23]. However, the mechanism of this regulation or the effect of extracellular calcium on a specific signalling molecule resulting in decreased thromboxane generation has not been studied.
In the present study, we show that ADP activates ERK2 downstream of P2Y receptors in a Src-family-kinase-dependent manner. ADP-induced ERK activation requires signalling from both P2Y1 and P2Y12 receptors but does not depend on outside-in signalling. Furthermore, we demonstrate that ERK plays an important role in ADP-mediated thromboxane generation. Finally, we show that extracellular calcium negatively regulates ADP-induced ERK activation and thus reduces agonist-induced thromboxane generation in platelets.
EXPERIMENTAL
Approval for this study was obtained from the Institutional Review Board of Temple University (Philadelphia, PA, U.S.A.).
Materials
Apyrase (Type VII), BSA (fraction V), aspirin (acetylsalicylic acid) and ERK antibodies (anti-phosphospecific and total ERK), 2MeSADP (2-methyl-thio-ADP), apyrase (type V), MRS-2179 and GR 144053 were obtained from Sigma. PLC inhibitor (U73122), dimethyl BAPTA [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid], GF109203X, PP3 (4-amino-7-phenylpyrazol[3,4-d]pyrimidine, PP2 {4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine} and U0126 were from Biomol. An alkaline phosphatase-labelled secondary antibody was from Kirkegaard and Perry Laboratories. CDP-Star® chemiluminescent substrates were purchased from Applied Biosystems. SFLLRN and AYPGKF were custom synthesized by Invitrogen. AR-C69931MX was a gift from Astra-Zeneca. YM-254890 was a gift from Yamanouchi Pharmaceuticals. All other reagents were reagent grade, and deionized water was used throughout.
Isolation of human platelets
Whole blood was drawn from healthy, consenting human volunteers into tubes containing one-sixth volume of ACD (2.5 g sodium citrate, 1.5 g citric acid and 2g glucose in 100 ml of deionized water). Blood was centrifuged (Eppendorf 5810R centrifuge) at 230 g for 20 min at room temperature (23 °C) to obtain PRP (platelet-rich plasma). PRP was incubated with 1 mM aspirin for 30 min at 37 °C. The PRP was then centrifuged at 980 g for 10 min at room temperature to pellet the platelets. Platelets were resuspended in Tyrode's buffer [138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM Hepes (pH 7.4) and 0.2% BSA] containing 0.01 units/ml of apyrase.Cells were counted using a Coulter Z1 Particle Counter and the concentration of cells was adjusted to 4×108 platelets/ml. All experiments using washed platelets were performed in the absence of extracellular calcium unless otherwise mentioned. In the experiments with thromboxane A2 measurements, the treatment of PRP with aspirin was omitted.
Aggregometry
Aggregation of 0.5 ml of washed platelets was analysed using a P.I.C.A. lumiaggregometer (Chrono-log). Aggregation was measured using light transmission while stirring (900 rev./min) at 37 °C. Agonists were added simultaneously for platelet stimulation, however platelets were pre-incubated with each inhibitor (where noted) at 37 °C. Each sample was allowed to aggregate for at least 3 min. The chart recorder (Kipp and Zonen) was set for 0.2 mm/s. All samples contained exogenously added human fibrinogen (1 mg/ml) without added calcium. Aggregation tracings are representative of results obtained from three separate experiments on three different donors.
Western blot analysis
Platelets were stimulated for the appropriate time with agonists, in the presence or absence of inhibitors, and the reaction was stopped by the addition of 3×SDS Laemmli's buffer. Platelet samples were boiled for 10 min and proteins were separated using SDS/PAGE (10% gel) and then transferred on to PVDF membrane. Non-specific binding sites were blocked by incubation in TBST [Tris-buffered saline-Tween 20; 20 mM Tris, 140 mM NaCl and 0.1% (v/v) Tween 20] containing 2% (w/v) BSA for 30 min at room temperature, and membranes were incubated overnight at 4 °C with the primary antibody (1:1000 dilution in TBST containing 2% BSA) with gentle agitation. After three washes of 5 min each with TBST, the membranes were probed with an alkaline phosphatase-labelled secondary antibody (1:5000 dilution in TBST containing 2% BSA) for 1 h at room temperature. After additional washing steps, membranes were incubated with the CDP-Star® chemiluminescent substrate (Tropix) for 10 min at room temperature and immunoreactivity was detected using a Fuji Film Luminescent Image Analyzer (LAS-1000 CH, Japan).
Measurement of thromboxane A2 generation
In the present study, all measurements were made as thromboxane B2 to reflect the quantification of thromboxane A2. This is due to the fact that thromboxane A2 is very unstable and is rapidly converted to thromboxane B2.
Washed, human platelets without aspirin treatment were prepared as noted above, and brought to a concentration of 2×108 platelets/ml. Stimulations were performed in a platelet aggregometer with stirring (900 rev./min) at 37 °C without added calcium. The signalling pathway inhibitors and the vehicle, as noted in the Figure legends, were added 10 min prior to addition of the agonist. Stimulations were performed for 3–5 min and the reaction was stopped by snap freezing. The samples were stored at −80 °C until thromboxane B2 analysis was performed. Levels of thromboxane B2 were determined in duplicate using a correlate-EIA thromboxane B2 enzyme immunoassay kit (Assay Designs), according to the manufacturer's instructions. Data represent the normalized data obtained from at least three donors±S.E.M.
Measurement of platelet secretion
Platelet secretion was determined by measuring the release of ATP using CHRONO-LUME® reagent. The activation of platelets was performed in a lumiaggregometer at 37 °C with stirring at 900 rev./min, and the secretion was measured and expressed as nmol of ATP released/108 platelets. In experiments where inhibitors were used, the platelet sample was incubated with the inhibitors for 10 min at 37 °C prior to the addition of agonists. The secretion was subsequently measured as described above.
RESULTS
Role of P2Y receptors in ADP-induced phosphorylation of ERK in platelets
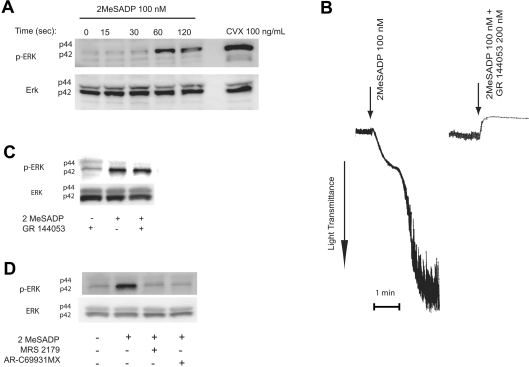
ERK1/2 have been reported to be activated in platelets upon agonist stimulation [13–19]. We initiated the present study to determine whether ADP activates ERK and to further evaluate the role of P2Y receptors in this event. We used 2MeSADP instead of ADP as an agonist in our studies as ADP is a weaker agonist than 2MeSADP [24] and also could have contaminating ATP that activates the P2X1 receptors [25] and interferes with lumi-chrome secretion assays. As shown in Figure 1(A), stimulation of washed and aspirin-treated platelets with the P2Y receptor agonist 2MeSADP resulted in the phosphorylation of ERK2 in a time-dependent manner. The phosphorylation of ERK was detected as early as 60 s. ERK1 was not activated even at 120 s with the maximal concentration of 2MeSADP (100 nM) (Figure 1A). A collagen receptor agonist, convulxin, was used as a positive control. The phosphorylation of ERK2 increased with increasing concentrations of 2MeSADP (results not shown). These data indicate that platelet P2Y receptor stimulation with ADP or 2MeSADP results in ERK2 activation.
Figure 1. Role of P2Y receptors in 2MeSADP-induced phosphorylation of ERK in platelets.
Washed and aspirin-treated human platelets were stimulated with 100 nM 2MeSADP for different time points or 100 ng/ml convulxin (CVX) with stirring as indicated at 37 °C and ERK phosphorylation was measured using Western blot analysis using a phospho-ERK-specific antibody (A). Washed human platelets, without aspirin-treatment, were stimulated with 2MeSADP for 60 s in stirring conditions in the presence or absence of the GPIIb/IIIa antagonist, GR 144053 without added calcium. Platelet aggregation (B) and ERK phosphorylation (C) were measured by lumi-aggregometry and Western blot analysis respectively. Washed and aspirin-treated platelets were stimulated with 100 nM 2MeSADP in the presence of a P2Y1 receptor antagonist, MRS2179, or a P2Y12 receptor antagonist, AR-C69931MX, and ERK phosphorylation was measured by Western blot analysis (D). Total ERK antibodies were used in the Western blot analysis to ensure similar protein loading in all lanes. The data are representative of experiments performed using platelets from at least three different donors.
Platelet stimulation with ADP resulted in platelet aggregation leading to outside-in signalling through the fibrinogen receptor [26]. In order to rule out the contribution of outside-in signalling to ERK phosphorylation, and to determine whether P2Y receptor stimulation alone can cause ERK phosphorylation, we blocked fibrinogen binding to its receptor on platelets using GR144053, an RGDS mimetic [27]. GR144053 at a concentration of 200 nM completely blocked 2MeSADP-induced platelet aggregation (Figure 1B) but did not affect 2MeSADP-induced ERK2 phosphorylaton (Figure 1C), indicating that P2Y receptor stimulation resulted in ERK2 phosphorylation independently of outside-in signalling.
As 2MeSADP activates both P2Y1 and P2Y12 receptors on platelets [24], we evaluated the relative contribution of these P2Y receptor subtypes on ERK2 phosphorylation. As shown in Figure 1(D), 100 nM 2MeSADP caused robust ERK2 phosphorylation in human platelets and this phosphorylation was abolished in the presence of 100 μM MRS-2179, a selective P2Y1 receptor antagonist, or 100 nM AR-C69931MX, a selective P2Y12 receptor antagonist. These results indicate that concomitant signalling from both P2Y1 and P2Y12 receptors is necessary for ADP-induced ERK2 phosphorylation in human platelets.
Effect of MEK (MAPK/ERK kinase) inhibition on ADP-induced platelet functional responses
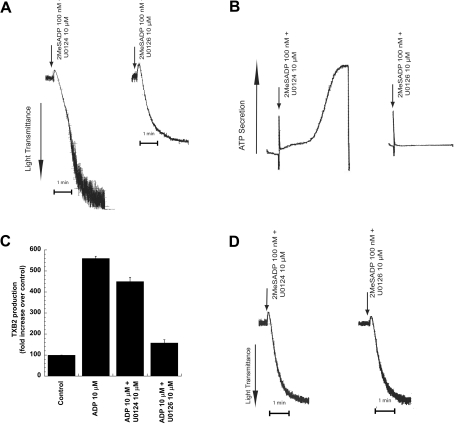
We evaluated the role of ERK2 in ADP-induced platelet functional responses using U0126, a selective inhibitor of MEK, an upstream kinase [17]. When platelets that were not pre-treated with aspirin were used, 2MeSADP-induced platelet responses were markedly suppressed by the MEK inhibitor. For example, 2MeSADP-induced platelet aggregation (Figure 2A), dense granule secretion (Figure 2B), and thromboxane generation (Figure 2C) were nearly abolished by 10 μM of the MEK inhibitor U0126, compared with the inactive analog U0124. In order to rule out the secondary effects of thromboxane on ADP-induced platelet aggregation, we evaluated the effect of U0126 on aspirin-treated human platelets. Addition of U0126, in contrast, had no detectable effect on 2MeSADP-induced aggregation (Figure 2D) or shape change (results not shown) in aspirin-treated platelets. These data indicate that inhibition of MEK and subsequent phosphorylation of ERK2 results in drastic inhibition of ADP-induced thromboxane generation. Thus ERK2 plays an important role in ADP-induced thromboxane generation.
Figure 2. Effect of MEK inhibition on ADP-induced platelet functional responses.
Washed human platelets were stimulated with 2MeSADP (100 nM) or ADP (10 μM), without added calcium, in the presence of the MEK kinase inhibitor, U0126 (10 μM) or its inactive analogue, U0124 (10 μM) as indicated. Pre-incubation times for U0126 and U0124 were 10 min at 37 °C. Platelet aggregation (A), platelet secretion (B) and thromboxane A2 generation (C) were measured as described in the Experimental section. Washed platelets treated with aspirin were stimulated with 2MeSADP and platelet aggregation was measured (D). All the above data are representative of experiments performed using platelets from at least three different donors. The maximum level of thromboxane seen upon stimulation with ADP was 4452 pg/ml. TXB2, thromboxane B2.
Role of Gq pathway signalling molecules in ADP-induced ERK phosphorylation and thromboxane generation
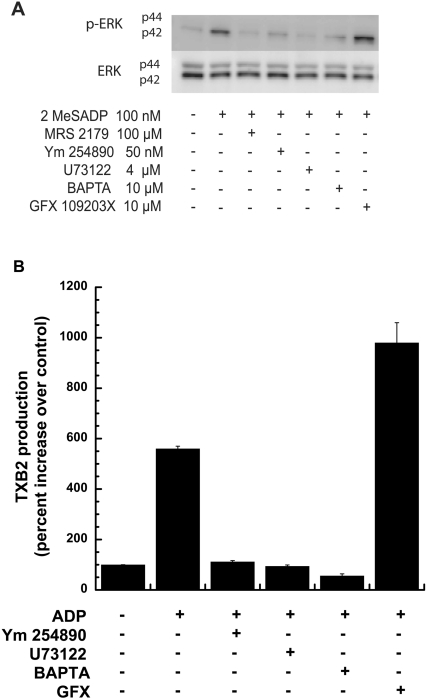
As P2Y1 receptor stimulation is required for ADP-induced ERK phosphorylation, we evaluated the role of the downstream signalling molecules in this event. P2Y1 receptor stimulation resulted in sequential activation of Gq and PLC-β, leading to an increase in intracellular calcium and activation of PKC [24]. As shown in Figure 3(A), 2MeSADP-induced ERK2 phosphorylation was completely inhibited by 100 μM MRS-2179, a P2Y1 receptor selective antagonist, 50 nM YM-254890, a selective inhibitor of Gq/11 [28], 4 μM U73122, an inhibitor of PLC [29], or 10 μM dimethyl BAPTA, an intracellular chelator of calcium ions [30]. We ensured that these inhibitors were functional in the system using aggregation and intracellular calcium mobilization as readouts (results not shown), as described in our previous studies [17,31]. However, inhibition of PKC using 10 μM GF109203X, a pan PKC inhibitor, caused potentiation of ERK2 phosphorylation.
Figure 3. Role of Gq pathway signalling mediators on ADP-induced ERK phosphorylation and thromboxane A2 generation.
Washed human platelets, treated with either aspirin or vehicle, were stimulated, without added calcium, with 2MeSADP (100 nM) or ADP (10 μM) in the presence or absence of a Gq blocker (YM-254890), a PLC inhibitor (U73122), a calcium chelator (BAPTA) or a PKC inhibitor (GFX) as indicated in the Figure. All of the inhibitors were incubated for 10 min at 37 °C prior to stimulation. ERK phosphorylation in aspirin-treated platelets (A) and thromboxane A2 generation in vehicle-treated platelets (B) were measured by Western blot analysis and ELISA respectively. All of the above data are representative of experiments performed using platelets from at least three different donors. TXB2, thromboxane B2.
As our previous experiments [17] suggested a role for ADP-induced ERK2 phosphorylation in thromboxane generation, we evaluated the effect of the P2Y1 receptor pathway inhibitors on ADP-induced thromboxane generation. As shown in Figure 3(B), consistent with the inhibition of ERK2 phosphorylation (Figure 3A), inhibition of Gq/11, PLC-β or release of intracellular calcium using YM-254890, U73122 or dimethyl BAPTA respectively, abolished thromboxane generation by ADP. These results suggest that ADP-induced ERK activation and thromboxane generation is dependent on calcium mobilization that occurs downstream of P2Y1-mediated Gq and PLC activation. Intriguingly, consistent with ERK2 phosphorylation (Figure 3A), inhibition of PKC isoforms with GF109230X resulted in potentiation of ADP-induced thromboxane generation. These results imply that one or more of the PKC isoforms negatively regulates ERK2 phosphorylation; as a result, inhibition of the PKC isoforms leads to potentiation of ERK2 phosphorylation as well as thromboxane generation.
Role of ERK in Gi+Gz-mediated thromboxane generation
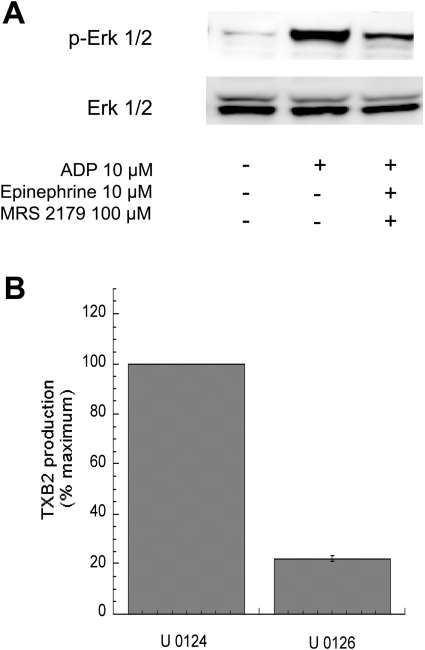
In platelets, ADP activates Gq and Gi pathways [3], whereas adrenaline (epinephrine), through α2A adrenergic receptors, activates Gz pathways [32]. We have previously shown [33] that in the absence of Gq signalling, co-stimulation of Gi and Gz pathways leads to fibrinogen receptor activation and thromboxane generation. Interestingly, co-stimulation of Gi and Gz pathways generates more thromboxane A2 than Gq and Gi pathways [33]. Hence the first question we asked was whether Gi+Gz stimulation led to ERK1/2 activation and whether ERK activation by Gi+Gz was greater than Gq+Gi stimulation. As established previously [33], Gq and Gi stimulation is achieved by activation of platelets with ADP, whereas Gi and Gz stimulation is accomplished by activation of platelets with ADP plus adrenaline, in the presence of MRS-2179. As shown in Figure 4(A), Gi+Gz stimulation leads to ERK2 phosphorylation albeit to a lesser extent than that by Gq+Gi stimulation. We evaluated the role of ERK2 in Gi+Gz-mediated thromboxane generation. As shown in Figure 4(B), U0126 markedly inhibited Gi+Gz-mediated thromboxane generation in platelets. Hence we concluded that ERK2 plays a key role in thromboxane generation by Gq+Gi pathways as well as Gi+Gz pathways.
Figure 4. Role of ERK in Gi+Gz-mediated activation of platelets.
Washed and aspirin-treated human platelets were stimulated with 10 μM ADP alone or with 10 μM ADP+100 μM MRS2179+10 μM adrenaline (epinephrine) for 1 min, without added calcium, with stirring at 37 °C, and ERK phosphorylation was measured using Western blot analysis using a phospho-ERK-specific antibody (A). Total ERK antibodies were used in the Western blot analysis to ensure similar protein loading in all lanes. The data are representative of experiments performed using platelets from at least three different donors. (B) Washed human platelets were stimulated with 10 μM ADP+100 μM MRS-2179+10 μM adrenaline in the presence of the MEK kinase inhibitor, U0126 (10 μM) or its inactive analogue, U0124 (10 μM) as indicated. Pre-incubation times for U0126 and U0124 were 10 min at 37 °C. Thromboxane A2 generation was measured as described in the Experimental section and normalized to 100%. All of the above data are generated using platelets from at least three different donors. TXB2, thromboxane B2.
Role of Src family kinases in ADP-induced ERK phosphorylation and thromboxane generation in platelets
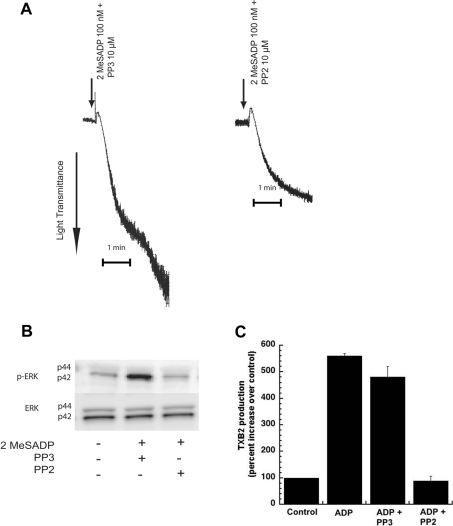
The Src family tyrosine kinases are activated downstream of P2Y1 and P2Y12 receptors in platelets [33,34]. They have been implicated in vWF (von Willebrand factor)-mediated thromboxane generation and ERK2 phosphorylation [17,35] and thromboxane generation by other agonists [33,36]. Hence, we investigated whether the Src family tyrosine kinases were involved in ADP-induced phosphorylation of ERK. In order to determine the role of Src family tyrosine kinases in platelet aggregation, we measured the 2MeSADP-induced platelet aggregation in non-aspirin-treated platelets, in the presence and absence of the Src family inhibitor PP2 (10 μM). The structural analogue PP3 (10 μM), with no Src inhibiting activity, was used as a negative control. As shown in Figure 5(A), the 2MeSADP-mediated secondary platelet aggregation was abolished without any effect on the primary aggregation by PP2. However, the negative control, PP3 had no effect on the 2MeSADP-induced secondary aggregation. Consistent with these results, we observed that PP2 abolished 2MeSADP-induced ERK2 phosphorylation whereas PP3 had no effect (Figure 5B). Furthermore, ADP-mediated thromboxane A2 generation was dramatically inhibited in the presence of the Src family kinase selective inhibitor, PP2 (Figure 5C). From the above results, it is evident that the Src family tyrosine kinases are activated by P2Y receptor activation and that these kinases are the upstream kinases that are involved in the ADP-induced ERK2 phosphorylation and thromboxane A2 generation in platelets.
Figure 5. Effect of Src kinase inhibition on ADP-mediated platelet aggregation, ERK phosphorylation and thromboxane A2 generation.
Washed human platelets, without aspirin treatment, were stimulated with 2MeSADP (100 nM) or ADP (10 μM) for 60 s, without added calcium, in the presence or absence of the Src kinase inhibitor (PP2). The inactive analogue, PP3, was used as a negative control. Both PP3 and PP2 were pre-incubated at 37 °C for 10 min prior to stimulation. Platelet aggregation (A), ERK phosphorylation (B), and thromboxane A2 (C) were measured by lumiaggregometry, Western blot analysis and ELISA respectively. All of the above data are representative of experiments performed using platelets from at least three different donors. TXB2, thromboxane B2.
Effect of extracellular calcium on 2MeSADP-induced ERK phosphorylation
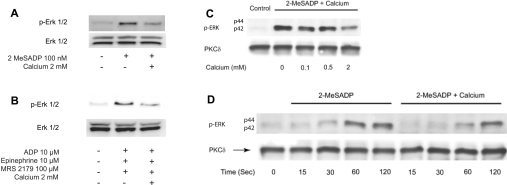
Previous studies [23] have established that agonist-induced thromboxane generation dramatically increases as the extracellular calcium concentrations are reduced. Furthermore, extracellular calcium is known to abolish thromboxane generation by weak agonists such as ADP [23]. The mechanism of this negative regulation is not known. As the present studies indicated an important role for ERK2 in ADP-induced thromboxane generation, we evaluated the effect of extracellular calcium on ADP-induced ERK2 phosphorylation. As shown in Figure 6(A), ADP-induced ERK2 phosphorylation was dramatically inhibited when platelets were stimulated in the presence of 2 mM extracellular calcium ions (84±3% inhibition). Similarly, extracellular calcium also dramatically blocked Gi+Gz-mediated ERK2 phosphorylation in platelets (Figure 6B). We further evaluated whether this inhibition by extracellular calcium was concentration-dependent. As shown in Figure 6(C), there was no effect of extracellular calcium until a concentration of 2 mM, but at this physiological concentration, calcium inhibited ERK2 phosphorylation. We also investigated whether the kinetics of ERK2 phosphorylation were altered in the presence of extracellular calcium. However, the phosphorylation of ERK2 occurred to a similar extent at various time points in the presence and absence of calcium (Figure 6D). As the inhibitor studies have shown that blockade of ERK2 is sufficient to abolish ADP-induced thromboxane generation, these data (Figure 6) indicate that extracellular calcium negatively regulates thromboxane A2 generation through inhibition of ERK2 phosphorylation.
Figure 6. Effect of extracellular calcium on (A) 2MeSADP- or (B) Gi+Gz-induced ERK phosphorylation.
Washed and aspirin-treated human platelets were resuspended in Tyrodes buffer with or without 2 mM extracellular calcium, and stimulated with 100 nM 2MeSADP (A) or 10 μM ADP+100 μM MRS-2179+10 μM adrenaline (epinephrine) (B) with stirring at 37 °C, and ERK phosphorylation was measured by Western blot analysis. The effect of various concentrations of extracellular calcium (C) and the time course of ERK2 phosphorylation in the presence or absence of extracellular calcium (D) were measured. Each Western blot is representative of experiments performed using platelets from at least three different donors.
DISCUSSION
The purpose of the present study was to elucidate the molecular mechanisms of ERK activation and the functional consequences downstream of ADP receptor stimulation in platelets. Although previous studies have examined the activation of ERK1/2 in platelets by a number of strong agonists such as thrombin and convulxin [13–19], their activation by ADP remains poorly understood. Hence in the present study we investigated: (i) the activation of the MAPKs, ERK1/2, by ADP; (ii) the mechanism of their activation; and (iii) their role in ADP-induced platelet activation.
Our data show that phosphorylation by ADP of predominantly ERK2, but not ERK1, occurs in platelets and this event occurs independently of outside-in signalling. Furthermore, signalling events downstream of both the P2Y1 and the P2Y12 receptors are required for ERK2 phosphorylation. Similarly, ERK2 can be activated by stimulation of Gi+Gz pathways. To the best of our knowledge, this is the first report of a phosphorylation event that requires concomitant signalling from both the P2Y receptors or two separate G proteins (Gq+Gi or Gi+Gz).
In contrast with our findings, two previous studies [21,22] have reported that little or no ERK phosphorylation occurs when platelets are stimulated with P2Y receptor agonists. We believe that the failure of other investigators to demonstrate ERK phosphorylation by P2Y receptor agonists is due to important differences in experimental conditions. For example, the reported lack of ERK phosphorylation in studies by Falker et al. [22] is attributable to the presence of extracellular calcium. Consistent with that finding, in the present study we have demonstrated that extracellular calcium blocks ADP-induced ERK phosphorylation (Figure 6A). Secondly, the presence of strong agonists, such as collagen, could overwhelm the signalling from weak agonists such as ADP and ERK phosphorylation in the studies by Roger et al. [21]. It is important to note that the same group of researchers [21], in agreement with our data, showed that 2MeSADP did cause ERK2 phosphorylation.
We have previously shown that concomitant signalling events from both of these P2Y receptors are required for fibrinogen receptor activation and thromboxane generation [8,37]. Hence, we evaluated the role of ERK2 in these physiological responses. Unlike the first generation inhibitors of MEK, such as PD098059, which have activity that block the cyclo-oxygenase pathway [38], U0126 is not known to exhibit such effects. We confirmed that ERK2 plays an important role in ADP-induced thromboxane generation and consequently affects ADP-induced platelet aggregation and dense granule secretion in non-aspirin-treated platelets. However, when platelets were treated with aspirin, there was no effect of MEK inhibition on ADP-induced platelet aggregation or shape change, indicating that ERK2 is primarily involved in thromboxane generation. We have recently shown that PAR-mediated thromboxane generation is only partially blocked by MEK inhibitors [20]. The lack of complete inhibition of PAR-mediated thromboxane generation is most likely due to the fact that PAR agonists are stronger agonists than ADP, causing much stronger Gq stimulation as well as activating G12/13 pathways [39].
ERK2 also appears to play an important role in Gi+Gz-mediated thromboxane generation. However, Gi+Gz stimulation leads to a greater extent of thromboxane generation compared with Gq+Gi stimulation. Interestingly, this greater thromboxane generation is not due to an increase in ERK2 phosphorylation. Thus Gi+Gz pathways may be activating other downstream signalling molecules that contribute to enhanced thromboxane generation. These pathways require further investigation.
Consistent with the role of P2Y1 stimulation in ERK2 phosphorylation and thromboxane generation, inhibitors of downstream signalling events, Gq, PLC-β and calcium, blocked both ERK2 phosphorylation and thromboxane generation. Interestingly, inhibition of all PKC isoforms with a pan-PKC inhibitor resulted in potentiation of ERK2 phosphorylation, suggesting that some of the PKC isoforms might be negatively regulating the ERK activation pathway (outlined in Figure 7). Such regulation of the Gq pathway by PKC isoforms is known; for example PKCα negatively regulates PLCβ1 in Swiss 3T3 cells [40]. Consistent with the role of ERK2 in thromboxane generation, the pan-PKC inhibition also resulted in dramatically increased thromboxane generation. The specific PKC isoforms involved in this phenomenon and the mechanism by which these isoforms regulate the ERK pathway remains under investigation.
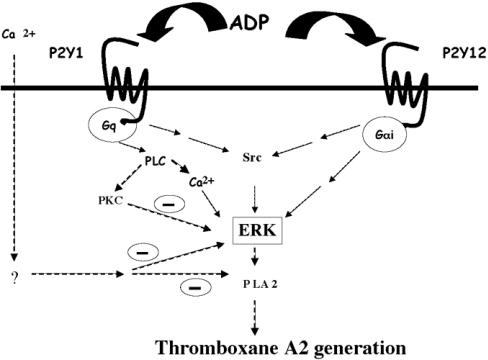
Figure 7. Model depicting the mechanism of activation and functional role of ERK in ADP-induced thromboxane A2 generation.
Signalling through both the P2Y1 and the P2Y12 receptors is essential for ADP-induced ERK2 activation in platelets. Activation of PLC and subsequent intracellular calcium increases are necessary for ADP-induced ERK2 activation. Src activation occurring downstream of the P2Y receptor activation is required for ADP-induced ERK activation. Extracellular calcium and specific PKC isoform(s) negatively regulate ADP-induced ERK2 phosphorylation and thromboxane generation through as yet undefined mechanisms. The identity of the specific PKC isoform(s) mediating this negative regulation is yet to be identified.
Src family tyrosine kinases regulate signal transduction pathways of various haemopoietic cells. Many members of Src family kinases are present in platelets [41]. It has also been reported that stimulation of G-protein-coupled receptors induce activation of Src kinases [42]. Lyn-deficient mice have impaired thromboxane generation in response to γ-thrombin when compared with wild-type mice [36]. Earlier studies have shown that Src family kinases are important in thromboxane generation downstream of the signalling pathways involving the inhibitory G-protein family (i.e. Gαi and Gαz) [33], PAR stimulation [43] and GPIb-mediated thromboxane A2 in platelets [17]. However, the functional roles of Src family kinases in ADP-induced platelet activation have not been well-defined. It has recently been reported that Src kinase is activated primarily by the Gq-coupled P2Y1 receptor in platelets [34]. Our previous work has shown that both Gi and Gz signalling can cause Src activation [33], and we have found that the Src kinase inhibitor PP2 inhibits ADP-mediated ERK2 phosphorylation and thromboxane generation. Thus the present study demonstrates that an Src family kinase downstream of either Gi or Gq, is a key signalling molecule in ADP-induced ERK2 phosphorylation (summarized in Figure 7). Further studies are required to identify which member of the Src family kinases is involved in this ERK2 phosphorylation and which G-protein it is downstream of.
Lin et al. [11,44] have shown that phosphorylation of cytosolic PLA2 at Ser505 is important for the hormonally regulated release of arachidonic acid from membrane-bound phospholipids, as the S505A mutation causes little or no increase in agonist-mediated arachidonic acid release. Using SB203580, an inhibitor of p38 MAPK, agonist-induced phosphorylation of cytosolic PLA2 [45,46] and thromboxane generation were shown to occur through p38 MAPK in platelets [47]. However, SB203580 has subsequently been shown to block both cyclo-oxygenases and thromboxane synthase, indicating that the effects of this inhibitor are not primarily on cytosolic PLA2 activation [38]. Furthermore, using VX-702, another structurally distinct inhibitor of p38 MAPK, it was concluded that p38 MAPK does not contribute to agonist-induced thromboxane generation in platelets [48]. Finally, we have shown that ADP-induced p38 MAPK activation is blocked by aspirin treatment [49]. Thus ADP does not directly activate p38 MAPK and depends on a cyclo-oxygenase product. Hence, p38 MAPK does not appear to play a significant role in ADP-induced thromboxane generation in platelets.
Thromboxane generation in platelets, induced by weak agonists such as ADP, is completely abolished in the presence of physiological concentrations of extracellular calcium concentrations [23]. Indeed, extracellular calcium has some inhibitory effect on thromboxane generation by stronger agonists as well [23]. However, the mechanism of the negative regulation of thromboxane generation by extracellular calcium has not been addressed to date. As the present study identified ERK2 as a key signalling molecule in ADP-induced thromboxane generation, we evaluated the effect of extracellular calcium on ADP-induced ERK2 activation. Interestingly, extracellular calcium dramatically blocked ADP- or Gi+Gz-induced ERK2 phosphorylaton. As ERK2 activation blockade resulted in inhibition of thromboxane generation by Gq+Gi or Gi+Gz pathways, we concluded that inhibition of ERK2 activation is a key mechanism through which extracellular calcium regulates agonist-induced thromboxane generation (outlined in Figure 7). However, the pathways linking increases in extracellular calcium to ERK pathways need further investigation.
It is clear that weaker agonists such as ADP cannot generate thromboxane in the presence of physiological levels of extracellular calcium. It is also true that extracellular calcium dampens the extent of thromboxane generated by stronger agonists. This might be a physiological mechanism of balancing haemostasis and thrombosis. However, it is obvious that the platelet is capable of generating thromboxane when stimulated with weaker agonists under certain conditions. This observation is more important when studies are performed with PRP under the conditions of low extracellular calcium and no aspirin-treatment. As many of the studies using knockout animal platelets are performed under these conditions, caution must be exercised in interpreting the results. A decrease in aggregation in the knockout mouse platelets should not be readily interpreted as a direct role of the signalling molecule in fibrinogen receptor activation. It is possible that the signalling molecule might have a role in the generation of thromboxane and the reduced aggregation is due to reduced positive feedback from the generated thromboxane. Additional experiments need to be performed with these mice to ensure that the defect is not due to thromboxane generation.
In conclusion, ADP-induced platelet activation leads to phosphorylation of ERK2 through Src family tyrosine kinases. This event depends on the signalling from both the P2Y1 and the P2Y12 receptors. This activation of ERK2 is important for thromboxane generation in ADP-induced platelet stimulation. We also show that extracellular calcium blocks ADP-induced thromboxane A2 generation through the inhibition of ERK2 activation. In addition to ADP, which activates Gq+Gi pathways, these pathways also appear to be important in Gi+Gz-mediated platelet activation.
Acknowledgments
This work was supported by Research Grants HL60683 and HL80444 from the NIH (National Institutes of Health) to S.P.K. and a predoctoral fellowship from the American Heart Association, Pennsylvania-Delaware affiliate to S.M and H.S. A.G. is supported by a NIH training grant T32 HL07777. We thank Dr G.L. Prasad (Department of Physiology, Temple University School of Medicine, Philadelphia, PA, U.S.A.) for his critical reading of the manuscript and helpful suggestions prior to submission.
References
- 1.Brass L. F., Manning D. R., Cichowski K., Abrams C. S. Signaling through G proteins in platelets: to the integrins and beyond. Thromb. Haemostasis. 1997;78:581–589. [PubMed] [Google Scholar]
- 2.Cattaneo M., Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler., Thromb., Vasc. Biol. 1999;19:2281–2285. doi: 10.1161/01.atv.19.10.2281. [DOI] [PubMed] [Google Scholar]
- 3.Kahner B. N., Shankar H., Murugappan S., Prasad G. L., Kunapuli S. P. Nucleotide receptor signaling in platelets. J. Thromb. Haemostasis. 2006;4:2317–2326. doi: 10.1111/j.1538-7836.2006.02192.x. [DOI] [PubMed] [Google Scholar]
- 4.Trumel C., Payrastre B., Plantavid M., Hechler B., Viala C., Presek P., Martinson E. A., Cazenave J. P., Chap H., Gachet C. A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood. 1999;94:4156–4165. [PubMed] [Google Scholar]
- 5.Nieswandt B., Schulte V., Zywietz A., Gratacap M. P., Offermanns S. Costimulation of Gi- and G1213-mediated signaling pathways induces integrin αIIbβ3 activation in platelets. J. Biol. Chem. 2002;277:39493–39498. doi: 10.1074/jbc.M207256200. [DOI] [PubMed] [Google Scholar]
- 6.Dorsam R. T., Kim S., Jin J., Kunapuli S. P. Co-ordinated signaling through both G12/13 and Gi pathways is sufficient to activate GPIIb/IIIa in human platelets. J. Biol. Chem. 2002;277:47588–47595. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- 7.Bennett J. S. Novel platelet inhibitors. Annu. Rev. Med. 2001;52:161–184. doi: 10.1146/annurev.med.52.1.161. [DOI] [PubMed] [Google Scholar]
- 8.Jin J., Quinton T. M., Zhang J., Rittenhouse S. E., Kunapuli S. P. Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires co-ordinated signaling through integrin α(IIb)β(3) and ADP receptors. Blood. 2002;99:193–198. doi: 10.1182/blood.v99.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Jin J., Daniel J. L., Kunapuli S. P. Molecular basis for ADP-induced platelet activation II: the P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 10.Halenda S. P., Banga H. S., Zavoico G. B., Lau L. F., Feinstein M. B. Synergistic release of arachidonic acid from platelets by activators of protein kinase C and Ca2+ ionophores. Evidence for the role of protein phosphorylation in the activation of phospholipase A2 and independence from the Na+/H+ exchanger. Biochemistry. 1989;28:7356–7363. doi: 10.1021/bi00444a031. [DOI] [PubMed] [Google Scholar]
- 11.Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 12.Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 13.Oury C., Toth-Zsamboki E., Vermylen J., Hoylaerts M. F. P2X(1)-mediated activation of extracellular signal-regulated kinase 2 contributes to platelet secretion and aggregation induced by collagen. Blood. 2002;100:2499–2505. doi: 10.1182/blood-2002-03-0812. [DOI] [PubMed] [Google Scholar]
- 14.Toth-Zsamboki E., Oury C., Cornelissen H., De Vos R., Vermylen J., Hoylaerts M. F. P2X1-mediated ERK2 activation amplifies the collagen-induced platelet secretion by enhancing myosin light chain kinase activation. J. Biol. Chem. 2003;278:46661–46667. doi: 10.1074/jbc.M308452200. [DOI] [PubMed] [Google Scholar]
- 15.Sun L., Feng S., Resendiz J. C., Lu X., Durante W., Kroll M. H. Role of the Pyk2-MAP kinase-cPLA2 signaling pathway in shear-dependent platelet aggregation. Ann. Biomed. Eng. 2004;32:1193–1201. doi: 10.1114/b:abme.0000039353.97347.28. [DOI] [PubMed] [Google Scholar]
- 16.Li Z., Zhang G., Feil R., Han J., Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin αIIbβ3. Blood. 2006;107:965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia A., Quinton T. M., Dorsam R. T., Kunapuli S. P. Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–3414. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tulasne D., Bori T., Watson S. P. Regulation of RAS in human platelets. Evidence that activation of RAS is not sufficient to lead to ERK1/2 phosphorylation. Eur. J. Biochem. 2002;269:1511–1517. doi: 10.1046/j.1432-1033.2002.02798.x. [DOI] [PubMed] [Google Scholar]
- 19.Kramer R. M., Roberts E. F., Strifler B. A., Johnstone E. M. Thrombin induces activation of p38 MAP kinase in human platelets. J. Biol. Chem. 1995;270:27395–27398. doi: 10.1074/jbc.270.46.27395. [DOI] [PubMed] [Google Scholar]
- 20.Shankar H., Garcia A., Prabhakar J., Kim S., Kunapuli S. P. P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation. J. Thromb. Haemostasis. 2006;4:638–647. doi: 10.1111/j.1538-7836.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- 21.Roger S., Pawlowski M., Habib A., Jandrot-Perrus M., Rosa J. P., Bryckaert M. Costimulation of the Gi-coupled ADP receptor and the Gq-coupled TXA2 receptor is required for ERK2 activation in collagen-induced platelet aggregation. FEBS Lett. 2004;556:227–235. doi: 10.1016/s0014-5793(03)01430-3. [DOI] [PubMed] [Google Scholar]
- 22.Falker K., Lange D., Presek P. ADP secretion and subsequent P2Y12 receptor signalling play a crucial role in thrombin-induced ERK2 activation in human platelets. Thromb. Haemostasis. 2004;92:114–123. doi: 10.1160/TH03-12-0729. [DOI] [PubMed] [Google Scholar]
- 23.Packham M. A., Bryant N. L., Guccione M. A., Kinlough-Rathbone R. L., Mustard J. F. Effect of the concentration of Ca2+ in the suspending medium on the responses of human and rabbit platelets to aggregating agents. Thromb. Haemostasis. 1989;62:968–976. [PubMed] [Google Scholar]
- 24.Kunapuli S. P., Dorsam R. T., Kim S., Quinton T. M. Platelet purinergic receptors. Curr. Opin. Pharmacol. 2003;3:175–180. doi: 10.1016/s1471-4892(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 25.Mahaut-Smith M. P., Ennion S. J., Rolf M. G., Evans R. J. ADP is not an agonist at P2X(1) receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br. J. Pharmacol. 2000;131:108–114. doi: 10.1038/sj.bjp.0703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shattil S. J. Signaling through platelet integrin αIIbβ3: inside-out, outside-in, and sideways. Thromb. Haemostasis. 1999;82:318–325. [PubMed] [Google Scholar]
- 27.Matsuno H., Kozawa O., Nagashima S., Kanamaru M., Uematsu T. Comparative antiplatelet effects of aspirin, vapiprost and GR144053, a GPIIb/IIIa antagonist, with a special reference to the role of platelet microaggregates. Br. J. Pharmacol. 1999;127:1129–1134. doi: 10.1038/sj.bjp.0702651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., Kobori M. A novel Gαq/11-selective inhibitor. J. Biol. Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- 29.Lockhart L. K., Pampolina C., Nickolaychuk B. R., McNicol A. Evidence for a role for phospholipase C, but not phospholipase A2, in platelet activation in response to low concentrations of collagen. Thromb. Haemostasis. 2001;85:882–889. [PubMed] [Google Scholar]
- 30.Paul B. Z., Daniel J. L., Kunapuli S. P. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. Role of p160 Rho-associated coiled-coil-containing protein kinase in platelet shape change. J. Biol. Chem. 1999;274:28293–28300. doi: 10.1074/jbc.274.40.28293. [DOI] [PubMed] [Google Scholar]
- 31.Kim S., Jin J., Kunapuli S. P. Relative contribution of G-protein-coupled pathways to protease-activated receptor-mediated Akt phosphorylation in platelets. Blood. 2006;107:947–954. doi: 10.1182/blood-2005-07-3040. [DOI] [PubMed] [Google Scholar]
- 32.Yang J., Wu J., Kowalska M. A., Dalvi A., Prevost N., O'Brien P. J., Manning D., Poncz M., Lucki I., Blendy J. A., Brass L. F. Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9984–9989.. doi: 10.1073/pnas.180194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorsam R. T., Kim S., Murugappan S., Rachoor S., Shankar H., Jin J., Kunapuli S. P. Differential requirements for calcium and Src family kinases in platelet GPIIb/IIIa activation and thromboxane generation downstream of different G-protein pathways. Blood. 2005;105:2749–2756. doi: 10.1182/blood-2004-07-2821. [DOI] [PubMed] [Google Scholar]
- 34.Hardy A. R., Jones M. L., Mundell S. J., Poole A. W. Reciprocal cross-talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood. 2004;104:1745–1752. doi: 10.1182/blood-2004-02-0534. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Pestina T., Berndt M. C., Jackson C. W., Gartner T. K. Botrocetin/vWf-induced signaling through GPIb-IX-V that produces TxA2 in an αIIbβ3 and aggregation-independent manner. Blood. 2005;106:2750–2756. doi: 10.1182/blood-2005-04-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho M. J., Pestina T. I., Steward S. A., Lowell C. A., Jackson C. W., Gartner T. K. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with γ-thrombin. Blood. 2002;99:2442–2447. doi: 10.1182/blood.v99.7.2442. [DOI] [PubMed] [Google Scholar]
- 37.Jin J., Kunapuli S. P. Co-activation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borsch-Haubold A. G., Pasquet S., Watson S. P. Direct inhibition of cyclooxygenase-1 and -2 by the kinase inhibitors SB 203580 and PD 98059. SB 203580 also inhibits thromboxane synthase. J. Biol. Chem. 1998;273:28766–28772. doi: 10.1074/jbc.273.44.28766. [DOI] [PubMed] [Google Scholar]
- 39.Offermanns S. The role of heterotrimeric G proteins in platelet activation. Biol. Chem. 2000;381:389–396. doi: 10.1515/BC.2000.051. [DOI] [PubMed] [Google Scholar]
- 40.Xu A., Wang Y., Xu L. Y., Gilmour R. S. Protein kinase Cα-mediated negative feedback regulation is responsible for the termination of insulin-like growth factor I-induced activation of nuclear phospholipase C β1 in Swiss 3T3 cells. J. Biol. Chem. 2001;276:14980–14986. doi: 10.1074/jbc.M009144200. [DOI] [PubMed] [Google Scholar]
- 41.Pestina T. I., Stenberg P. E., Druker B. J., Steward S. A., Hutson N. K., Barrie R. J., Jackson C. W. Identification of the Src family kinases, Lck and Fgr in platelets. Their tyrosine phosphorylation status and subcellular distribution compared with other Src family members. Arterioscler., Thromb., Vasc. Biol. 1997;17:3278–3285. doi: 10.1161/01.atv.17.11.3278. [DOI] [PubMed] [Google Scholar]
- 42.Bauer M., Maschberger P., Quek L., Briddon S. J., Dash D., Weiss M., Watson S. P., Siess W. Genetic and pharmacological analyses of involvement of Src-family, Syk and Btk tyrosine kinases in platelet shape change. Src-kinases mediate integrin αIIbβ3 inside-out signalling during shape change. Thromb. Haemostasis. 2001;85:331–340. [PubMed] [Google Scholar]
- 43.Murugappan S., Shankar H., Bhamidipati S., Dorsam R. T., Jin J., Kunapuli S. P. Molecular mechanism and functional implications of thrombin-mediated tyrosine phosphorylation of PKCδ in platelets. Blood. 2005;106:550–557. doi: 10.1182/blood-2004-12-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borsch-Haubold A. G., Kramer R. M., Watson S. P. Cytosolic phospholipase A2 is phosphorylated in collagen- and thrombin-stimulated human platelets independent of protein kinase C and mitogen-activated protein kinase. J. Biol. Chem. 1995;270:25885–25892. doi: 10.1074/jbc.270.43.25885. [DOI] [PubMed] [Google Scholar]
- 46.Borsch-Haubold A. G., Kramer R. M., Watson S. P. Phosphorylation and activation of cytosolic phospholipase A2 by 38-kDa mitogen-activated protein kinase in collagen-stimulated human platelets. Eur. J. Biochem. 1997;245:751–759. doi: 10.1111/j.1432-1033.1997.t01-1-00751.x. [DOI] [PubMed] [Google Scholar]
- 47.Canobbio I., Reineri S., Sinigaglia F., Balduini C., Torti M. A role for p38 MAP kinase in platelet activation by von Willebrand factor. Thromb. Haemostasis. 2004;91:102–110. doi: 10.1160/TH03-02-0083. [DOI] [PubMed] [Google Scholar]
- 48.Kuliopulos A., Mohanlal R., Covic L. Effect of selective inhibition of the p38 MAP kinase pathway on platelet aggregation. Thromb. Haemostasis. 2004;92:1387–1393. doi: 10.1160/TH04-03-0187. [DOI] [PubMed] [Google Scholar]
- 49.Dangelmaier C., Jin J., Daniel J. L., Smith J. B., Kunapuli S. P. The P2Y1 receptor mediates ADP-induced p38 kinase-activating factor generation in human platelets. Eur. J. Biochem. 2000;267:2283–2289. doi: 10.1046/j.1432-1327.2000.01235.x. [DOI] [PubMed] [Google Scholar]