Abstract
Nicotine replacement products are commonly used to promote smoking cessation, but alternative and complementary methods may increase cessation rates. The current experiment compared the short-term effects of a transdermal nicotine patch to voucher-based reinforcement of smoking abstinence on cigarette smoking. Fourteen heavy smokers (7 men and 7 women) completed the four 5-day phases of the study: baseline, patch treatment, voucher treatment, and return to baseline. The order of the two treatment phases was counterbalanced across participants. In the patch treatment condition, participants wore a 14-mg transdermal nicotine patch every day. In the voucher treatment condition, participants received vouchers contingent on abstinence from smoking, defined as producing carbon monoxide (CO) readings of ≤4 parts per million. Participants e-mailed two video clips per day showing them breathing into a CO monitor and the resulting CO reading to clinic staff. In the voucher treatment, 24% of samples were negative, and 5% of samples were negative in the patch treatment. Results suggest that contingent vouchers were more effective than transdermal nicotine patches in promoting abstinence.
Keywords: cigarette smoking, transdermal nicotine patch, voucher reinforcement, abstinence, nicotine
Tobacco use contributes to 440,000 deaths each year, making it the leading cause of preventable death and disease in the United States (Centers for Disease Control, 2002). These preventable conditions cost an estimated $157 billion in health-related economic losses annually (Centers for Disease Control, 2002). Despite the detrimental effects of cigarettes, 46.2 million adults in the U.S. smoke (“The Health Consequences of Smoking,” 2004). Of those smokers, however, 70% report a desire to quit smoking. Given the substantial health and economic costs attributable to smoking, it is critical to develop effective smoking cessation programs. The primary purpose of the current study was to examine the efficacy of two different smoking cessation aids, voucher-based abstinence reinforcement and a transdermal nicotine patch.
Abstinence reinforcement therapy refers to the arrangement of reinforcement, often through the use of contingent vouchers, for objectively verified drug abstinence (Bigelow, Stitzer, Griffiths, & Liebson, 1981; Boudin, 1972; Crowley, 1986; Higgins et al., 1991; Hunt & Azrin, 1973; Silverman, 2004). Vouchers have monetary value and are exchangeable for goods and services. Many studies have demonstrated that abstinence reinforcement is effective in initiating and maintaining drug abstinence (Budney, Higgins, Radonovich, & Novy, 2000; Dallery, Silverman, Chutuape, Bigelow, & Stitzer, 2001; Katz, Chutuape, Jones, & Stitzer, 2002; Silverman, Chutuape, Bigelow, & Stitzer, 1999; Silverman, Higgins, et al., 1996; Silverman, Wong, et al., 1996), including cigarette smoking abstinence (Dallery, Glenn, & Raiff, in press; Rand, Stitzer, Bigelow, & Mead, 1989; Roll, Higgins, Steingard, & McGinley, 1998; Stitzer & Bigelow, 1985; Stitzer, Rand, Bigelow, & Mead, 1986). In one of the first studies to apply contingent reinforcement for reduced carbon monoxide (CO) levels in cigarette smokers, Stitzer and Bigelow (1982) delivered $5 payments to participants who submitted CO samples with at least a 50% decrease from the average baseline readings. This contingency effectively decreased CO levels, decreased number of self-reported cigarettes per day, and increased latency to first cigarette of the day.
Other methods of promoting smoking cessation are more common than abstinence reinforcement therapy, including nicotine replacement products (e.g., nicotine gum, nicotine lozenges, and nicotine patches; Bansal, Cummings, Hyland, & Giovino, 2004; Fiore, Smith, Jorenby, & Baker, 1994; Hughes, 1993), on-line support groups (e.g., de Vries & Brug, 1999; Strecher, 1999), pharmacotherapies (e.g., buproprion; Swanson, Burroughs, Long, & Lee, 2003), and cognitive behavioral therapy (e.g., Garcia-Vera, 2004; Schnoll et al., 2005). Although these treatments produce positive outcomes, there is still considerable room for improvement in cessation rates. For example, Fiore et al. (1994) conducted a meta-analysis of 17 studies that investigated effects of the transdermal nicotine patch for smokers trying to quit. The authors compared overall abstinence rates of the patch relative to a placebo patch. At the end of treatment, 27% of participants in the active patch group and 13% of those in the placebo patch group were abstinent, as determined by biochemical analysis.
Recently, Tidey, O'Neill, and Higgins (2002) examined the effects of contingent monetary reinforcement on smoking, with and without 21-mg transdermal nicotine patches, in outpatients with schizophrenia. Both 5-day conditions were equally effective in reducing smoking, which suggests that the nicotine patch did not enhance the effects of the abstinence contingency for persons with schizophrenia. Similarly, Wiseman, Williams, and McMillan (2005) compared the effectiveness of contingent payment and the patch in cocaine-abusing outpatients in a 2-week intervention. Participants were assigned to receive either contingent vouchers or noncontingent vouchers. Within these two groups, participants wore a placebo patch or a 21-mg transdermal nicotine patch in a randomized crossover design. Although the nicotine patch resulted in lower CO levels than the placebo patch, only contingent payment was effective in promoting abstinence (defined as CO ≤ 8 ppm).
The manner of collecting CO samples by Wiseman et al. (2005) and Tidey et al. (2002), which required participants or researchers to travel multiple times per day, is not practical on a long-term basis. However, it is important that researchers continue to collect at least two CO samples per day from participants due to its estimated half-life of 6 hr (Crowley, MacDonald, Zerbe, & Petty, 1991; Middleton, 2000). Dallery and Glenn (2005) demonstrated the feasibility of an Internet-based method for collecting CO samples. This method allows participants to submit CO readings via Internet-based technology from their own homes. Participants received vouchers (exchangeable for items on the Internet) contingent on meeting criteria for decreasing CO and then maintaining low CO levels. This method overcomes distance as a barrier to treatment and allows CO samples to be collected without putting excessive demands on participants or researchers.
To our knowledge, no study has compared the effects of voucher reinforcement and those of the nicotine patch in a typical smoking population. The current study used the Internet-based monitoring and treatment method developed by Dallery and Glenn (2005) to compare the effects of contingent vouchers and transdermal nicotine patches on CO level. We used a 14-mg patch, rather than a higher dose, in light of evidence that that 15-mg and 25-mg transdermal nicotine patches were equally effective in reducing smoking in heavy smokers in an outpatient study (Killen, Fortmann, Davis, Strausberg, & Varady, 1999).
Method
Participants
Fourteen smokers (7 men and 7 women) participated. Participant characteristics are presented in Table 1. Smokers were recruited from the local and surrounding communities using flyers, print media, and word of mouth. Interested smokers were given a brief interview over the phone to ensure that they met basic inclusion criteria and were provided information about the study. Inclusion criteria consisted of the following: being between 18 and 60 years old, smoking a minimum of 15 cigarettes per day, having smoked for at least 2 years, not residing with another smoker who smoked inside the home, and expressing a desire to quit smoking. Interested smokers who met the inclusion criteria were scheduled for in-person interviews. The local institutional review board approved all study procedures.
Table 1. Participant Characteristics.
| Participant | Age (years) | Sex | Ethnicity | Weekly income ($) | Cigarettes per day | Years smoking | FTND | URICA |
| S0057 | 57 | F | W | 401–500 | 20 | 45 | 5 | 8.8 |
| A0058 | 53 | M | W | 301–400 | 40 | 35 | 10 | 11.0 |
| C0058 | 53 | M | W | 201–300 | 30 | 36 | 8 | 12.7 |
| S0060 | 23 | F | W | 201–300 | 20 | 10 | 7 | 9.8 |
| K0062 | 26 | F | W | < 100 | 20 | 10 | 6 | 8.8 |
| J0063 | 45 | M | W | < 100 | 18 | 25 | 7 | 9.2 |
| B0064 | 28 | M | W | 201–300 | 18 | 17 | 3 | 11.4 |
| T0064 | 27 | M | W | 601–700 | 20 | 12 | 6 | 12.2 |
| K0065 | 21 | M | W | 401–500 | 25 | 10 | 8 | 9.6 |
| B0069 | 49 | F | W | 401–500 | 25 | 34 | 4 | 8.0 |
| A0073 | 25 | M | W | 201–300 | 15 | 7 | 3 | 9.6 |
| L0075 | 21 | F | W | 301–400 | 20 | 6 | 4 | 9.6 |
| D0076 | 43 | F | W | < 100 | 20 | 19 | 8 | 8.3 |
| M0079 | 42 | F | W | 201–300 | 40 | 30 | 8 | 11.9 |
Interview
Participants read and signed an informed consent, and were then required to pass a quiz about the informed consent to ensure comprehension of the procedures (Silverman et al., 1999). A University of Rhode Island Change Assessment (URICA; Prochaska & DiClemente, 1983) and a psychosocial history were then administered. The URICA is a self-report measure that assesses motivation to change (in this case, motivation to quit smoking). The URICA contains four subscales that measure the stages of change: precontemplation, contemplation, action, and maintenance. Participants responded on a 5-point Likert scale (1 = strongly disagree to 5 = strongly agree). The psychosocial history contains questions related to demographics, smoking history, prior drug use and abuse, general health, and medication use; the Fagerstrom Test for Nicotine Dependence (FTND); and the University of Florida Functional Assessment of Smoking (UFFAS). The FTND (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) is a six-item questionnaire that assesses nicotine dependence. Scores can range from 0 to 10, with 0 representing very low addiction and 10 representing very high addiction. The UFFAS (data not presented) is a 44-item questionnaire intended to identify the controlling variables of smoking. Urine and CO samples were also collected. The presence of cocaine, benzodiazepines, or opiates (verified by self-report and urinalysis) or a CO reading of < 20 ppm disqualified participants. Women were disqualified if they were pregnant or breastfeeding (verified by self-report and urinalysis).
Procedure
Co Monitoring
A laptop computer, a Web camera, and a CO monitor were loaned to qualifying participants, and an e-mail address was established for each participant. Research staff set up the equipment in each participant's home and demonstrated its use. For security purposes, computer-tracking devices were installed on laptops, copies of participants' drivers' licenses were taken, and participants were required to sign off-campus property certificates and contracts stating that they would return the equipment.
Participants were responsible for recording and e-mailing two video clips each day to research staff (Dallery & Glenn, 2005). The video clips needed to show the CO monitor on and ready to take a reading, the participant taking a breath and pushing the start button on the CO monitor, the participant holding his or her breath for 15 s and then exhaling fully into the CO monitor, and the final CO reading (in parts per million). An interval of at least 8 hr between submissions was required. Each phase lasted 5 days; no readings were taken on the weekends to minimize carryover effects. In addition, participants were notified that attempts to falsify a sample were easily detected and would lead to dismissal from the study. Microsoft Windows® automatically date- and time-stamped all video clips and attempts to alter them, so researchers could determine if the files were altered. Computers were administratively locked so that the participant could not easily change the date and time of the computer.
Participants were also asked to record the number of cigarettes smoked per day during the study. They were instructed to be honest, that this information would not be collected until the end of the study, and that this information would not affect their study participation or voucher earnings in any way. Once per phase, participants completed two craving and withdrawal questionnaires: The first was derived from the Withdrawal Symptoms Questionnaire (WSQ; Hughes & Hatsukami, 1986) and the second was the Questionnaire of Smoking Urges (QSU; Tiffany & Drobes, 1991). The WSQ assesses nicotine withdrawal and consists of 13 questions relating to “urges to smoke,” “irritable,” “anxious,” “difficulty concentrating,” “restless,” “impatient,” “excessive hunger,” “tremor,” “heart racing,” “sweating,” “dizziness,” “craving cigarettes,” “insomnia/disturbed sleep,” “increased eating,” “drowsiness,” “headache,” “bowel or stomach problems,” and “depressed.” Answers were scored on a 100-point visual analogue scale. Each participant was asked to put a mark on the scale related to how he or she felt, with the far left side labeled “not at all” and the far right side labeled “extremely.” The QSU assesses smoking urges through a 32-item questionnaire. The questionnaire is divided into Factor 1 and Factor 2. Factor 1 items represent intention and desire to smoke and anticipation of pleasure from smoking. Factor 2 items represent anticipation of relief from negative affect, nicotine withdrawal, and urgent and overwhelming desire to smoke. Participants were asked to score their answers on 7-point scales (1 = strong disagreement and 7 = strong agreement).
Behavioral counseling sessions were conducted once per phase. All researchers followed an established procedure for conducting the behavioral counseling sessions to ensure consistency across researchers and participants. The counseling sessions followed a semistructured script based on Public Health Service guidelines (Fiore et al., 2000). An identical script was used in each phase. Behavioral counseling sessions included such topics as current tobacco use and advice to assist the participant in quitting smoking. Participants' smoking histories were reviewed, and participants were strongly advised to quit smoking. Researchers helped participants to develop a quit plan including such topics as getting the support of family and friends and advice to remove ashtrays and other smoking paraphernalia. Research technicians were trained by a licensed psychologist (the second author). Participants were informed that use of nicotine replacement products other than those provided by the researcher would result in disqualification (no participant reported use of such products during the study).
Participants could visit a study Web site to access links pertaining to information about quitting smoking and the health effects of smoking. Each participant also had his or her own Web page that showed a graph of CO readings, vouchers earned to date, the amount of vouchers left to spend, and a link to a Web page with a list of vendors from which they could purchase items with their vouchers. Participants notified research staff when they wished to purchase items with their vouchers. If they had enough voucher earnings, research staff ordered and delivered the item. Firearms, weapons, drugs, and alcohol could not be purchased with voucher earnings. In addition to vouchers, participants received $100 for completing the study.
Phases
Half of the participants experienced the patch treatment before the contingent voucher treatment, and the other half experienced the contingent voucher treatment before the patch treatment. Assignment to treatment order was random.
Baseline (a)
Participants earned $5 per day contingent on correctly submitting two CO readings per day.
Contingent Voucher Treatment (b)
Vouchers were earned contingent on submitting CO samples of ≤ 4 ppm. A CO reading of 8 to 10 ppm is a common measure of abstinence, but recent research has suggested that CO readings less than 4 ppm may best classify those who are abstinent (Javors, Hatch, & Lamb, 2005). As with the voucher schedule used by Dallery and Glenn (2005), the first CO sample ≤ 4 ppm resulted in a $3 voucher. The value increased by $0.25 for each successive negative sample. Bonus vouchers ($5) were delivered for every third consecutive negative sample. If a participant failed to submit a sample or submitted a positive sample, the value of the next voucher was reset to $3. After three consecutive negative samples following a reset, the value of the next voucher returned to the highest value previously obtained. On this schedule, participants have progressively more to gain by continued abstinence and more to lose if they lapse. This schedule of voucher earnings was modeled after other studies that have been effective in promoting abstinence (Roll & Higgins, 2000; Roll, Higgins, & Badger, 1996). Participants could earn up to $56.25 if they were continuously abstinent during the contingent voucher phase.
Nicotine Patch Treatment (c)
Participants were given five 14-mg transdermal nicotine patches (NicoDerm CQ®) at the beginning of the study. Researchers informed participants not to use the patches until notified. Participants were called the day before the patch condition was to start. They were asked to put on a new patch every morning for the next 5 days. If participants experienced any adverse effects from the patch (e.g., pain, irritation, nausea) they were advised to take off the patch and were withdrawn from the study (1 participant experienced skin irritation and was withdrawn from the study; data are not presented). Compliance with wearing the patch was assessed over the phone; all participants reported wearing the patch as instructed.
Return To Baseline (d)
Participants earned $5 per day as long as they correctly submitted two CO readings per day.
Statistical Analyses
Statistical analyses were conducted using SPSS 11.0 professional edition. A repeated measures analysis of variance (ANOVA), with Huynh-Feldt correction, was conducted with condition and order as factors. Analyses were conducted for Factor 1 and Factor 2 of the QSU, the 13 components of the WSQ, the numbers of self-reported cigarettes per day, the average CO level per day, and the percentage of negative CO samples (missing samples were considered positive). Alpha was set at .05 for all statistical tests. In addition, to compare the CO results with the self-reports of smoking status, we calculated a Pearson's correlation coefficient relating the number of self-reported cigarettes smoked per day with the average CO per day (i.e., the average of the two COs collected). Correlation coefficients were also calculated relating participants' URICA scores to their average CO values in the patch and voucher conditions.
Results
Figure 1 presents each participant's average CO and self-reported number of cigarettes smoked per day across the four phases of the study. The voucher treatment appeared to be effective for Participants A073, C058, and S060. Participants A058, B069, J063, S057, and K065 decreased their smoking after the initial baseline phase, but no clear control was seen across subsequent phases. Data from Participants K062, M079, T064, B064, and L075 showed no clear effects during any phase of the study.
Figure 1. Average CO (ppm) per day and self-reported number of cigarettes smoked per day.
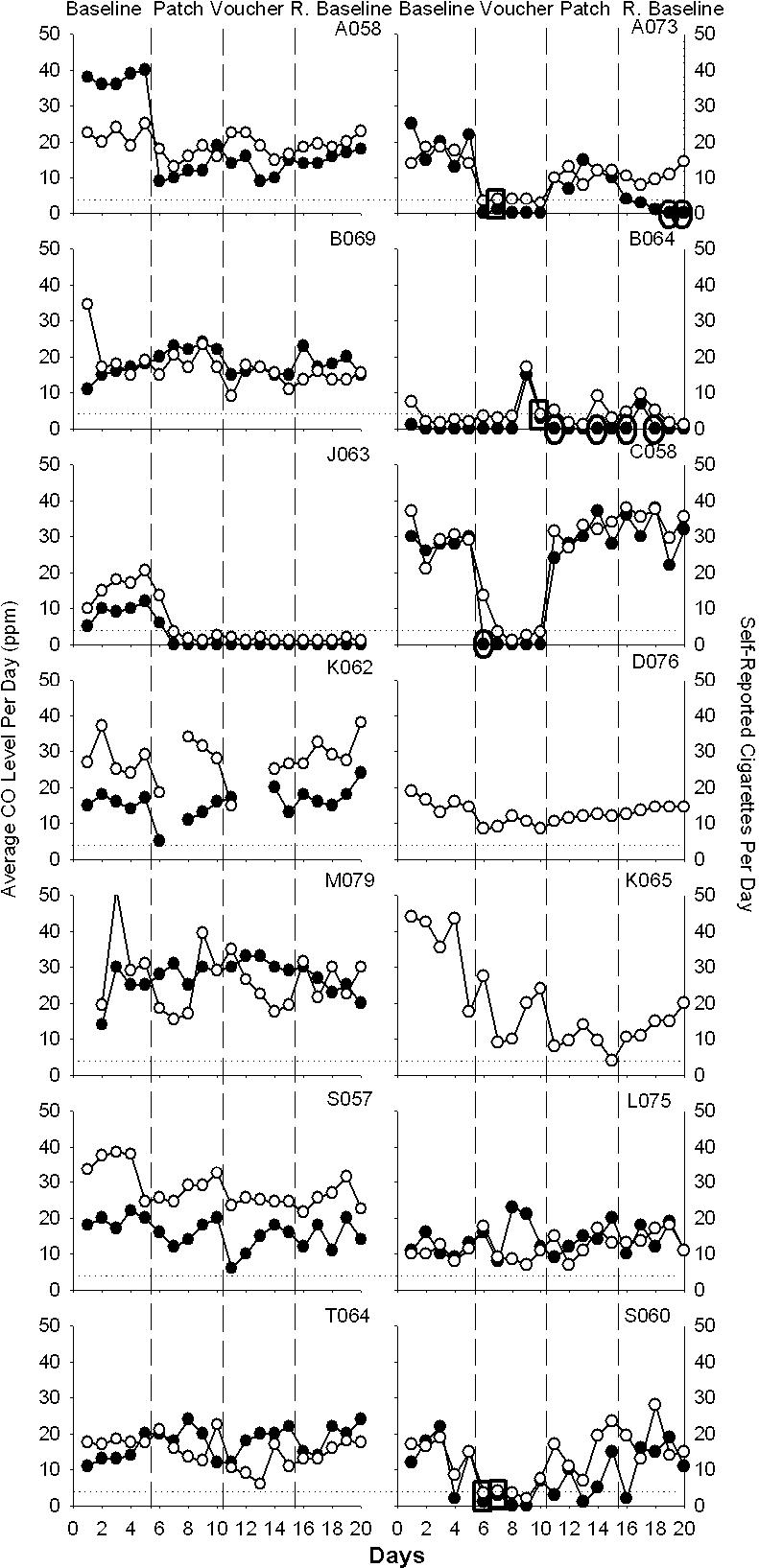
The left y axis presents average CO level as the average of the morning and afternoon CO levels for the day and is represented by open data points. The right y axis presents number of self-reported cigarettes smoked per day and is represented by solid data points. The dashed horizontal line indicates the abstinence criterion of 4 ppm. Ovals around data points reflect self-reports of nonsmoking with CO levels above the abstinence criterion. Rectangles reflect self-reports of smoking with CO levels meeting the abstinence criterion. Missing data points reflect data not submitted. The two graphs without cigarettes smoked per day represent Participants D076, and K065, who failed to submit self-report data on number of cigarettes smoked per day.
Figure 2 presents the average number of self-reported cigarettes per day, the average CO level per day, and the average percentage of negative samples. The average (and SEM) number of cigarettes smoked per day was 17.3 (1.3), 14.1 (1.3), 11.0 (1.3), and 14.4 (1.3) across the baseline, patch, voucher, and return to baseline phases, respectively. A repeated measures ANOVA of cigarettes per day showed a significant effect of Condition, F(3, 11) = 28.68, p < .001. Bonferroni's post hoc contrasts showed a significant decrease in cigarettes smoked in the voucher condition compared to baseline (p < .001), patch (p < .001), and return to baseline (p < .05). A significant effect of Treatment Order, F(3, 11) = 95.25, p < .001, was found, suggesting that participants who received the voucher treatment first were more successful in decreasing the number of cigarettes smoked per day. The average (and SEM) COs were 21.2 (1.0), 16.7 (0.9), 12.1 (0.9), and 17.5 (1.0) ppm. A repeated measures ANOVA of the average CO level per day showed a significant effect of Condition, F(3, 13) = 21.40, p < .001. Bonferroni's post hoc contrasts showed a significant decrease in the average CO level per day in the voucher condition compared to baseline (p < .001), patch (p < .05), and return to baseline (p < .001); there was also a decrease from baseline compared to both patch (p < .05) and return to baseline (p < .05). A significant effect of Treatment Order, F(3, 13) = 8.79, p < .05, was found, suggesting that participants who received the voucher treatment first were more successful in decreasing their average CO level per day.
Figure 2. The average number of self-reported cigarettes per day (top), the average CO level per day (middle), and the average percentage of negative samples (bottom).

Each point represents data from an individual participant, and shaded bars represent condition means.
The percentage of samples meeting the abstinence criterion were 7.9% (5.8), 5.5% (4.3), 24.4% (10.1), and 12.1% (8.4) across the baseline, patch, voucher, and return to baseline phases, respectively. A repeated measures ANOVA on the percentage of abstinent samples revealed a significant effect of Condition, F(3, 13) = 3.74, p < .05, with an increase in the percentage of samples ≤4 ppm in the voucher phase compared to the patch phase (p < .05). No statistically significant difference was found for a treatment order effect.
A Pearson's correlation coefficient revealed a significant correlation between CO and self-reported number of cigarettes per day (r = .72). A Pearson's correlation coefficient did not reveal a significant correlation between participants' URICA scores and their CO values in the patch and voucher conditions.
A repeated measures ANOVA did not reveal any significant effects for Factor 1 or Factor 2 of the QSU. Table 2 presents the means and standard deviations for items from the WSQ that were found to be statistically significant. Irritability and frustration, F(3, 13) = 3.50, p < .05, and difficulty concentrating, F(3, 13) = 3.84, p < .05, showed a significant effect of Condition. Bonferroni's post hoc contrasts showed a significant increase in the patch condition as compared to the baseline condition (p < .05) for these two items. Anxious, F(3, 13) = 5.42, p < .01, depression or feeling blue, F(3, 13) = 3.26, p < .05, and desire for sweets, F(3, 13) = 4.23, p < .05, showed a significant effect of Condition, suggesting an increase in this measure for the patch phase compared to baseline, voucher, and return to baseline. Restlessness, F(3, 13) = 5.42, p < .01, showed a significant effect of Condition. Bonferroni's post hoc contrasts showed a significant increase in restlessness in the patch, voucher, and return to baseline phases compared to baseline (p < .05). Insomnia or disturbed sleep, F(3, 13) = 3.26, p < .05, showed a significant effect of Condition, with a linear increase across the baseline, patch, voucher, and return to baseline phases. A significant effect of Treatment Order was found for craving a cigarette or nicotine, F(3, 13) = 5.11, p < .05, suggesting an increase in craving a cigarette or nicotine in the group that experienced the voucher treatment first.
Table 2. Means and Standard Deviations for Statistically Significant Items from the Minnesota Nicotine Withdrawal Scale.
| Dependent variable | Condition |
|||
| Baseline | Patch | Voucher | Return to baseline | |
| Irritability/frustration/anger | 29.6 ± 27.2 | 50.4 ± 32.2 | 45.7 ± 29.6 | 39.8 ± 29.8 |
| Anxious | 31.0 ± 25.8 | 51.6 ± 29.9 | 47.0 ± 24.5 | 47.6 ± 30.0 |
| Difficulty concentrating | 28.8 ± 27.4 | 48.6 ± 34.5 | 40.8 ± 30.2 | 42.5 ± 35.2 |
| Restlessness | 23.0 ± 26.1 | 50.4 ± 29.2 | 49.1 ± 29.8 | 52.4 ± 28.9 |
| Insomnia/disturbed sleep | 30.3 ± 35.0 | 48.6 ± 32.2 | 49.2 ± 34.0 | 51.0 ± 35.0 |
| Depression/feeling blue | 18.8 ± 25.7 | 39.6 ± 27.4 | 28.8 ± 24.6 | 32.2 ± 34.2 |
| Desire for sweets | 23.6 ± 22.1 | 38.1 ± 28.7 | 41.8 ± 34.5 | 39.2 ± 32.0 |
Discussion
Relative to baseline, the contingent voucher treatment reduced CO levels and self-reported number of cigarettes smoked. These results are consistent with two studies that suggest that Internet-based abstinence reinforcement is feasible and effective in promoting smoking abstinence (Dallery & Glenn, 2005; Dallery et al., in press). Relative to baseline, the 14-mg transdermal nicotine patch did not produce significant changes in CO and reported number of cigarettes smoked. Overall, during the voucher treatment 24% of samples were negative and 5% were negative during the patch treatment.
In a previous study, Dallery and Glenn (2005) found that 60% of samples were negative during Internet-based abstinence reinforcement, whereas 24% met the criterion for abstinence in the present study. There are two procedural differences between the two studies. First, the current study did not incorporate a shaping phase in the contingent-voucher condition. Shaping refers to reinforcing successively closer approximations to the desired behavior (Galbicka, 1994). With smokers, this entails reinforcing successively lower CO readings (Dallery & Glenn, 2005; Dallery et al., in press; Lamb, Morral, Kirby, Iguchi, & Galbicka, 2004). Shaping could be a crucial step because if reinforcement is available only after the relatively large transition to abstinence, participants may not initially contact the reinforcer and therefore may not initiate abstinence (Lamb, Kirby, Morral, Galbicka, & Iguchi, 2004; Lamb, Morral, Kirby, Iguchi, & Galbicka, 2004). Therefore, it is possible that if the current study had contained a shaping phase, more participants might have initiated abstinence.
Second, the treatment duration was longer and the maximum amount of voucher earnings was higher in Dallery and Glenn (2005) than in the present study. Participants in Dallery and Glenn's study could earn $137.50 over 10 days in the contingent voucher phase, whereas participants in the current study could earn only $56.25 over 5 days in the contingent voucher phase. The shorter duration and the lower maximum earnings could have contributed to the modest effects noted in the current study. However, the schedule of voucher earnings was the same: The value of vouchers started at the same amount and increased according to the same schedule. Thus, if the voucher phase had been 10 days in the current study, participants could have earned an amount equivalent to that earned by participants in Dallery and Glenn's study. Nevertheless, it is possible that the duration and maximum amount that can be earned contribute to treatment success. Indeed, one study suggests that the reinforcing potency of cigarette smoking decreases with longer periods of abstinence produced by alternative sources of reinforcement (Lussier, Higgins, & Badger, 2005).
Another factor that should be considered in interpreting the present findings is that relatively heavy smokers were enrolled. In a similar study using an Internet-based treatment to decrease smoking, Dallery et al. (in press) found a correlation between the number of self-reported cigarettes per day at intake and CO during treatment. The more cigarettes a person smoked prior to treatment, the higher his or her CO was likely to be during treatment, suggesting that heavier smokers are less likely to quit than are lighter smokers. It may be that a higher reinforcer magnitude is necessary to produce changes in smoking for these smokers. Indeed, in studies of voucher reinforcement for other drugs of abuse (e.g., cocaine, heroin; Dallery et al., 2001; Silverman et al., 1999), relatively low magnitudes of voucher earnings produced smaller changes in drug use. When the magnitude was increased, greater gains were observed. Thus, it may be necessary to increase the voucher magnitude from the one used in the present study to promote cessation in heavy smokers.
The comparison between vouchers and transdermal nicotine patches in the present study employed a moderate (14-mg) dose of nicotine, which is commonly considered to be the middle dose in treatment plans. The manufacturers of nicotine patches recommend that people who smoke more than 10 cigarettes per day and wish to use the patch should use a 21-mg patch for 6 weeks followed by a 14-mg patch for 2 weeks, and then to use the 7-mg patch for 2 weeks (www.nicodermcq.com). Although it is possible that the dose of nicotine used in the current study was too low, it was deemed a good starting point for safety purposes (Kouri, Stull, & Lukas, 2001). Killen, Fortmann, Davis, Strausberg, and Varady (1999) determined that 15-mg and 25-mg transdermal nicotine patches were equally effective at reducing smoking by heavy smokers in an outpatient study. However, other research does support using higher dose patches (Daughton et al., 1999; Hughes et al., 1999; Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006). Just as the voucher magnitude could be increased, the maximally effective dose of nicotine replacement should be identified, particularly for heavy smokers (Shiffman et al.). The Internet-based method could provide a rigorous and convenient way to examine the effects of different doses of the transdermal nicotine patch, and to compare the maximally effective dose to contingent vouchers and the combination of vouchers and pharmacotherapy.
In addition to the CO data, participants self-reported smoking significantly fewer cigarettes in the contingent voucher phase than in baseline, patch phase, or return to baseline. There was an order effect such that participants who received the voucher treatment first smoked fewer cigarettes than did participants who received the patch treatment first. Of particular interest in Figure 1 are those instances either when participants reported smoking no cigarettes and yet submitted a positive sample (seven instances; ovals) or when participants reported smoking at least one cigarette and submitted a negative sample (four instances; rectangles). Several possibilities could account for the discrepancies. One possibility is that participants did not record how many cigarettes they smoked on each day, but instead recorded this information the next day or days later, and had forgotten whether or not they smoked. Alternatively, for participants who reported not smoking cigarettes but submitted a positive CO sample, these participants may have been exposed to high levels of ambient CO or may have smoked another substance such as marijuana.
Many treatment options are available for people who want to quit smoking; however, the costs of these treatments vary. For example, a 4-week supply of transdermal nicotine patches costs about $53.28 (Song et al., 2002), a 30-day supply of Nortriptyline (25 mg) plus associated doctor fees costs about $118.65, and a 30-day supply of Buproprion (150 mm SR) plus associated doctor fees costs about $277.44 (Hall et al., 2005). A 5-day supply of nicotine patches as used in the present study costs $13 (Ronckers, Groot, & Ament, 2005); however, patches are sold in 14-day kits. The maximum cost of the vouchers in the current study was $56.25, an amount similar to a 4-week supply of nicotine patches. It would be informative to equate the costs of each type of treatment over 4 weeks of treatment and examine their effects on smoking.
Cost is also one concern in considering an alternative biochemical measure of smoking status. Cotinine, a metabolite of nicotine that can be detected in urine or saliva, can provide a highly specific and sensitive assay for smoking status (Ahijevych, Tyndale, Dhatt, Weed, Browning, 2002; Hughes et al., 2003). Cotinine analysis, however, is relatively expensive and would require in-person visits for sample collection. Cotinine could be collected once per week to minimize costs (Ahijevych et al.), which may have advantages in certain settings, but the frequency of and delay to reinforcement may reduce effectiveness. A CO-based method increases the contiguity between behavior and reinforcement, which may be particularly important during shaping and the initiation of abstinence. In light of these considerations, it may be useful to transition from CO to cotinine (Higgins et al., 2004). New low-cost urinary dipstick techniques, although somewhat early in development, may prove to be useful (Parker et al., 2002). Nevertheless, for obvious reasons, cotinine cannot be used to measure smoking status in conjunction with nicotine replacement therapy.
The results suggest that contingent vouchers are more effective than transdermal nicotine patches in a group of heavy smokers (see also Wiseman et al., 2005). In comparison to previous studies (Dallery & Glenn, 2005; Dallery et al., in press), the results also serve to highlight features of voucher reinforcement treatment that may be critical for initiating abstinence. For example, the effects of shaping procedures, voucher magnitude, and treatment duration may warrant further assessment.
Acknowledgments
This research was supported by NIH Grant R21DA015289. We thank Steven Meredith for his help with data collection and Bethany Raiff, Darragh Devine, Tim Hackenberg, Julie Marusich, and Steven Meredith for their helpful suggestions on a previous version of this manuscript.
References
- Ahijevych K.L, Tyndale R.F, Dhatt R.K, Weed H.G, Browning K.K. Factors influencing cotinine half-life in African American and Caucasian women. Nicotine and Tobacco Research. 2002;4:423–431. doi: 10.1080/1462220021000018452. [DOI] [PubMed] [Google Scholar]
- Bansal M.A, Cummings M, Hyland A, Giovino G.A. Stop-smoking medications: Who uses them, who misuses them, and who is misinformed about them? Nicotine and Tobacco Research. 2004;6:S303–S310. doi: 10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- Bigelow G.E, Stitzer M.L, Griffiths R.R, Liebson I.A. Contingency management approaches to drug self-administration and drug abuse: Efficacy and limitations. Addictive Behaviors. 1981;6:241–252. doi: 10.1016/0306-4603(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Boudin H.M. Contingency contracting as a therapeutic tool in the deceleration of amphetamine use. Behavior Therapy. 1972;3:604–608. [Google Scholar]
- Budney A.J, Higgins S.T, Radonovich K.J, Novy P.L. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States 1995–1999. Morbidity and Mortality Weekly Report. 2002;51:300–303. [PubMed] [Google Scholar]
- Crowley T.J. Doctors' drug abuse reduced during contingency-contracting treatment. Alcohol and Drug Research. 1986;6:299–307. [PubMed] [Google Scholar]
- Crowley T.J, MacDonald M.J, Zerbe G.O, Petty T.L. Reinforcing breath carbon monoxide reductions in chronic obstructive pulmonary disease. Drug and Alcohol Dependence. 1991;29:47–62. doi: 10.1016/0376-8716(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Dallery J, Glenn I.M. Effects of an Internet-based voucher reinforcement program for smoking abstinence: A feasibility study. Journal of Applied Behavior Analysis. 2005;38:349–357. doi: 10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Glenn I.M, Raiff B.R. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence. in press doi: 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Dallery J, Silverman K, Chutuape M.A, Bigelow G.E, Stitzer M.L. Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcer magnitude. Experimental and Clinical Psychopharmacology. 2001;9:317–325. doi: 10.1037//1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- Daughton D.M, Fortmann S.P, Glover E.D, Hatsukami D.K, Heatley S.A, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post-quit day. Preventive Medicine. 1999;28:113–188. doi: 10.1006/pmed.1998.0391. [DOI] [PubMed] [Google Scholar]
- de Vries H, Brug J. Computer-tailored interventions motivating people to adopt health promoting behaviors: Introduction to a new approach. Patient Education and Counseling. 1999;36:99–105. doi: 10.1016/s0738-3991(98)00127-x. [DOI] [PubMed] [Google Scholar]
- Fiore M.C, Bailey W.C, Cohen S.J, Dorfman S.F, Goldstein M.G, Gritz E.R, et al. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 2000. [Google Scholar]
- Fiore M.C, Smith S.S, Jorenby D.E, Baker T.B. The effectiveness of the nicotine patch for smoking cessation. Journal of the American Medical Association. 1994;271:1940–1947. [PubMed] [Google Scholar]
- Galbicka G. Shaping in the 21st century: Moving percentile schedules into applied settings. Journal of Applied Behavior Analysis. 1994;27:739–760. doi: 10.1901/jaba.1994.27-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Vera M.P. Clinical utility of the combination of cognitive-behavioral techniques with nicotine patches as a smoking-cessation treatment: Five-year results of the “Ex-Smoker” program. Journal of Substance Abuse Treatment. 2004;27:325–333. doi: 10.1016/j.jsat.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hall S.M, Lightwood J.M, Humfleet G.L, Bostrom A, Reus V.I, Munoz R. Cost-effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services and Research. 2005;32:381–392. doi: 10.1007/BF02384199. [DOI] [PubMed] [Google Scholar]
- The health consequences of smoking—A report of the Surgeon General. 2004. Retrieved April 15, 2005, from www.cdc.gov/tobacco/sgr/sgr_2004/ [PubMed] [Google Scholar]
- Heatherton T.F, Kozlowski L.T, Frecker R.C, Fagerstrom K.O. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Delaney D.D, Budney A.J, Bickel W.K, Hughes J.R, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Heil S.H, Solomon L.J, Lussier J.P, Abel R.L, Lynch M.E, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine and Tobacco Research. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Hughes J.R. Pharmacotherapy for smoking cessation: Unvalidated assumptions, anomalies, and suggestions for future research. Journal of Consulting and Clinical Psychology. 1993;61:751–760. doi: 10.1037//0022-006x.61.5.751. [DOI] [PubMed] [Google Scholar]
- Hughes J.R, Hatsukami D.K. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes J.R, Keely J.P, Niaura R.S, Ossip-Klein D.J, Richmond R.L, Swan G.E. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine and Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Hughes J.R, Lesmes G.R, Hatsukami D.K, Richmond R.L, Lichtenstein E, Jorenby D, et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine and Tobacco Research. 1999;1:169–174. doi: 10.1080/14622299050011281. [DOI] [PubMed] [Google Scholar]
- Hunt G.M, Azrin N.H. A community-reinforcement approach to alcoholism. Behavior Research and Therapy. 1973;11:91–104. doi: 10.1016/0005-7967(73)90072-7. [DOI] [PubMed] [Google Scholar]
- Javors M.A, Hatch J.P, Lamb R.J. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Katz E.C, Chutuape M.A, Jones H.E, Stitzer M.L. Voucher reinforcement for heroin and cocaine abstinence in an outpatient drug-free program. Experimental and Clinical Psychopharmacology. 2002;10:136–142. doi: 10.1037//1064-1297.10.2.136. [DOI] [PubMed] [Google Scholar]
- Killen J.D, Fortmann S.P, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Experimental and Clinical Psychopharmacology. 1999;7:226–233. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- Kouri E.M, Stull M, Lukas S.E. Nicotine alters some of cocaine's subjective effects in the absence of physiological or pharmacokinetic changes. Pharmacology Biochemistry and Behavior. 2001;69:209–217. doi: 10.1016/s0091-3057(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Kirby K.C, Morral A.R, Galbicka G, Iguchi M.Y. Improving contingency management programs for addiction. Addictive Behaviors. 2004;29:507–523. doi: 10.1016/j.addbeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Morral A.R, Kirby K.C, Iguchi M.Y, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lussier J.P, Higgins S.T, Badger G.J. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology. 2005;181:486–495. doi: 10.1007/s00213-005-0008-5. [DOI] [PubMed] [Google Scholar]
- Middleton E.T. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117:758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- Parker D.R, Lasater T.M, Windsor R, Wilkins J, Upegui D.I, Heimdal J. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine and Tobacco Research. 2002;4:305–309. doi: 10.1080/14622200210142715. [DOI] [PubMed] [Google Scholar]
- Prochaska J, DiClemente C. Stages and process of self-change of smoking: Toward and integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Rand C.S, Stitzer M.L, Bigelow G.E, Mead A.M. The effects of contingent payment and frequent workplace monitoring on smoking abstinence. Addictive Behaviors. 1989;14:121–128. doi: 10.1016/0306-4603(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T, Badger G.J. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T, Steingard S, McGinley M. Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: A feasibility study. Experimental and Clinical Psychopharmacology. 1998;6:157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Ronckers E.T, Groot W, Ament A.J.H.A. Systematic review of economic evaluations of smoking cessation: Standardizing the cost-effectiveness. Medical Decision Making. 2005;25:437–448. doi: 10.1177/0272989X05278431. [DOI] [PubMed] [Google Scholar]
- Schnoll R.A, Rothman R.L, Wielt D.B, Lerman C, Pedri H, Wang H, et al. A randomized pilot study of cognitive-behavioral therapy versus basic health education for smoking cessation among cancer patients. Annals of Behavioral Medicine. 2005;30:1–11. doi: 10.1207/s15324796abm3001_1. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson S.G, Gwaltney C.J, Balabanis M.H, Shadel W.G. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Silverman K. Exploring the limits and utility of operant conditioning in the treatment of drug addiction. The Behavior Analyst. 2004;27:209–230. doi: 10.1007/BF03393181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Chutuape M.A, Bigelow G.E, Stitzer M.L. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins S.T, Brooner R.K, Montoya I.D, Cone E.J, Schuster C.R, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong C.J, Higgins S.T, Brooner R.K, Montoya I.D, Contoreggi C, et al. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug and Alcohol Dependence. 1996;41:157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- Song F, Raftery J, Aveyard P, Hyde C, Barton P, Woolacott N. Cost-effectiveness of pharmacological interventions for smoking cessation: A literature review and a decision analytic analysis. Medical Decision Making. 2002;22:S26–S37. doi: 10.1177/027298902237708. [DOI] [PubMed] [Google Scholar]
- Stitzer M.L, Bigelow G.E. Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addictive Behaviors. 1982;7:403–412. doi: 10.1016/0306-4603(82)90010-7. [DOI] [PubMed] [Google Scholar]
- Stitzer M.L, Bigelow G.E. Contingent reinforcement for reduced breath carbon monoxide levels: Target-specific effects on cigarette smoking. Addictive Behaviors. 1985;10:345–349. doi: 10.1016/0306-4603(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Stitzer M.L, Rand C.S, Bigelow G.E, Mead A.M. Contingent payment procedures for smoking reduction and cessation. Journal of Applied Behavior Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecher V.J. Computer-tailored smoking cessation materials: A review and discussion. Patient Education and Counseling. 1999;36:107–117. doi: 10.1016/s0738-3991(98)00128-1. [DOI] [PubMed] [Google Scholar]
- Swanson N.A, Burroughs C.C, Long M.A, Lee R.W. Controlled trial for smoking cessation in a Navy shipboard population using nicotine patch, sustained-release buproprion, or both. Military Medicine. 2003;168:830–834. [PubMed] [Google Scholar]
- Tidey J.W, O'Neill S.C, Higgins S.T. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology. 2002;10:241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Tiffany S.T, Drobes D.J. The development and initial validation of a questionnaire of smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Wiseman E.J, Williams D.K, McMillan D.E. Effectiveness of payment for reduced carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine-abusing outpatients wearing nicotine or placebo patches. Experimental and Clinical Psychopharmacology. 2005;13:102–110. doi: 10.1037/1064-1297.13.2.102. [DOI] [PubMed] [Google Scholar]


