Abstract
Cell repulsion responses to Eph receptor activation are linked to rapid actin cytoskeletal reorganizations, which in turn are partially mediated by Rho–ROCK (Rho kinase) signalling, driving actomyosin contractility. In the present study, we show that Rho alone is not sufficient for this repulsion response. Rather, Cdc42 (cell division cycle 42) and its effector MRCK (myotonic dystrophy kinase-related Cdc42-binding kinase) are also critical for ephrinB-induced cell retraction. Stimulation of endothelial cells with ephrinB2 triggers rapid, but transient, cell retraction. We show that, although membrane retraction is fully blocked by blebbistatin (a myosin-II ATPase inhibitor), it is only partially blocked by inhibiting Rho–ROCK signalling, suggesting that there is ROCK-independent signalling to actomyosin contractility downstream of EphBs. We find that a combination of either Cdc42 or MRCK inhibition with ROCK inhibition completely abolishes the repulsion response. Additionally, endocytosis of ephrin–Eph complexes is not required for initial cell retraction, but is essential for subsequent Rac-mediated re-spreading of cells. Our data reveal a complex interplay of Rho, Rac and Cdc42 in the process of EphB-mediated cell retraction–recovery responses.
Keywords: actomyosin contraction, cell division cycle 42 (Cdc42), EphB receptor, myotonic dystrophy kinase-related cell division cycle 42-binding kinase (MRCK), Rho kinase (ROCK)
Abbreviations: Cdc42, cell division cycle 42; HUVEC, human umbilical-vein endothelial cell; KD, kinase dead; MLC2, myosin light chain II; MRCK, myotonic dystrophy kinase-related Cdc42-binding kinase; NA, numerical aperture; p-Eph, phosphorylated Eph; ROCK, Rho kinase; TRITC, tetramethylrhodamine β-isothiocyanate
INTRODUCTION
Eph receptors and their cell-surface-anchored ligands, ephrins, regulate a wide variety of cell-migration-related processes in developing and adult tissues [1,2]. Signalling pathways triggered by Eph receptors and ephrins promote cell contact-dependent repulsion that prevents mixing of receptor- and ligand-expressing cells. The mechanisms underlying these cell repulsion responses are complex and not fully characterized, but are known to involve cell–cell detachment by loss of high-affinity Eph–ephrin interactions, either due to proteolytic cleavage [3] or by their internalization [4,5], and concomitant increased contractility of Eph-expressing cells resulting in the withdrawal of cell extensions [6–8]. Both of these component steps have been shown to involve activation of the Rho family GTPases, Rho (contractility) and Rac (Eph endocytosis), by Eph receptor recruitment and activation of GEFs (guanine-nucleotide-exchange factors), ephexin1 and Vav2 [9–11].
Although Rho, acting through ROCK (Rho kinase) and MYPT1 (myosin phosphatase target subunit 1) phosphorylation, was initially established as the Rho GTPase family member that was associated with actomyosin contractility and cell retraction episodes [12], it has been demonstrated recently that another Rho family GTPase, Cdc42 (cell division cycle 42), acting through MRCK (myotonic dystrophy kinase-related Cdc42-binding kinase), can also drive cell contractility [13,14] and, indeed, co-operates with Rho–ROCK signalling in mediating cell invasion [15]. Cdc42 has been shown to be activated by Eph receptors, but, rather than being linked to contractility, is proposed to regulate actin polymerization during dendritic spine morphogenesis [16]. Rac also appears not to regulate cell contractility downstream of Eph receptors, but it, and its exchange factor Vav2, mediate repulsion responses to ephrins by regulating endocytosis of ephrin–Eph complexes [4,11].
In the present study, we have investigated how Rho family GTPases and ephrin–Eph endocytosis regulate endothelial cell retraction and subsequent membrane re-spreading triggered by ephrinB2. We show that the retraction response is entirely dependent on myosin-II ATPase activity and, in line with previous studies, is only partially dependent on Rho–ROCK signalling [6,7]. Microinjection of dominant-negative constructs reveals that Cdc42–MRCK signalling plays a critical role in endothelial cell contractility triggered by ephrinB2 and, in combination with ROCK inhibition, can entirely prevent Eph-mediated endothelial cell retraction. Interestingly, we find that blocking endocytosis of ephrin–Eph complexes, through expression of dominant-negative dynamin, had no effect on actomyosin-driven cell retraction; rather, it inhibits cell re-spreading, suggesting that removal of surface-active Eph receptors is required to terminate actomyosin contractility signals before cell recovery from repulsion responses. Rac does not appear to play a role in the retraction event, but is necessary for the recovery of cells to a fully spread morphology. These findings suggest an important role for Cdc42 in repulsive signalling responses to activated Ephs.
MATERIALS AND METHODS
Reagents and expression plasmids
Antibodies for cell staining and Western blotting were anti-c-Myc (9E10, Serotec, dilution 1:200), anti-dynamin (HUDY1, Upstate Biotechnology, dilution 1:50), anti-FLAG (M2, Sigma, dilution 1:50), anti-tubulin (YL1/2, Serotec, dilution 1:10000), anti-phospho-MLC2 (myosin light chain II) (Ser19, New England Biolabs, dilution 1:1000) and anti-MLC2 (FL-172, Santa Cruz Biotechnology, dilution 1:500). Anti-phospho-Eph receptor was as described previously [4]. All secondary antibodies were from Jackson ImmunoResearch. All reagents were purchased from Sigma unless otherwise stated.
The following constructs were received as gifts: pcDNA3-dynamin1 (K44A Dyn; Dr J. M. Edwardson, Department of Pharmacology, University of Cambridge, Cambridge, U.K.) and pEfFLAG-CDC42BPA-isobK106M-FL [MRCKα KD (kinase-dead); Dr C. J. Marshall, Cancer Research UK, London, U.K.].
Cell culture and microinjection
HUVECs (human umbilical-vein endothelial cells) (TCS Cell Works) were cultured as described previously [4] and were used at 50–80% confluence. Cell nuclei were microinjected with DNA expression constructs (100–300 μg/ml) and an injection marker [FITC-, TRITC (tetramethylrhodamine β-isothiocyanate)- or biotin-conjugated lysinated dextrans (Molecular Probes)] and cells were allowed 1.5–4 h to express recombinant protein as indicated.
Cells were stimulated with 1 μg/ml ephrinB2–Fc (R+D Systems) or 1 μg/ml Fc (R+D Systems) pre-clustered (20 min) with 5 μg/ml goat anti-human IgG (Stratech). Cells were incubated with 100 μM blebbistatin (Tocris) for 30 min, or with Y-27632 (Calbiochem) for 2 h, before, and also during, stimulation.
Time-lapse movies were made using an inverted microscope (Zeiss Axiovert 200M) with a Zeiss 40× 0.6 NA (numerical aperture) lens, a digital camera (C4742-95, Hamamatsu) and Openlab (Improvision) software. Cells were maintained at 37 °C with 5% CO2 during recording.
Immunocytochemistry
Cultured cells were fixed, permeabilized and quenched as described previously [4] and incubated with primary antibodies for 45 min, followed by appropriate secondary antibodies for 25 min. TRITC- or FITC-conjugated phalloidin was used to stain F-actin (filamentous actin) when required. All antibody dilutions were in PBS and incubations were at room temperature (22 °C). Confocal images were collected using a Leica TCS-NT confocal laser-scanning microscope attached to a Leica DM IRBE inverted microscope with a HCX 63× 1.32 NA oil-immersion lens using Leica confocal software and were subsequently handled with Adobe Photoshop.
To examine internalization of ephrin–Eph complexes, two protocols were used. (i) After fixation, cells were incubated with anti-goat-Cy3 antibodies to label external IgG–ephrinB2–Eph complexes. Cells were then washed, post-fixed, permeabilized and incubated with anti-(goat FITC) antibodies to label internalized IgG–ephrinB2–Fc. (ii) Alternatively, cells were acid-washed twice for 5 min at 4 °C in DMEM (Dulbecco's modified Eagle's medium) (Gibco) containing 0.2% (w/v) BSA, 10 mM Mes and 1 mM sodium pyruvate, pH 2, before fixation to remove any externally bound ephrinB2–Fc before immunostaining.
Biotinylation and immunoblotting
The biotinylation method used was as described previously [17]. Briefly, following biotinylation of cell surfaces at 4 °C for 10 min using EZ-Link NHS (N-hydroxysuccinimido)-SS-biotin (Pierce), cells were maintained at 4 °C, and incubated in a Tris/amine buffer (25 mM Tris/HCl, 137 mM NaCl, 5 mM KCl, 2.3 mM CaCl2, 0.5 mM MgCl2 and 0.143 g/l Na2HPO4, pH 7.4) for 10 min. Cells were stimulated as normal at 37 °C, and, after the required internalization time, any non-internalized biotin was removed from cell surfaces using a glutathione solution (75 mM NaCl, 75 mM NaOH, 1 mM EDTA, 0.1% BSA and 50 mM glutathione, pH 9) twice for 20 min, which was neutralized by washing with a Tris/glycine buffer (0.192 M glycine and 25 mM Tris/HCl, pH 7.4). Cells were washed several times in TBS (Tris-buffered saline: 20 mM Tris/HCl and 150 mM NaCl, pH 7.4) before lysates were prepared in a modified RIPA buffer [125 mM NaCl, 20 mM Tris/HCl, 10% (v/v) glycerol, 1% (v/v) Nonidet P40, 50 mM NaF, 200 mM vanadate, 100 μg/ml PMSF and Complete™ protease inhibitor cocktail (Roche)]. Biotinylated proteins were precipitated using streptavidin-conjugated agarose beads (Upstate Biotechnology) and were resuspended in Laemmli loading buffer and analysed by SDS/PAGE (??% gels) and Western blotting.
Analysis of results
ImageJ (version 1.33U; public domain software available from http://rsb.info.nih.gov/ij) was used to quantify results from Western blots. All graphs were drawn using Microsoft Excel.
RESULTS AND DISCUSSION
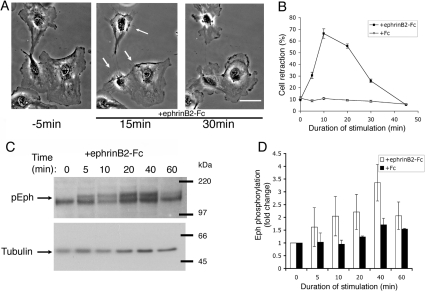
Previous studies have shown that ephrin-induced cell retraction and growth cone collapse is only partially blocked by inhibitors of Rho GTPase and its effector ROCK [6,7,18], suggesting that additional parallel pathways regulate this component of the repulsion response. To investigate signalling pathways underlying ephrin-regulated cell retraction, we stimulated HUVECs with soluble pre-clustered ephrinB2–Fc. We found that non-confluent HUVECs display a dynamic reproducible retraction–recovery response to ephrinB2–Fc exposure within minutes (Figure 1A and Supplementary Movie 1 at http://www.BiochemJ.org/bj/404/bj4040023add.htm). Cells rapidly undergo plasma membrane retraction (within 10 min), but then subsequently, despite the continued presence of ephrin, recover to a spread state (within 35 min) by lamellipodial growth along the retraction fibres left behind (Figures 1A and 1B). These early changes in cell morphology correlate with the initial phase of activation of Eph receptors as revealed by phosphorylation of Eph juxtamembrane tyrosine residues (Figures 1C and 1D). Interestingly, although increased phosphorylation was observed after 5 min, maximal phosphorylation of Eph receptors was not until 40 min after stimulation (Figures 1C and 1D), by which time most cells had recovered from their retracted state. This suggests that dephosphorylation of Eph receptors at the juxtamembrane sites is not a driver of, or even required for, cell re-spreading.
Figure 1. Retraction–recovery response of HUVECs to ephrinB2–Fc.
(A) Time-lapse movie stills (pre-clustered ephrinB2–Fc added at 0 min; arrows indicate regions of cell retraction; see also Supplemental Movie S1 at http://www.BiochemJ.org/bj/404/bj4040023add.htm). Scale bar, 30 μm. (B) Cell retraction in response to pre-clustered ephrinB2–Fc or control Fc (n=5, >100 cells/condition). Molecular masses are given in kDa. (C, D) Time course of phosphorylation of Eph receptors (pEph) in response to ephrinB2–Fc. Results are means±S.E.M. (n=3).
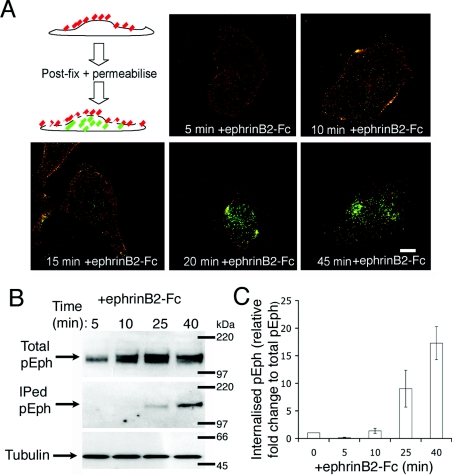
Since the switch from retraction to re-spreading appears not to involve deactivation of Eph receptors, we investigated whether it requires Eph receptor internalization. Previously, it has been shown that removal of ephrinB–EphB complexes at cell–cell contacts, through their internalization, is required for cell separation during the repulsion response [4,5]. More recent evidence suggests that endocytosis of EphA receptors may direct, rather than simply facilitate, ephrin-triggered repulsion responses, since Vav2 both regulates endocytosis of ephrin–Eph complexes and is necessary for growth cone collapse [11]. Using both immunocytochemistry and biochemical techniques, we have determined the temporal relationship between ephrin–Eph complex internalization and membrane retraction–recovery events. HUVECs were stimulated with IgG-pre-clustered ephrinB2–Fc for various times as indicated, fixed in the absence of detergents and immunostained for ephrinB2–Fc/IgG on the cell surface (red staining). Cells were then post-fixed, permeabilized and stained for internalized ephrinB2–Fc/IgG (green staining). Ephrin–Eph complex endocytosis is relatively slow compared with the cell-retraction response, and most internalized ephrin/IgG appears approx. 20–45 min after initial exposure to ephrinB2–Fc (Figure 2A). These data are corroborated by surface biotinylation studies that also show the time course of phosphorylated Eph (p-Eph) internalization (Figures 2B and 2C). In these experiments, all surface proteins were biotinylated for 10 min at 4 °C, washed and returned to 37 °C for various times in the presence of ephrinB2–Fc to allow endocytosis. Biotinylated proteins remaining on the surface after incubation were debiotinylated by incubating with glutathione buffer at 4 °C leaving only the endocytosed proteins biotinylated. The amount of biotinylated p-Eph was determined by binding cell lysate to streptavidin–agarose beads and Western blotting with anti-p-Eph. These experiments show that very little p-Eph is internalized within 10 min of ephrinB2 stimulation (Figures 2B and 2C), the time at which we observe maximal cell retraction (Figure 1B). Moreover, most internalization of activated Eph receptors is between 25 and 40 min after ephrinB2 exposure when we observe withdrawn lamellipodia subsequently extending along retraction fibres. These experiments address the temporal relationship between ephrinB2-triggered repulsive signalling and endocytosis of activated Eph receptors. They show that internalization of activated Eph receptors coincides with the recovery of retracted cells rather than the retraction phase itself.
Figure 2. EphB–ephrinB2 internalization coincides with cell recovery, and not with cell retraction.
(A) The schematic diagram shows extracellular ephrinB2–Fc (red) and intracellular ephrinB2–Fc (green). Confocal images show that most ephrinB2–Fc internalization occurs between 20 and 45 min. Scale bar, 20 μm. (B, C) Surface biotinylation experiments to assay the time course of internalization of active Eph receptors. Results are means±S.E.M. (n=3). Molecular masses are given in kDa. IPed, immunoprecipitated.
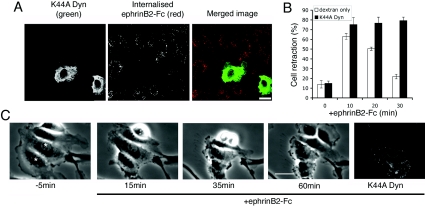
Next we tested whether the cell re-spreading response is dependent on internalization of ephrin–Eph complexes by expressing a dominant-negative mutant form of dynamin, dynamin-1 (K44A). We show that expression of dominant-negative dynamin inhibits internalization of soluble ephrinB2 (Figure 3A). Importantly, blocking dynamin activity had no effect on cell retraction triggered by ephrinB2, but did abrogate subsequent cell re-spreading (Figures 3B and 3C). Time-lapse microscopy shows that dominant-negative-dynamin-expressing cells remain contracted for up to 60 min after stimulation with ephrinB2–Fc (Figure 3C and Supplementary Movie 2 at http://www.BiochemJ.org/bj/404/bj4040023add.htm). The movie demonstrates, however, that contracted cells are able to produce small, localized ruffles but were unable to re-spread lamellipodia. In order to control whether dominant-negative dynamin may be acting by inhibiting actin filament polymerization required for cell spreading, we tested whether dynamin-1 (K44A) interfered with lamellipodial extension after cell trypsinization, and found that dynamin-1 (K44A)-expressing cells adhere and spread by extending lamellipodia similarly to uninjected controls after re-plating (results not shown). These experiments show that endocytosis of ephrin–Eph complexes is not necessary for ephrinB2-induced cell retraction in HUVECs, but rather is required for the subsequent recovery of cells to a fully spread morphology. In line with our results, Mann et al. [19] showed that internalization of EphB receptors is not necessary for collapse of Xenopus retinal growth cones. In contrast, Vav2-dependent internalization of EphA receptors is necessary for collapse of retinal growth cones [11], indicating that there may be differences between EphB and EphA signalling pathways that control internalization and retraction in ways that are not cell-type-specific.
Figure 3. Internalization of ephrinB2–Fc is necessary for cell re-spreading.
(A) Cells expressing K44A Dyn (green) do not internalize EphB–ephrinB2 complexes (red); acid-washing the cells immediately before fixation removed external ephrinB2–Fc, therefore red staining represents internalized ephrinB2–Fc. (B) EphrinB2-triggered retraction in cells injected with K44A Dyn, or with control injection marker (dextran). Results are means±S.E.M. (n=3–5, >30 cells/condition). (C) Time-lapse stills showing that K44A Dyn-injected cells do not re-spread lamellipodia after ephrinB2–Fc stimulation (see Supplemental Movie S2 at http://www.BiochemJ.org/bj/404/bj4040023add.htm). Scale bars, 20 μm (A), 30 μm (B).
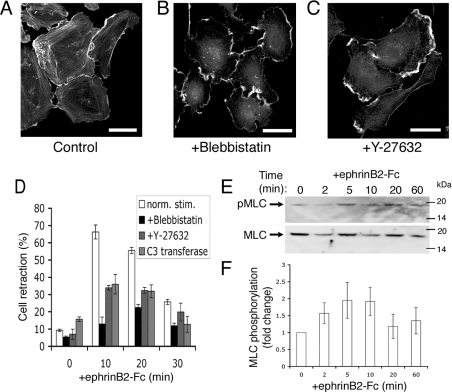
Many cellular contraction events are actomyosin-dependent and can be blocked by the pharmacological agent blebbistatin, which inhibits myosin-II ATPase activity [20,21]. HUVECs treated with 100 μM blebbistatin lose their stress fibres but remain well spread with membrane ruffles (Figures 4A and 4B). At 10 min after ephrinB2–Fc stimulation, when we generally see most cell retraction, blebbistatin completely blocked the retraction response (Figure 4D). These data demonstrate that rapid retraction triggered by ephrinB2 is strictly dependent on myosin activity and increased actomyosin contractility. We next tested the effect of inhibiting Rho–ROCK signalling on ephrin-induced endothelial cell retraction. Inhibition of ROCK with 10 μM Y-27632 had the predicted effect of removing actin stress fibres (Figure 4C), as did expression of C3 transferase (results not shown). In line with previous studies of ephrin-induced collapse of retinal ganglion cell growth cones and rounding of PC-3M cells [6,7,18], endothelial cell retraction was only partially inhibited by Y-27632 or C3 transferase. Pre-treatment of cells for 2 h with 10 μM Y-27632 or microinjection of 20 μg/ml C3 transferase (2 h expression time) attenuated the retraction response by approx. 50% (Figure 4D) compared with a greater than 90% suppression by blebbistatin. The blebbistatin-sensitive, but Y-27632/C3-insensitive, component of ephrin-triggered cell contractility was most clear at 10 min (Figure 4D). Actomyosin-driven cell retraction involves phosphorylation of MLC2 [22]. We found that MLC2 was phosphorylated 2 min after stimulation of cells with ephrinB2, reached a peak at 5–10 min, and then returned to control levels at 20–60 min (Figures 4E and 4F). Taken together, these results show that endothelial cell retraction rapidly triggered by ephrinB2–Fc is strictly actomyosin-dependent, but that there is Rho/ROCK-independent signalling to this actomyosin contractility.
Figure 4. EphrinB2–Fc-induced retraction is myosin II-dependent, but only partially blocked by Rho/ROCK inhibition.
(A–C) Treatment of HUVECs with 100 μM blebbistatin or 10 μM Y-27632 results in the loss of actin stress fibres. Scale bars, 40 μm. (D) Quantification of cellular retraction in response to ephrinB2–Fc in the presence of blebbistatin and Y-27632 (n=3, >100 cells/time point) and in cells injected with C3 transferase (n=8, >40 cells/experiment). norm. stim., normal stimulation. (E, F) Phosphorylation of MLC2 during the retraction response. Molecular masses are given in kDa. Results are means±S.E.M. (n=6).
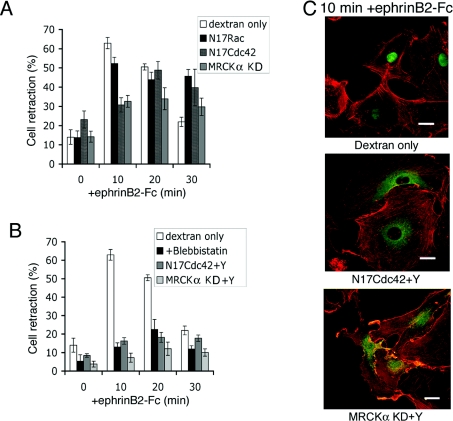
Next we investigated the involvement of other Rho family GTPases in endothelial cell retraction triggered by ephrinB2. We injected dominant-negative expression constructs (pRK5mycN17Rac and pRK5mycN17Cdc42) to inhibit the activity of Rac and Cdc42 and again examined the response to pre-clustered ephrinB2–Fc stimulation. Expression of dominant-negative Rac barely altered the cell-retraction phase, but cells remained retracted and did not subsequently re-spread (Figure 5A). A sustained retraction phase is not unexpected because Rac-regulated lamellipodia extension is known to be required for cell spreading from a rounded morphology [23]. In contrast, expression of N17Cdc42 significantly reduced the retraction response. At 10 min after ephrinB2 exposure, when control injected cells displayed maximum retraction, expression of N17Cdc42 attenuated the retraction response by approx. 50%. However, by 20 min, the retraction response of N17Cdc42-injected cells was similar to control-injected cells, suggesting that Cdc42 signalling to actomyosin contraction is a component of the initial phase of Eph signalling to cell contractility. Recent studies have demonstrated that Cdc42, like Rho, can regulate actomyosin contractility, through its effector MRCK [24], to regulate cell invasion and nuclear movements during cell polarization [15,25]. MRCK, like ROCK, phosphorylates MLC2. We have shown that expression of dominant-negative MRCK (MRCKα KD) inhibits cell retraction (Figure 5A). To determine whether the Cdc42–MRCK signalling pathway may interact in a parallel non-redundant role with Rho–ROCK signalling to regulate the cell retraction response, we combined N17Cdc42 and MRCKα KD microinjection experiments with the addition of Y-27632 and, in both cases, this resulted in the inhibition of retraction levels to those obtained with blebbistatin (Figures 5B and 5C). Taken together, these data imply that Rho–ROCK and Cdc42–MRCK signalling pathways play complementary/additive roles in the retraction of HUVECs in response to EphB activation.
Figure 5. Cdc42 and Rho pathways co-operate in ephrinB2–Fc-induced retraction.
(A) Time course of ephrinB2-induced retraction in cells injected with injection marker (dextran), N17Rac, N17Cdc42 or MRCKα KD (n=3–7). (B) Quantification of the combined effects of N17Cdc42 with Y-27632 or MRCKα KD with Y-27632 (n=7) on retraction induced by ephrinB2–Fc compared with injection marker and cells pre-treated with blebbistatin. Results are means±S.E.M. (C) Representative images of cells (actin in red) injected with either N17Cdc42 (middle panel) or MRCKα KD (bottom panel) and incubated with 10 μM Y-27632 as compared with FITC–dextran-injected cell (top panel). Shown green is the expression marker (top panel), anti-myc or anti-FLAG (in the middle and bottom panels). Scale bars, 20 μm.
In summary, the data presented demonstrate that Rho and Cdc42 function in concert to mediate ephrinB2-induced cell retraction. While blocking Rho–ROCK signalling with C3 transferase and Y-27632 and Cdc42–MRCK signalling with dominant-negative constructs, only partially inhibited ephrin-triggered cell contractility, blocking both signalling pathways mirrored the absolute inhibition achieved with blebbistatin, a myosin II ATPase inhibitor. The recovery to a fully spread morphology, on the other hand, requires functioning Rac and the internalization of ephrin–Eph complexes. The removal of activated Eph receptors from the membrane may be vital to enable cells to reset their signalling machinery and to continue migrating after a temporary Eph-mediated contact-inhibition response.
Multimedia
Acknowledgments
We thank Chris Marshall, Mike Olson and Stefan Martin for reagents and discussion, Iwan Evans for help with the manuscript preparation and Paul Martin for comments on the manuscript. This work was funded by the Medical Research Council. C. D. N. is an MRC Senior Research Fellow.
References
- 1.Poliakov A., Cotrina M., Wilkinson D. G. Diverse roles of Eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Pasquale E. B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 3.Hattori M., Osterfield M., Flanagan J. G. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 4.Marston D. J., Dickinson S., Nobes C. D. Rac-dependent trans-endocytosis of ephrinBs regulates Eph–ephrin contact repulsion. Nat. Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 5.Zimmer M., Palmer A., Kohler J., Klein R. EphB–ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 6.Wahl S., Barth H., Ciossek T., Aktories K., Mueller B. K. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Q., Sasaki Y., Shoji M., Sugiyama Y., Tanaka H., Nakayama T., Mizuki N., Nakamura F., Takei K., Goshima Y. Cdk5/p35 and Rho-kinase mediate ephrin-A5-induced signaling in retinal ganglion cells. Mol. Cell. Neurosci. 2003;24:632–645. doi: 10.1016/s1044-7431(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 8.Riedl J., Brandt D., Batlle E., Price L., Clevers H., Bos J. Downregulation of Rap1 activity is involved in ephrinB1-induced cell contraction. Biochem. J. 2005;389:465–469. doi: 10.1042/BJ20050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shamah S. M., Lin M. Z., Goldberg J. L., Estrach S., Sahin M., Hu L., Bazalakova M., Neve R. L., Corfas G., Debant A., Greenberg M. E. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;10:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 10.Sahin M., Greer P. L., Lin M. Z., Poucher H., Eberhart J., Schmidt S., Wright T. M., Shamah S. M., O'Connell S., Cowan C. W., et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Cowan C. W., Shao Y. R., Sahin M., Shamah S. M., Lin M. Z., Greer P. L., Gao S., Griffith E. C., Brugge J. S., Greenberg M. E. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Riento K., Ridley A. J. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 13.Dong J. M., Leung T., Manser E., Lim L. Cdc42 antagonizes inductive action of cAMP on cell shape, via effects of the myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) on myosin light chain phosphorylation. Eur. J. Cell Biol. 2002;81:231–242. doi: 10.1078/0171-9335-00238. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z. S., Manser E. PAK and other Rho-associated kinases: effectors with surprisingly diverse mechanisms of regulation. Biochem. J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson S., Paterson H. F., Marshall C. J. Cdc42–MRCK and Rho–ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat. Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 16.Irie F., Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat. Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- 17.Martin S., Henley J. M. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J. 2004;23:4749–4759. doi: 10.1038/sj.emboj.7600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harbott L. K., Nobes C. D. A key role for Abl family kinases in EphA receptor-mediated growth cone collapse. Mol. Cell. Neurosci. 2005;30:1–11. doi: 10.1016/j.mcn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Mann F., Miranda E., Weinl C., Harmer E., Holt C. E. B-type Eph receptors and ephrins induce growth cone collapse through distinct intracellular pathways. J. Neurobiol. 2003;57:323–336. doi: 10.1002/neu.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs M., Toth J., Hetenyi C., Malnasi-Csizmadia A., Sellers J. R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 21.Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 22.Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 23.Price L. S., Leng J., Schwartz M. A., Bokoch G. M. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung T., Chen X. Q., Tan I., Manser E., Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol. Cell. Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes E. R., Jani S., Gundersen G. G. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.