Abstract
Neurogranin (Ng) is a 78-amino-acid-long protein concentrated at dendritic spines of forebrain neurons that is involved in synaptic plasticity through the regulation of CaM (calmodulin)-mediated signalling. Ng features a central IQ motif that mediates binding to CaM and is phosphorylated by PKC (protein kinase C). We have analysed the subcellular distribution of Ng and found that it associates to cellular membranes in rat brain. In vitro binding assays revealed that Ng selectively binds to PA (phosphatidic acid) and that this interaction is prevented by CaM and PKC phosphorylation. Using the peptide Ng-(29–47) and a mutant with an internal deletion (Ng-IQless), we have shown that Ng binding to PA and to cellular membranes is mediated by its IQ motif. Ng expressed in NIH-3T3 cells accumulates at peripheral regions of the plasma membrane and localizes at intracellular vesicles that can be clearly visualized following saponin permeabilization. This distribution was affected by PLD (phospholipase D) and PIP5K (phosphatidylinositol 4-phosphate 5-kinase) overexpression. Based on these results, we propose that Ng binding to PA may be involved in Ng accumulation at dendritic spines and that Ng could modulate PA signalling in the postsynaptic environment.
Abbreviations: CaM, calmodulin; CaMKII, Ca2+/CaM-dependent protein kinase II; DGK, diacylglycerol kinase; DIG, detergent-insoluble glycosphingolipid and cholesterol-rich complex; GAP-43, growth-associated protein of 43 kDa; GFP, green fluorescent protein; HRP, horseradish peroxidase; IF, immunofluorescence; LTD, long-term depression; LTP, long-term potentiation; NA, numerical aperture; Ng, neurogranin; PA, phosphatidic acid; PBS-S, PBS containing 0.2% (w/v) saponin; PC, phosphatidylcholine; PIP5K, phosphatidylinositol 4-phosphate 5-kinase; PKC, protein kinase C; PLD, phospholipase D; PMRSS, plasma membrane ring-shaped structure; PP1, protein phosphatase 1; TBST, Tris-buffered saline containing 0.1% (w/v) Tween 20
Keywords: calmodulin; neurogranin; phosphatidic acid; phosphatidylinositol 4,5-bisphosphate; phospholipase D (PLD); protein kinase C (PKC)
INTRODUCTION
Neurogranin (Ng) (also named RC3, p17 or BICK) is a small protein originally identified in rat brain [1–3] that is abundantly expressed in the cerebral cortex, hippocampus, amygdala and basal ganglia. Ng expression develops postnatally in most areas, and peaks during the second and third postnatal week in rodents [4], coincident with periods of active dendrite branching, synaptogenesis and the maturation of synaptic plasticity signalling cascades [5]. Ng binds CaM (calmodulin) in the absence or at low levels of Ca2+ [6] and is phosphorylated by PKC (protein kinase C) [1]. CaM binding and PKC phosphorylation occur at a central region of the molecule that shows high homology with the IQ motif present in GAP-43 (growth-associated protein of 43 kDa), an axonal protein abundantly expressed in developing and regenerating neurons [7]. CaM binding and PKC phosphorylation at the IQ motif are mutually exclusive events.
Ng-knockout mice show severe deficits in performing hippocampus-dependent tasks [8,9]. It has been proposed that these cognitive deficits are caused by the attenuation of Ca2+ and Ca2+/CaM-mediated signalling [10] according to the following scheme. In the postsynaptic environment of resting neurons, Ng would be bound to CaM. Following excitatory stimulation, a rise in Ca2+ levels and PKC activation would release CaM from Ng stores to participate in Ca2+/CaM-mediated pathways. In this way, the amount of CaM available locally for signalling purposes would be drastically increased after stimulation. Actually, mice lacking Ng have lower amounts of phosphorylated CaMKII (Ca2+/CaM-dependent protein kinase II) in their brains [8,11], suggesting an attenuated signalling through this pathway. Also, they show a decreased response of PKA (protein kinase A) and PKC to excitatory stimulation [12], which would most probably be related to the marked anomalies found in these mice for LTP (long-term potentiation)–LTD (long-term depression) induction [13].
During the last two decades, it has become evident that cellular lipids not only form bilayers to separate compartments, but also participate in signal transduction and events that lead to cellular polarization, endocytosis, exocytosis, etc. Phosphoinositides have received major attention at this respect, but other phospholipids such as PA (phosphatidic acid) are quickly emerging as signalling molecules [14]. The spatiotemporal distribution of cellular PA is tightly regulated by localized synthesis, mediated by PLDs (phospholipases D), DGKs (diacylglycerol kinases) or acyltransferases, and quick and efficient degradation catalysed by lipid phosphate phosphatases [15]. PA participates in a variety of signalling events. It activates PIP5K (phosphoinositide 4-phosphate 5-kinase) [16] or the mTOR (mammalian target of rapamycin) [17] and inhibits PP1 (protein phosphatase 1) [18]. It can also act as a binding partner for protein translocation and recruitment, as in the case of Raf-1 kinase [19], and has been shown to be important in cytoskeleton remodelling and vesicle trafficking.
Previous immunocytochemical work has established that Ng localizes to neuronal cell bodies and dendrites, distributes in multiple small aggregates (hence its name, neurogranin) and concentrates at dendritic spines [20–23]. Ng sorting in the somatodendritic compartment can be adequately explained based on the dendritic targeting of its mRNA [24,25] and regulated local translation [26]. However, its characteristic distribution in small aggregates cannot be solely justified by its interaction with CaM. In the present work, we have analysed in detail the subcellular localization of Ng and found that substantial amounts of Ng associate to membranes. A search for interacting partners has revealed that Ng selectively binds to PA, and that free CaM or PKC phosphorylation prevents this interaction. Using an optimized immunostaining method, we have shown that Ng concentrates in peripheral and presumptively highly dynamic regions of the plasma membrane and that this localization is altered after experimental manipulation of cellular PA production and distribution. The relevance of the interaction between Ng and PA is discussed in the context of synaptic plasticity.
EXPERIMENTAL
Antibodies, peptides and recombinant proteins
Polyclonal antibodies against Ng were developed in rabbits using the peptide Ng-(9–25) covalently linked to KLH (keyhole-limpet haemocyanin) (Imject KLH, Pierce) (Ab205) or recombinant rat Ng (Ab756) as immunogens. Affinity-purified antibodies were obtained from clarified immune sera using affi-gel resins (Bio-Rad) coupled to the relevant immunogens. Polyclonal antibody against GAP-43 (Ab797) was raised in rabbits against recombinant rat GAP-43 and affinity-purified as described above. Monoclonal mouse antibody to CaM was kindly provided by Dr J.M. McDonald (University of Alabama, Birmingham, AL, U.S.A.). Other antibodies are commercially available. The peptides biotin–AAKIQASFRGHMARKKIK and biotin–AAKIQAS(P)FRGHMARKKIK (phosphoserine version), corresponding to Ng-(29–47), were synthesized in an Applied Biosystems peptide synthesizer (model 431A) using Fmoc (fluoren-9-ylmethoxycarbonyl) chemistry, purified by gel-filtration and HPLC and verified by MS. Recombinant rat Ng and GAP-43 were prepared as described in [27]. cDNAs of Ng mutants were subcloned into pGEX-2T (Pharmacia) and expressed in Rosetta Competent Cells (Stratagene). Recombinant Ng mutants were purified using glutathione–agarose affinity chromatography and thrombin digestion.
Immunocytochemistry
Rats were deeply anaesthetized by intraperitoneal administration of a mixture of ketamine, valium and atropine (5:4:1, by vol.) and transcardially perfused with PBS for 10 min and then with cold 4% paraformaldehyde in PBS for 30 min. Brain pieces were post-fixed overnight in the same fixative and 100 μm sections were cut on a vibratome. Endogenous peroxidase activity was blocked with 0.1% H2O2 in 0.12 M phosphate buffer, pH 7.3, for 20 min, and free-floating sections were successively incubated in (i) 5% heat-inactivated horse serum and 0.2% (w/v) saponin in 0.12 M phosphate buffer, pH 7.3, for 2 h, (ii) affinity-purified anti-Ng antibody (1:500) in the same buffer overnight, (iii) biotinylated goat anti-rabbit IgG (1:200) for 2 h, and (iv) ABC reagent (Vector) for 1 h. Immunostaining was visualized using DAB (3,3′-diaminobenzidine) (Sigma) and H2O2 in 0.12 M phosphate buffer, pH 7.3. Immunostained sections were either dehydrated and mounted with Eukitt (Merck) for wide-field light microscopy or enhanced with osmium tetroxide and cut in an ultramicrotome to obtain semithin sections (1–2 μm thick) that were observed under an optical microscope and cut further to obtain ultrathin sections (60–100 nm thick) that were analysed at the electron microscope (JEOL JEM1010), according to established methods [28]. Images were captured with a Photometrics Coolsnap FX digital camera attached to a Zeiss Axiovert 200M microscope or, for electron microscopy, with a Gatan BioScan camera.
Ng–CaM binding assay
Serial dilutions of purified recombinant Ng or mutants (250 ng–10 μg) were prepared in a volume of 100 μl containing 12 mM Tris/HCl, pH 7.0, 75 mM NaCl, 1 mM EGTA, 2 mM EDTA and 5 mM 2-mercaptoethanol and added to 30 μl of CaM–agarose (Sigma) which had been washed twice in the same buffer. Following incubation for 1 h at 4 °C in a Thermomixer (Eppendorf), non-bound Ng (supernatant) and CaM-bound Ng (pellet) were separated by centrifugation at 2500 g for 1 min. Representative aliquots were analysed by Western blot and the ratio between bound and non-bound Ng (pellet/supernatant ratio) was calculated for each sample.
Protein–lipid overlay assay
All assays were performed at 4 °C in the dark. Recombinant Ng and GAP-43 were used to study their binding to phospholipids. To screen for possible interactions, PIP strips (Echelon Biosciences) were used according to the manufacturer's protocol. Hybond-C Extra nitrocellulose (Amersham Biosciences) and PA from different sources were used to prepare in situ PA-spotted strips. In brief, freeze-dried PA was dissolved in methanol/chloroform (1:1, v/v) at 1 mM and stored at −80 °C (stocks). Working PA solutions were prepared in methanol/chloroform/water (2:1:0.8, by vol.) and 1 μl aliquots containing 50, 25 and 12.5 pmol of PA were spotted on membrane strips (Amersham Biosciences). The spotted strips were dried under nitrogen and left overnight at room temperature (22 °C). The following day, the strips were blocked with 3% BSA (essentially fatty-acid-free) in TBST [Tris-buffered saline containing 0.1% (w/v) Tween 20] for 60 min at 4 °C and incubated overnight at 4 °C with gentle rocking in fresh TBST containing 0.5 μg/ml Ng. The next day, the strips were washed four times over 40 min in TBST, incubated with anti-Ng antibody (1:20000 dilution) in TBST for 2 h, washed ten times over 1 h in TBST and incubated with HRP (horseradish peroxidase)-conjugated anti-rabbit antibody (1:12000 dilution) (Jackson ImmunoResearch Laboratories) diluted in TBST for 1 h. This was followed by a final washing step of 12 times in TBST over 60 min and visualization of HRP activity by ECL® (enhanced chemiluminescence) (Amersham Biosciences).
Liposomes
Stock solutions of PC (phosphatidylcholine) and PA were prepared in methanol/chloroform (1:1, v/v) at 5 μg/μl and stored at −80 °C. PC and PA from stocks were pipetted into glass tubes containing 100 μl of chloroform to generate mixtures of 125 μg of total lipid at different PA/PC ratios (0–50% of PA). The glass tubes had been washed previously with ethanol and water and dried in an oven. The PA/PC mixtures were dried under a nitrogen stream and kept for 1 h more at room temperature. Then, 250 μl of 150 mM NaCl and 10 mM Tris/HCl, pH 7.5, were added to each tube and the liposomes were generated by three 1 min cycles of vortex agitation at maximum and two 1 min cycles in a bath sonicator. At this point, 5 μg of purified recombinant Ng was added to the liposomes and the mixture was incubated at 25 °C for 30 min with agitation. Incubations were terminated by centrifugation at 200000 g for 25 min. Pellets and supernatants were separated and their Ng content was analysed by Western blot.
Expression vectors
The cDNAs for Ng, Ng-C3,4,9S (C3S/C4S/C9S mutant Ng), Ng-S36A and Ng-S36D were a gift from Dr Dan Gerendasy (Scripps Institute, La Jolla, CA, U.S.A.). The cDNA for Ng-I33Q was donated by Dr Dan Storm (University of Washington, Seattle, WA, U.S.A.). Ng cDNAs were subcloned into pcDNA3 (Invitrogen) for protein expression. Ng-IQless and double-Ng mutant cDNAs were made by PCR cloning in pcDNA3. Ng-IQless has an internal deletion between residues 30 and 45 of the rat sequence. PIP5KIα-myc-pCMV5 was a gift from Dr Helen Yin (University of Texas, Austin, TX, U.S.A.). Constructs for PLD expression, p-EGFP-C1-mPLD2 and p-EGFP-C1-hPLD1b, were kindly provided by Dr Michael A. Frohman (State University of New York, Stony Brook, NY, U.S.A.).
Cell culture, transfection and IF (immunofluorescence)
NIH-3T3 cells were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% fetal calf serum, 1 mM glutamine and antibiotics (penicillin and streptomycin at 50 units/ml). For transfection, 1.5×105 cells were plated in P35 dishes containing (or not) four round coverslips and the following day were transfected with Lipofectamine™ 2000 (Invitrogen) (2:1 ratio) in OptiMem for 2.5 h. Then, the cells were returned to their normal growth medium and processed 24 h later. Coverslips used for IF were cleaned with 65% nitric acid overnight, washed extensively with distilled water and heat-sterilized. Affinity-purified antibodies against Ng were used at 1:500. Two different methods were used for IF.
Conventional IF method
The whole procedure was performed at room temperature. After a quick rinse with PBS, cells were fixed with 4% (w/v) paraformaldehyde in PBS for 20 min, washed three times with PBS and incubated with 0.1 M glycine, pH 8.0, to inactivate free aldehydes. Blocking non-specific binding and permeabilization was achieved by incubating in blocking buffer [5% (v/v) heat-inactivated horse serum and 0.1% (w/v) Nonidet P-40 in PBS] for 30 min. Primary and secondary antibody incubations were performed in blocking buffer for 60 and 45 min respectively, followed by three 5 min washing steps in PBS. Finally, coverslips were rinsed sequentially in distilled water and ethanol and mounted in Mowiol after the ethanol had evaporated completely.
Cool IF method
Cells grown on coverslips in multiwell (×24) plates were quickly washed with Hanks medium (137 mM NaCl, 5.3 mM KCl, 0.45 mM KH2PO4, 0.35 mM Na2HPO4, 1.25 mM CaCl2, 0.8 mM MgSO4, 1 mM NaHCO3, 1 mM pyruvate, 0.6% D-glucose and 10 mM Hepes, pH 7.3) and fixed by addition of 1 ml of 4% (w/v) paraformaldehyde in Hanks medium at room temperature. After 10 min of fixation, 1 ml of ice-cold fixative was added slowly to each well and fixation continued at 4 °C for an additional 30 min. All subsequent steps were performed at 4 °C. Cells were rinsed quickly with PBS-S [PBS containing 0.2% (w/v) saponin], incubated with 50 mM glycine in PBS-S for 10 min and rinsed again with PBS-S. Incubation with blocking buffer [1% (v/v) heatinactivated horse serum and 0.1% (w/v) BSA in PBS-S] was extended for 30 min. Primary (90 min) and secondary (45 min) antibody incubations in blocking buffer were followed by three 5 min washing steps in PBS. On many occasions, cells were counterstained with phalloidin–coumarin or phalloidin–tetramethylrodamine (1:1000 dilution) for 10 min in PBS. Finally, coverslips were rinsed with distilled water, drained and left to dry before mounting in Mowiol. Visual inspection and image collection was carried out using 63× PlApo 1.4 NA (numerical aperture) and 100× PlApo 1.4 NA objectives in a Zeiss Axiovert 200 M microscope equipped with a Photometrics Coolsnap FX monochrome camera and MetaMorph 6.1 software (Universal Imaging). Digital image processing (averaging and background subtraction) and montage were carried out using ImageJ software (http://rsb.info.nih.gov/ij/).
Extracts, density fractionation and Western blots
Rats were killed by a lethal dose of sodium pentobarbital (intraperitonal injection), and their brains were removed quickly and cleaned from meninges and blood clots. All subsequent steps were performed at 4 °C. For density fractionation, rat forebrains (without cerebellum and brain stem) were homogenized (1:10, w/v) in 0.32 M sucrose, 1 mM EDTA and 10 mM Mops, pH 7.4, and centrifuged at 1300 g for 10 min (Sorvall SS-34 rotor) to obtain a pellet (P1) and a supernatant (S1) that was centrifuged for 15000 g for 15 min (Sorvall SS-34 rotor). The supernatant (S2) was centrifuged at 150000 g for 60 min (Beckman TL100.3 rotor) to obtain a soluble (S3) and particulate (P3) fraction. The pellet (P2) was resuspended in homogenization buffer, layered on top of a discontinuous sucrose gradient consisting of 4 ml of 1.2 M sucrose and 3.5 ml of 0.8 M sucrose in the same buffer. After centrifugation at 100000 g for 30 min in a swinging bucket rotor (Beckman SW40i), the material accumulated at the upper (My) and lower (Syn) interphases was collected. For hypotonic extraction, tissue was homogenized in a buffer containing 10 mM Tris/HCl, pH 7.4, 2 mM EDTA, 10 mM 2-mercaptoethanol, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin and 1 μg/ml pepstatin. Homogenates were then centrifuged at 1200 g for 10 min to obtain a pellet (P1) and a supernatant (S1). The pellet P1 was resuspended quickly in homogenization buffer plus 0.1% (w/v) Triton X-100 and centrifuged again at 1200 g for 10 min to obtain a soluble (N sup) and a particulate (N pel) nuclear fraction. The supernatant S1 was centrifuged at 100000 g for 40 min (Beckman TL100.3 rotor) to obtain a particulate (P3) fraction and a soluble (S3) fraction. DIGs (detergent-insoluble glycosphingolipid and cholesterol-rich complexes) were prepared from rat brain homogenized in ice-cold lysis buffer (25 mM Tris/HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA and 1% Triton X-100) as described in [29]. The homogenate was brought to 40% (w/v) sucrose in a final volume of 4 ml and placed at the bottom of a discontinuous gradient of sucrose composed of two more layers: an intermediate 6 ml layer (30% sucrose) and an upper 2 ml layer (5% sucrose). After equilibrium centrifugation at 100000 g (Beckman SW40i rotor), the interface between 5 and 30% sucrose was collected by aspiration and separated as the DIG fraction. The fraction at the bottom (40% sucrose) was taken and defined as the Triton X-100-soluble fraction. For cell extracts, cells were scraped and homogenized in cold medium (10 mM Tris/HCl, pH 7.5, 2 mM EDTA, 10 mM 2-mercaptoethanol, 1 μg/ml pepstatin, 1 μg/ml aprotinin, 1 μg/ml leupeptin and 1 mM PMSF) (250 μl/P35 dish) and centrifuged at 1000 g for 10 min at 4 °C. The pellet (P1) was separated and the supernatant was centrifuged again at 100000 g for 40 min at 4 °C (Beckman TL100.3 rotor) to obtain a soluble fraction (S3) and a particulate fraction (P3). Final resuspension volumes were identical for all fractions. Aliquots from different fractions were mixed with electrophoresis sample buffer, heated at 90 °C for 1 min, separated by SDS/PAGE [13% (w/v) polyacrylamide gel] and processed for Western blot as described in [27]. Polyclonal antibodies against Ng were used at 1:12000 dilution. Immunoreactive bands were visualized using HRP-conjugated secondary antibodies and ECL® detection.
General methods
Restriction enzyme digestions, DNA ligations, site-directed mutagenesis and other recombinant DNA procedures were performed according to standard protocols. All DNA constructs were verified by DNA sequencing.
RESULTS
Ng associates to intracellular membranes in neurons
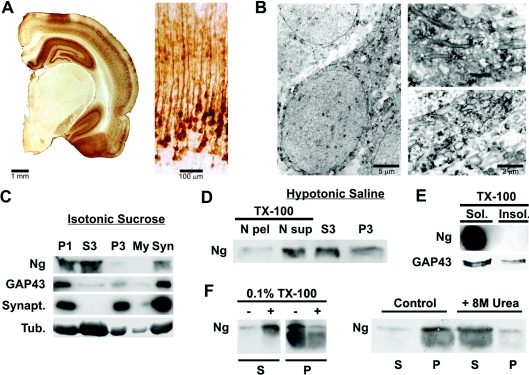
Previous studies have shown that Ng is typically distributed in small aggregates throughout the somatodendritic compartment of neurons [20,21,23]. In the present study, we confirmed these data on immunostained sections, using light (Figure 1A) and electron microscopy (Figure 1B) and additionally found significant amounts of Ng localizing at intracellular membranes (Figure 1B). This observation prompted us to study the association of Ng with cellular membranes. Thus rat brains homogenized in isotonic sucrose were density-fractionated and their Ng content was analysed by Western blot. More than 50% of brain Ng was recovered in the high-speed supernatant (S3) (Figure 1C). However, Ng was also present in the synaptosomal (Syn) fraction and, more abundantly, in the nuclei-enriched fraction (P1). Since, as seen with the light and electron microscopes, Ng was not so prevalent within nuclei, and, since other non-nuclear proteins, such as GAP-43, tubulin and synaptophysin, were also abundantly recovered at P1 fractions, we investigated the presence of Ng in P1 fractions in more detail. Rat brain homogenates were prepared in hypotonic saline and the low-speed pellets (P1) were resuspended in the same buffer with 0.1% (w/v) Triton X-100 and quickly centrifuged again to obtain a nuclear pellet (N pel) and a nuclear soluble (N sup) fraction. As shown in Figure 1(D), Ng in the P1 fraction was almost totally extracted after resuspension with Triton X-100. Similar results were obtained for GAP-43, tubulin and synaptophysin (not shown), indicating that these proteins are not really localized within nuclei, but have been retained in associated membranes. Figure 1(C) also shows that more than 20% of brain Ng was recovered in the synaptosomal fraction. When synaptosomes were broken by hypotonic shock, Ng remained tightly associated to membranes. Ng could be released from these membranes only with detergents (0.1% Triton X-100) or chaotropic agents (8 M urea) (Figure 1F), but not by changes in ionic strength or pH (results not shown). These results indicate a strong interaction between Ng and synaptosomal membranes that could be mediated by lipids, membrane proteins or both. Finally, to find out whether Ng was part of lipid rafts enriched in sphingolipids and cholesterol, DIGs were isolated from rat brains. As shown in Figure 1(E), Ng was not present in DIGs, unlike GAP-43.
Figure 1. Ng is associated to neuronal membranes in rat brain.
Brains from young adult Wistar rats were processed for immunocytochemistry as described in the Experimental section. (A) Left: stereomicroscope print showing half a coronal section displaying abundant Ng staining at the hippocampus, cerebral cortex and amygdala and none in the thalamus. Right: bright-field print showing Ng immunostaining distribution across somatosensory cortical layers. (B) Electron microscopy prints showing typical Ng immunostaining of cortical pyramidal neurons. Ng is distributed as small aggregates along an apical dendrite and frequently labels the cytoplasmic face of intracellular membranes. (C) Brains were density-fractionated in isotonic sucrose and processed by Western blotting using specific antibodies. Synapt., synaptophysin; Tub., tubulin. P1, S3, P3, My and Syn are described in the Experimental section. (D) Brains were density-fractionated in hypotonic saline and processed by Western blotting. Most of the Ng present at the P1 fraction was recovered in a soluble form after Triton X-100 (TX-100) extraction. N pel, N sup, S3 and P3 are described in the Experimental section. (E) Triton X-100 (TX-100)-insoluble (Insol.) (lipid rafts) and -soluble (Sol.) fractions were analysed by Western blotting. No Ng could be detected in the lipid raft fraction, whereas GAP-43 was clearly partitioned between Triton X-100-soluble and -insoluble fractions. (F) Synaptosomes from sucrose density fractionation were washed with isotonic sucrose and homogenized in 20 ml of a solution containing 1 mM EDTA and 10 mM Mops, pH 7.4. After stirring for 10 min at 4 °C, the mixture was centrifuged at 15000 g for 10 min at 4 °C and the pellet was resuspended and frozen in the same buffer at 1.5 mg/ml total protein. For assays, aliquots of synaptosomal membranes were thawed, centrifuged, resuspended in the same buffer with different additions, incubated for 30 min at 4 °C with gentle stirring and centrifuged again. Aliquots of supernatant (S) and pellet (P) were analysed by Western blotting. TX-100, Triton X-100.
Ng binds to PA
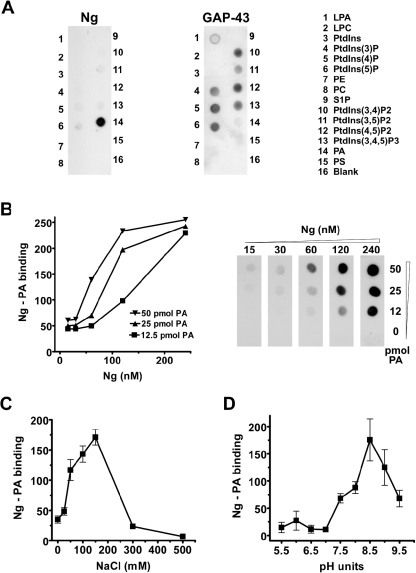
To date, the only Ng-interacting protein known is CaM [6,30–32]. However, characteristic Ng distribution within neurons and tight attachment to synaptosomal membranes suggest additional interactions. Using Ng immunoprecipitation and cell-permeable cross-linkers, we were unable to identify additional binding partners. We then explored the possibility of a direct interaction between Ng and membrane lipids using a lipid–protein overlay assay (PIP strips). Figure 2(A) shows that Ng selectively bound to PA, whereas GAP-43, which shares with Ng a similar IQ motif, bound not to PA, but to several other phosphoinositides. To discard the possibility of non-specific binding, two different affinity-purified anti-Ng antibodies were used and similar results were obtained. To determine the specificity and affinity of the interaction, we prepared our own PA-spotted Hybond-C nitrocellulose strips using dipalmitoyl-PA, PA from egg yolk and dioleoyl-PA. We found that Ng bound to all of them, but showed lower affinity for dioleoyl-PA (results not shown). This result may be related to the different adsorption of the lipids to the nitrocellulose support. From assays using several concentrations of Ng and egg-yolk PA, we calculated the affinity of the interaction [Kd=83±16 nM (mean±S.E.M.); n=4] (Figure 2B). Since the Ng concentration within neurons is estimated at 10 μM [10], it could be stated that, in the absence of competing interactions, most Ng would be complexed to PA. Next, to investigate the forces involved in stabilizing the interaction, we tested the effect of ionic strength and pH. Figures 2(C) and 2(D) show that Ng binding to PA was maximal at physiological salt concentrations and peaks at pH values between 8.0 and 8.5. These results indicate that electrostatic forces presumably established between the phosphate group of PA and basic sequences of Ng are important to stabilize the interaction, although the concurrence of hydrophobic interactions cannot be ruled out.
Figure 2. Ng binds to PA in vitro.
(A) PIP strips were blocked with 3% fatty acid-free BSA and incubated overnight with recombinant Ng or GAP-43 at 0.5 μg/ml. After extensive washing with TBST, anti-GAP-43 (797) or anti-Ng (756) affinity-purified antibodies were used to analyse protein content. The experiments were performed independently at least three times with similar results. A representative blot is shown. LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; PE, phosphatidylethanolamine; S1P, sphingosine 1-phosphate. (B) Hybond-C nitrocellulose strips were spotted with different amounts of egg-yolk PA and incubated with different concentrations of Ng. Ng–PA binding was quantified by densitometry of films exposed for different lengths of time and is expressed in arbitrary units. (C) The effect of ionic strength on Ng binding to PA was analysed by changing the NaCl concentration during overnight incubations with Ng as indicated. (D) The effect of pH on Ng binding to PA was analysed using a Tris/glycine buffer during overnight incubations with Ng. Results are means±S.E.M. for at least three independent experiments.
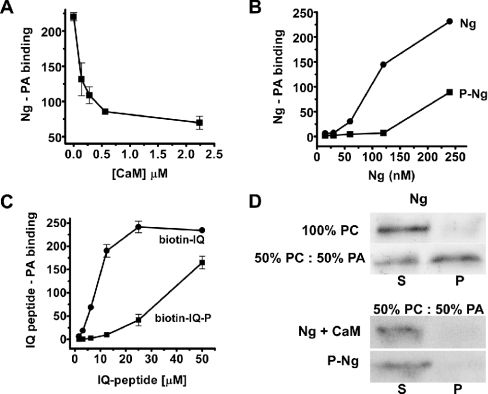
The IQ motif, a central stretch 15–20 amino acids long, concentrates most of the basic residues of Ng. This motif is also the binding site for CaM and hosts the PKC phosphorylation site (Ser36) [1]. Therefore we wished to know whether CaM or phosphorylation by PKC interfered with the interaction. Figure 3(A) shows that Ng pre-incubation with increasing concentrations of CaM substantially reduced its binding to PA, indicating that Ng–CaM and Ng–PA interactions are mutually exclusive. To check the phosphorylation effect, purified recombinant Ng was phosphorylated with PKC and the reaction mixture was passed through a CaM–agarose column to remove non-phosphorylated Ng. Since only non-phosphorylated Ng binds CaM, the flowthrough contained phosphorylated Ng exclusively. Figure 3(B) shows that phosphorylated Ng displayed less affinity for PA than non-phosphorylated Ng, suggesting that phosphorylation interferes with the interaction. The anti-Ng antibodies used were insensitive to the phosphorylation state of Ng. To evaluate the contribution of the IQ motif, a peptide corresponding to Ng-(29–47) was synthesized and biotinylated at the N-terminus. As shown in Figure 3(C), the biotin–IQ peptide bound to PA in a saturable fashion, whereas the same peptide phosphorylated at Ser36 (biotin–IQ-P) did not display significant binding. The relative affinity of the peptide for PA was lower than that of Ng, suggesting that either the spatial conformation and/or additional Ng residues could play a role in the interaction. The possibility of co-operative binding between Ng oligomers and multiple PA molecules should not be disregarded either. To confirm the data obtained with the lipid–protein overlays, we used liposomes, which more faithfully reconstitute the physiological environment of protein–lipid interactions. Using liposomes made of PC alone or mixtures of PA and PC, we found that Ng only binds to liposomes that contain PA (Figure 3D, upper panel). Maximal levels of Ng binding were obtained with liposomes containing dioleoyl-PA or egg-yolk PA, whereas less binding was observed using liposomes containing dipalmitoyl-PA (results not shown). This could be explained by different surface exposure of the PAs in the liposomes. We then checked whether CaM could compete with PA for Ng binding or whether phosphorylated Ng would bind to liposomes containing PA. As shown in Figure 3(D) (lower panel), pre-incubation with CaM prevented Ng binding and phosphorylated Ng did not bind to PA-containing liposomes, in good agreement with data obtained previously. In summary, using different preparations, we have shown that Ng selectively binds to PA and that the interaction is prevented by CaM and inhibited by PKC phosphorylation.
Figure 3. Regulation of Ng binding to PA.
(A) Ng binding to PA was analysed in protein–lipid overlays as described in the text, except that Ng was incubated previously with different amounts of CaM for 30 min at 25 °C before the mixture was added to egg-yolk PA-spotted strips. (B) Recombinant Ng was phosphorylated by PKC and separated from non-phosphorylated Ng by affinity chromatography in CaM–agarose columns. Binding of Ng (●) and phospho-Ng (■) was assayed as described in the text. (C) Binding of biotin–IQ peptide (●), corresponding to Ng-(29–47), and its phosphorylated form (biotin–IQ-P) (■) to egg-yolk PA-spotted strips was analysed using the method described for protein–lipid overlay assays, except that the primary and secondary antibodies were replaced by an incubation with HRP-labelled streptavidin (1:2000 dilution) for 1 h. Results in (A), (B) and (C) are means±S.E.M. for at least three independent experiments. (D) To analyse CaM competition, 5 μg of CaM was added to liposomes of the indicated composition along with Ng to the binding mixture. Typical Western blots representative of three independent experiments are shown. S, supernatant; P, pellet.
Ng associates to intracellular membranes in NIH-3T3 cells
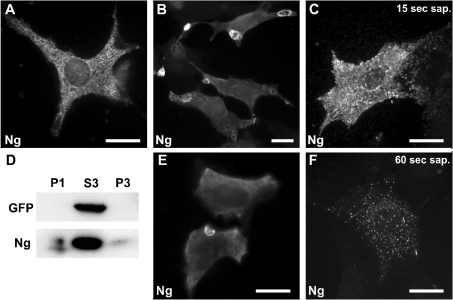
To study the physiological relevance of Ng association to cellular membranes, we looked for cell lines with Ng expression, but did not find any. Primary cultures of hippocampal or cortical neurons show very low Ng expression, even after 2 weeks in vitro [33]. Therefore we transfected a variety of commonly used cell lines, analysed their Ng expression and decided to use NIH-3T3 cells, since expression was not very high and subcellular distribution resembled that observed in tissue (Figure 4A). Ng in NIH-3T3 cells showed typical intracellular punctate labelling and was observed in small intracellular vesicles and, less commonly, at peripheral dorsal ruffles and edges of lamellipodia. The peripheral localization was not consistently reproduced between experiments. Since Ng is more a peptide than a protein (78 amino acid long), we thought that some Ng could be lost during the fixation and/or permeabilization steps. To test this hypothesis, we compared different IF protocols and found that cooling the cells during fixation and maintaining the procedure thereafter at low temperatures (4–10 °C) is fundamental to fully reveal Ng peripheral localization (see the Experimental section). Using this method, anti-Ng antibodies strongly labelled PMRSSs (plasma membrane ring-shaped structures) located at the cell periphery (Figure 4B and Supplementary Figure 1 at http://www.BiochemJ.org/bj/404/bj4040031add.htm). These ring-shaped concave structures resembled open craters at the dorsal plasma membrane that formed at the cellular edges (Figure 4E). Ng-labelled PMRSSs were more abundant in cells with moderate to high Ng expression, were particularly present in motile elongated cells, and were less abundant in contact-inhibited cells. All Ng-expressing cells still exhibited the typical intracellular punctate labelling, although sometimes the intense labelling of PMRSSs made it difficult to notice. Additionally, it was common to observe Ng labelling at lamellipodia edges, short membrane ruffles and tips of trailing tails of motile cells. This labelling pattern is specific since it was not seen in non-transfected cells and could be reproduced with either of two different anti-Ng antibodies. To investigate the association of Ng to cellular compartments, we analysed its distribution after in situ permeabilization with saponin. Saponin (0.2%), added to live cells, slowly and non-uniformly extracts cellular membranes because of its avidity for cholesterol. Thus, during saponin permeabilization, strictly soluble and cytosolic proteins quickly diffuse out of cells, whereas membrane or cytoskeleton-associated proteins are retained or released more slowly. For these experiments, GFP (green fluorescent protein) was always co-expressed with Ng and used as internal reporter of a typically soluble protein. At 15 s after saponin addition, GFP was completely washed out from transfected cells (results not shown), whereas Ng labelling was still strong and displayed a granular pattern (Figure 4C). After 60 s of permeabilization, a portion of Ng remained attached and visible, associated to multiple disseminated spots throughout the cytosol (Figure 4F). Longer extractions (>2 min) resulted in total loss of Ng. When non-ionic detergents such as Nonidet P-40 or Triton X-100 (also at 0.2%) were used instead of saponin, both GFP and Ng were totally extracted after 15 s of permeabilization (results not shown). These results again point to an association between Ng and cellular membranes. To support this, homogenates of transfected cells were density-fractionated and the contents of Ng and GFP were analysed by Western blotting. As expected, minor, but significant, amounts of Ng were found in the particulate fractions P1 and P3, whereas GFP was found only in the soluble fraction S3 (Figure 4D).
Figure 4. Ng subcellular distribution in NIH-3T3 cells.
NIH-3T3 cells were transfected with Ng alone or in conjunction with GFP. (A) Typical distribution of Ng in cells processed using the conventional IF method, showing a characteristic punctate intracellular labelling. (B, E) Ng distribution in cells processed using the cool IF method. Ng accumulates at ring-shaped structures that appear to form at the cell periphery. (C, F) Ng-transfected cells were permeabilized in situ with 0.2% saponin (sap.) for 15 s (C) or 60 s (F) and then processed using the cool IF method. Note the clustered distribution of Ng-labelled granules that spread out from the cell after 15 s of permeabilization and the spotty vesicular distribution observed after 60 s. Scale bar, 25 μm. (D) Cellular extracts from Ng- and GFP-transfected cells were density-fractionated and analysed by Western blotting. Note that, while GFP was completely recovered in the S3 fraction, visible amounts of Ng were present in the P1 and P3 fractions. P1, S3 and P3 are described in the Experimental section.
The IQ motif is involved in Ng translocation to the plasma membrane
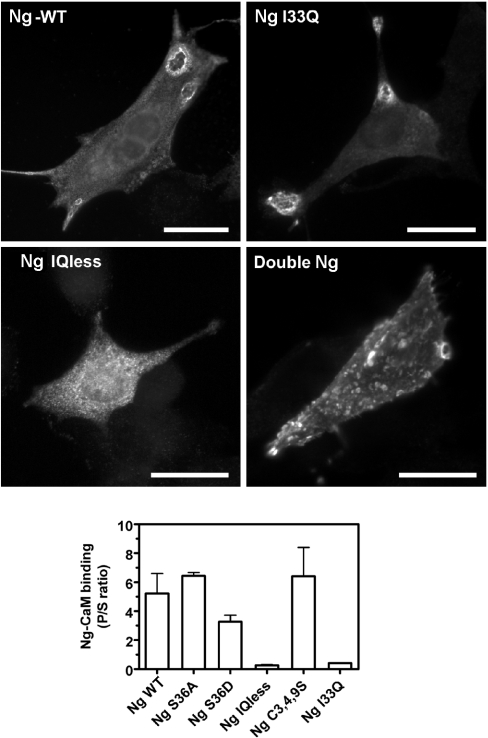
We next wished to find out the region of the Ng molecule responsible for its peripheral localization. Initially, we used several Ng mutants with mutations at Ser36 and N-terminal cysteine residues, and found that Ng-S36A, Ng-S36D and Ng-C3,4,9S localize at the plasma membrane in the same way as does Ng. Ng-S36A and Ng-S36D mutants are meant to mimic the non-phosphorylated and phosphorylated forms of Ng respectively. Therefore we reasoned that, if Ng binding to PA is responsible for association to membranes, then Ng-S36D should be cytosolic. Another explanation could be that Ng mutants bind PA with equal affinity to that of Ng. To test these hypotheses, recombinant Ng-S36A, Ng-S36D and Ng-C3,4,9S were purified and their binding to PA was compared with that of Ng in protein–lipid overlays. No differences in affinity were found between Ng and the mutants, thus suggesting that Ser36 and Cys3, Cys4 and Cys9 residues are not directly involved in Ng–PA interaction. Mutagenesis at phosphorylatable residues is frequently used to study the physiological relevance of particular phosphorylation sites. However, it is not uncommon to find that mutations of serine/threonine to aspartate do not reproduce the behaviour of the phosphorylated protein. In fact, it has been shown that, although phosphorylated Ng showed negligible binding to CaM, Ng-S36D displayed significant binding to CaM, whereas Ng-I33Q, another unrelated mutant, is unable to bind CaM [32]. We confirmed these data by comparing the affinities of the Ng mutants for CaM in vitro. Figure 5 (lower panel) shows that Ng-S36D still retained a substantial amount of binding to CaM, whereas Ng-I33Q did not bind to CaM at all. Then, to confirm whether the Ng IQ motif is involved in Ng peripheral localization, we made two additional mutants: one with a deletion of 15 amino acids corresponding to the IQ motif (Ng-IQless) and another with two Ng molecules linked in tandem (double-Ng). Figure 5 (upper panel) shows that both Ng and Ng-I33Q have a similar distribution characterized by their peripheral localization and PMRSSs labelling. Ng-IQless, on the other hand, displayed a typical punctate intracellular distribution with no peripheral labelling. The double-Ng mutant showed a characteristic distribution featuring an abundant localization throughout the plasma membrane. In situ permeabilization with saponin for 60 s completely washed out Ng-IQless from transfected cells, whereas Ng-I33Q and double-Ng displayed the typical spotty distribution found earlier for Ng (results not shown). Based on these results, we propose that the IQ motif is involved in Ng translocation to the plasma membrane.
Figure 5. Ng localization at the plasma membrane is mediated by the IQ motif.
Upper panels: NIH-3T3 cells were transfected with several wild-type Ng (Ng-WT) and Ng mutants as indicated. Cells transfected with Ng and Ng-I33Q displayed a typical distribution with strongly labelled craters, whereas Ng-IQless labelling was limited to intracellular puncta. Double-Ng distribution was characterized by its abundant presence at the plasma membrane. Scale bars, 25 μm. Lower panel: binding between CaM–agarose and several Ng mutants was assayed as described in the text. The results are expressed as the ratio between bound (P) and unbound (S) Ng in each assay. Note that Ng, Ng-S36A and Ng-C3,4,9S showed a similar binding to CaM, and Ng-IQless and Ng-I33Q did not bind CaM. Ng-S36D showed an intermediate level of CaM binding. Results are means±S.E.M. for four independent experiments.
PLD and PIP5KIα overexpression alters intracellular distribution of Ng
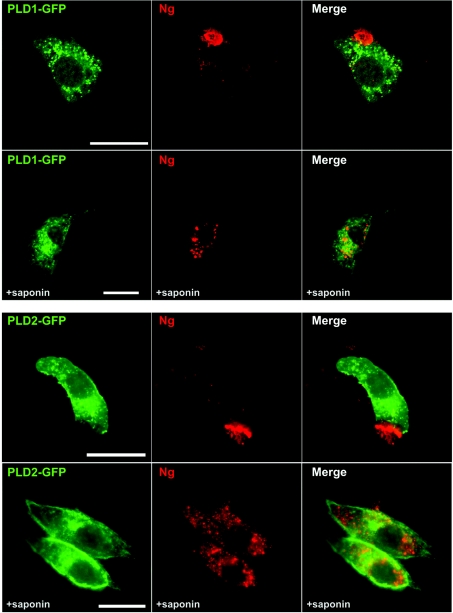
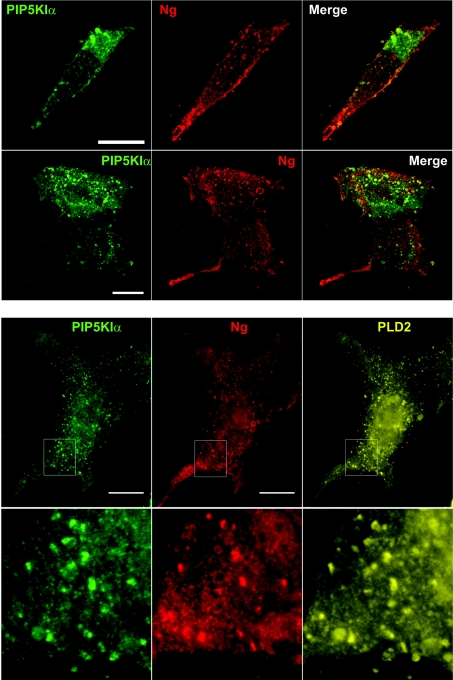
Having identified and characterized the interaction between Ng and PA, we were interested in investigating its physiological relevance. PLD1 and PLD2 catalyse PA production from PC in mammals and, along with DGKs, constitute the major source of cellular PA for signalling purposes [15]. To analyse the effect of an increased production of cellular PA, we overexpressed GFP-tagged PLDs and analysed Ng distribution. PLD2 and, to a lesser extent, PLD1 overexpression visibly altered Ng distribution (Figure 6). Following PLD overexpression, Ng changed from labelling multiple and small intracellular puncta to a distribution characterized by bigger aggregates disseminated within the cytoplasm or a distribution where practically all Ng was concentrated in one strongly labelled peripheral patch. The patches resembled previously observed PMRSSs, but now appeared to be practically filled with Ng. After in situ permeabilization, Ng was observed in intracellular vesicles that were bigger and less numerous than those found in cells expressing only Ng (see Figure 4). PLD2 and PLD1 localized to either the plasma membrane or intracellular vesicles respectively, even after in situ permeabilization, according to what has been described previously [34,35]. No co-localization could be detected between Ng and PLD1 or PLD2. It has been shown that PA and PtdIns(4,5)P2 production are mutually and positively regulated, for example in membrane ruffles, through cross-activation of PIP5KIα and PLD with PA and PtdIns(4,5)P2 respectively [36]. It is also known that PLD2 has a high catalytic activity which is almost exclusively dependent on PtdIns(4,5)P2 [37]. To find out how an increase in cellular PtdIns(4,5)P2 affects Ng distribution, we overexpressed PIP5KIα. As illustrated in Figure 7 (upper panel), no Ng-labelled PMRSSs were observed in PIP5KIα-expressing cells. Instead, Ng labelled multiple small protrusions or blebs decorating the dorsal surface of the plasma membrane where PIP5KIα was also observed. When Ng was co-expressed with PIP5KIα and PLD2, the same peripheral distribution was found for all three proteins (Figure 7, lower panel). Since the interaction between PIP5KIα and PLD2 has been clearly documented [38], it is suggested that Ng localization in PIP5KIα-overexpressing cells is mediated by local generation of PtdIns(4,5)P2 and up-regulation of PLD2 activity.
Figure 6. Effects of PLD overexpresion on Ng subcellular distribution.
NIH-3T3 cells were transfected with Ng and either PLD1–GFP (upper panels) or PLD2–GFP (lower panels) and analysed using the cool IF method before (upper rows) or after (lower rows) 60 s of in situ permeabilization with saponin. Note that Ng labelling at the plasma membrane is restricted to a peripheral patch that showed strong fluorescence. After saponin extraction, Ng is observed in perinuclear (PLD1) or dispersed cytoplasmic (PLD2) vesicles. These vesicles were bigger than those observed without PLD overexpression. No co-localization between Ng and PLDs was observed. Scale bar, 25 μm.
Figure 7. Effect of PtdIns(4,5)P2 availability on Ng subcellular distribution.
Upper panels: NIH-3T3 cells were transfected with Ng and PIP5KIα and their distribution was analysed using the cool IF method. In both motile (upper row) and quiescent (lower row) cells, Ng showed a predominant distribution at the plasma membrane, characterized by its presence in multiple small protrusions or blebs that displayed some co-localization with PIP5KIα. Lower panels: NIH-3T3 cells were transfected with Ng, PIP5KIα and PLD2–HA (haemagglutinin) and their distribution was analysed as described above. The expression of PIP5KIα also alters PLD2–HA localization at the plasma membrane. PIP5KIα, PLD2–HA and Ng show a typical spotty distribution, characterized by their presence in multiple small protrusions or blebs at the cell periphery. Outlines in the upper row are shown magnified in the lower row. Co-localization of P5KIα, PLD2–HA and Ng can be observed at the protrusions.
DISCUSSION
In recent years, the study of Ng participation in neuronal plasticity events has been addressed from multiple approaches [12,39–44]. In most of them, the general understanding that Ng solely acts as a local CaM chelator concentrating and releasing CaM in a calcium- and PKC-activity-dependent fashion has dominated the discussions. Intracellular signalling depends not only on protein modification, but also on restricted subcellular localization and regulated translocation. This is especially relevant in highly complex and morphologically asymmetrical cells such as neurons, where compartmentalization is fundamental for proper function. In the present study, we have undertaken a different approach that relies on the analysis of Ng subcellular localization in a variety of conditions and environments.
Using immunocytochemistry combined with electron microscopy, we have confirmed that Ng typically distributes in small aggregates and strongly labels intracellular membranes in neurons. The presence of Ng in aggregates has been previously observed by others [1] and could be related to its strong tendency to form disulfide-linked oligomers. Ng could also be part of multimeric complexes of yet undefined composition and functionality. Regarding membrane association, earlier work has already reported Ng at membranes [20–22,30], although no binding partners were identified. Here, we show that Ng associates tightly to synaptosomal membranes and selectively binds to PA in vitro. Since no other binding partners were found, we propose that Ng binding to PA is required for membrane association. In protein–lipid overlays, Ng binding to PA is stabilized by ionic interactions. However, Ng association to synaptosomal membranes is not equally affected by changes of ionic strength or pH. Therefore we believe that additional interactions should be involved. Since non-ionic detergents effectively extracted Ng from synaptosomal membranes, we propose that these interactions could be hydrophobic in nature. Further, cellular Ng could interact with membranes as an oligomer and co-operatively bind to PA clusters or be assembled further in a multimeric complex at the PSD. Our data also indicate that the IQ motif mediates Ng binding to PA. There are several proteins whose PA-binding motifs have been identified [45,46]. Although there is no strict PA-binding consensus sequence, PA-binding motifs are most commonly short sequences that contain one or more basic residues. Ng IQ motif is a short (15–20 amino acids) amphiphilic (basic and hydrophobic) central region of the molecule that makes a good candidate for a PA-binding motif. Since the Ng IQ motif is also the site of CaM binding and PKC phosphorylation, this finding has interesting regulatory implications.
Evidence supporting Ng association with membranes was also obtained in NIH-3T3 cells. In these experiments, the design of an optimized IF method was necessary to see Ng accumulations at the cell periphery (PMRSSs). The morphology and localization of Ng-labelled PMRSSs led us to think that they could be related to macropinocytic vesicles that originated in ruffling areas that collapsed upon cell fixation [47]. Furthermore, in situ permeabilization experiments demonstrated that Ng, although mostly soluble, also associates to multiple intracellular vesicles. Looking for the domains involved in membrane association, we found that Ng-IQless, a mutant without the IQ motif, does not localize at the plasma membrane and completely disappears after in situ permeabilization. Furthermore, double-Ng, a mutant that features two Ng molecules linked in tandem, and consequently two IQ motifs, displays a plasma membrane localization even more evident than Ng. These observations suggest that the IQ motif is important in defining Ng intracellular distribution. On the other hand, Ng-I33Q, a mutant unable to bind CaM, is also widely localized at the plasma membrane with a distribution similar to that of Ng. Since the IQ motif is the site of CaM interaction, it can be concluded that CaM is not necessary for Ng association to membranes. This is in good agreement with our previous in vitro results showing that Ng binding to CaM and to PA are mutually exclusive. Evidence supporting an in vivo interaction between Ng and PA came from co-expression experiments. Expression of PLD2–GFP and, to a certain extent, PLD1–GFP induced visible Ng accumulation at the plasma membrane and localization at bigger intracellular vesicles after in situ permeabilization. Local PA and PtdIns(4,5)P2 levels at the plasma membrane are closely linked through cross-activation of PIP5KIα and PLD2. In cells overexpressing PIP5KIα, Ng localizes at the plasma membrane labelling multiple mini-protrusions, many of them also containing PIP5KIα. When PLD2 is overexpressed in these cells, a high degree of co-localization is observed among PIP5KIα, PLD2 and Ng. This suggests that PIP5KIα recruits PLD2 to the miniprotrusions and stimulates its activity by local production of free PtdIns(4,5)P2. As a result, Ng would be trapped in these sites by PA synthesized locally by PLD2. In summary, based on our results, we propose that Ng dynamically translocates between intracellular compartments and the plasma membrane and that newly synthesized PA, most probably derived from PtdIns(4,5)P2-stimulated PLD, is an essential factor in regulating its subcellular distribution.
Assessing the physiological relevance of the interaction between Ng and PA is not a straightforward task, mainly because very little is known of the relevance of PA as a second messenger in a neuronal context [14]. Most likely, intraneuronal distribution of PA would also be tightly regulated by multiple synthesizing and degrading enzymes and, possibly, PA-binding proteins. Imaging PA distribution in vivo using PA-binding domains fused to fluorescent proteins has been problematical for many groups (M. Frohman, personal communication), although there have been some reports of success [48]. With the data available, we could speculate on the functional relevance of the Ng–PA interaction at least in two directions. First, PA could act as a membrane anchor to locally recruit and concentrate Ng at specific sites in dendrites. In this way, Ng would benefit from the dynamic and tightly regulated spatiotemporal distribution of PA. This could also explain the selective concentration of Ng at dendritic spines and PSDs (post-synaptic densities), in spite of being a predominantly cytosolic protein [21,22]. Secondly, since newly synthesized PA has a short half-life, binding to Ng could prevent its degradation and contribute to create a PA reservoir that could be quickly, locally and massively released, for example, after Ng phosphorylation by PKC. Ng, because of its small size and high intracellular mobility, would be a suitable molecule to achieve this task. In this scenario, Ng would be an important element to potentiate PA-dependent signalling.
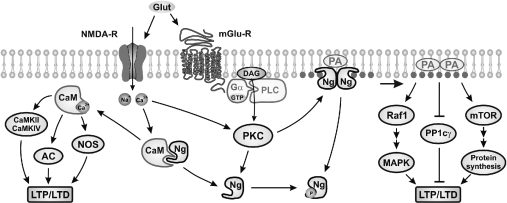
Ng-knockout mice suffer from learning and memory deficits. Our current knowledge on learning and memory at the molecular level is based on what we know about LTP and LTD mechanisms, which dictate the signalling context that leads to neurotransmission amplification or attenuation. The balance between kinase and phosphatase activity is crucial for the regulation of the LTP/LTD switch, and CaMKII and PP1 are two key actors in this interplay. Much attention has been paid to Ng's ability to release free CaM and its effect on CaMKII activity [44,49,50]. However, kinases are not the only important factors for LTP, as inhibition of phosphatase activity has been demonstrated to be at least as powerful as kinase stimulation to promote LTP [51,52]. In this context, it is revealing to find out that PP1γ, the predominant PP1 isoform in the postsynaptic compartment [53], is potently inhibited by PA [18]. Therefore Ng participation in synaptic plasticity mechanisms could be as illustrated in Figure 8. Under resting conditions, most Ng would be associated to CaM (cytosol) or PA (membranes). Following excitatory stimulation, intracellular Ca2+ increase and activation of PKC would release CaM and PA from Ng at different sites. CaM would activate CaMKII and promote its autophosphorylation, whereas PA would inhibit PP1γ activity and prolong the effects of LTP. In the absence of Ng, no CaM or PA would be sufficiently concentrated at the postsynaptic site and a limited CaMKII activation and PP1γ inhibition would lead to a diminished potentiation. From the evidence accumulated using knockout mice, it seems clear that Ng deficit has a big impact on cognition, spatial learning and memory tasks. This should be recognized as a good opportunity to study the cellular and molecular basis of learning and memory in an animal model without undesired interferences. A more precise knowledge of Ng localization, specific interactions and signalling cascades involved will contribute to a better understanding of the mechanisms of neuronal plasticity and the molecular basis of learning and memory processes.
Figure 8. Model depicting Ng participation in postsynaptic signal transduction.
Under resting conditions (low Ca2+, low PKC activity), Ng locally traps free CaM and PA. Following glutamate receptor activation [NMDA-R (N-methyl-D-aspartate receptor) and mGlu-R (metabotropic glutamate receptor)], both CaM and PA are released and participate in signal transduction pathways that favour LTP over LTD. AC, adenylylate cyclase; DAG, diacylglycerol; mTOR: mammalian target of rapamycin; NOS, nitric oxide synthase; PP1c, PP1 catalytic subunit.
Online data
Acknowledgments
This work was supported by a grant from the Spanish Ministry of Science and Technology (BFI2002-01581). We thank “Fundación Ramón Areces” for institutional support. I. D.-G. was the recipient of a fellowship from the Comunidad Autónoma de Madrid (CAM).
References
- 1.Baudier J., Deloulme J. C., Van Dorsselaer A., Black D., Matthes H. W. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17): identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J. Biol. Chem. 1991;266:229–237. [PubMed] [Google Scholar]
- 2.Watson J. B., Battenberg E. F., Wong K. K., Bloom F. E., Sutcliffe J. G. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J. Neurosci. Res. 1990;26:397–408. doi: 10.1002/jnr.490260402. [DOI] [PubMed] [Google Scholar]
- 3.Baudier J., Bronner C., Kligman D., Cole R. D. Protein kinase C substrates from bovine brain: purification and characterization of neuromodulin, a neuron-specific calmodulin-binding protein. J. Biol. Chem. 1989;264:1824–1828. [PubMed] [Google Scholar]
- 4.Alvarez-Bolado G., Rodriguez-Sanchez P., Tejero-Diez P., Fairen A., Diez-Guerra F. J. Neurogranin in the development of the rat telencephalon. Neuroscience. 1996;73:565–580. doi: 10.1016/0306-4522(96)00061-9. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda H., Barth A. L., Stellwagen D., Malenka R. C. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- 6.Gerendasy D. D., Herron S. R., Watson J. B., Sutcliffe J. G. Mutational and biophysical studies suggest RC3/neurogranin regulates calmodulin availability. J. Biol. Chem. 1994;269:22420–22426. [PubMed] [Google Scholar]
- 7.Gerendasy D. D., Herron S. R., Jennings P. A., Sutcliffe J. G. Calmodulin stabilizes an amphiphilic α-helix within RC3/neurogranin and GAP-43/neuromodulin only when Ca2+ is absent. J. Biol. Chem. 1995;270:6741–6750. doi: 10.1074/jbc.270.12.6741. [DOI] [PubMed] [Google Scholar]
- 8.Pak J. H., Huang F. L., Li J., Balschun D., Reymann K. G., Chiang C., Westphal H., Huang K. P. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyakawa T., Yared E., Pak J. H., Huang F. L., Huang K. P., Crawley J. N. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 10.Huang K. P., Huang F. L., Jager T., Li J., Reymann K. G., Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J. Neurosci. 2004;24:10660–10669. doi: 10.1523/JNEUROSCI.2213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krucker T., Siggins G. R., McNamara R. K., Lindsley K. A., Dao A., Allison D. W., De Lecea L., Lovenberg T. W., Sutcliffe J. G., Gerendasy D. D. Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus. J. Neurosci. 2002;22:5525–5535. doi: 10.1523/JNEUROSCI.22-13-05525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J., Li J., Huang K. P., Huang F. L. Attenuation of protein kinase C and cAMP-dependent protein kinase signal transduction in the neurogranin knockout mouse. J. Biol. Chem. 2002;277:19498–19505. doi: 10.1074/jbc.M109082200. [DOI] [PubMed] [Google Scholar]
- 13.Huang F. L., Huang K. P., Wu J., Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J. Neurosci. 2006;26:6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Devaiah S. P., Zhang W., Welti R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins G. M., Frohman M. A. Phospholipase D: a lipid centric review. Cell. Mol. Life Sci. 2005;62:2305–2316. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moritz A., De Graan P. N., Gispen W. H., Wirtz K. W. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J. Biol. Chem. 1992;267:7207–7210. [PubMed] [Google Scholar]
- 17.Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 18.Jones J. A., Hannun Y. A. Tight binding inhibition of protein phosphatase-1 by phosphatidic acid: specificity of inhibition by the phospholipid. J. Biol. Chem. 2002;277:15530–15538. doi: 10.1074/jbc.M111555200. [DOI] [PubMed] [Google Scholar]
- 19.Rizzo M. A., Shome K., Watkins S. C., Romero G. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J. Biol. Chem. 2000;275:23911–23918. doi: 10.1074/jbc.M001553200. [DOI] [PubMed] [Google Scholar]
- 20.Neuner-Jehle M., Denizot J. P., Mallet J. Neurogranin is locally concentrated in rat cortical and hippocampal neurons. Brain Res. 1996;733:149–154. [PubMed] [Google Scholar]
- 21.Watson J. B., Sutcliffe J. G., Fisher R. S. Localization of the protein kinase C phosphorylation/calmodulin-binding substrate RC3 in dendritic spines of neostriatal neurons. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8581–8585. doi: 10.1073/pnas.89.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson J. B., Szijan I., Coulter P. M., 2nd Localization of RC3 (neurogranin) in rat brain subcellular fractions. Mol. Brain Res. 1994;27:323–328. doi: 10.1016/0169-328x(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 23.Represa A., Deloulme J. C., Sensenbrenner M., Ben-Ari Y., Baudier J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J. Neurosci. 1990;10:3782–3792. doi: 10.1523/JNEUROSCI.10-12-03782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang J. W., Schumacher E., Coulter P. M., 2nd, Vinters H. V., Watson J. B. Dendritic translocation of RC3/neurogranin mRNA in normal aging, Alzheimer disease and fronto-temporal dementia. J. Neuropathol. Exp. Neurol. 1997;56:1105–1118. doi: 10.1097/00005072-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Mori Y., Imaizumi K., Katayama T., Yoneda T., Tohyama M. Two cis-acting elements in the 3′ untranslated region of α-CaMKII regulate its dendritic targeting. Nat. Neurosci. 2000;3:1079–1084. doi: 10.1038/80591. [DOI] [PubMed] [Google Scholar]
- 26.Pinkstaff J. K., Chappell S. A., Mauro V. P., Edelman G. M., Krushel L. A. Internal initiation of translation of five dendritically localized neuronal mRNAs. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2770–2775. doi: 10.1073/pnas.051623398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tejero-Diez P., Rodriguez-Sanchez P., Diez-Guerra F. J. Microscale purification of proteins exhibiting anomalous electrophoretic migration: application to the analysis of GAP-43 phosphorylation. Anal. Biochem. 1999;274:278–282. doi: 10.1006/abio.1999.4292. [DOI] [PubMed] [Google Scholar]
- 28.DeFelipe J., Fairen A. A simple and reliable method for correlative light and electron microscopic studies. J. Histochem. Cytochem. 1993;41:769–772. doi: 10.1177/41.5.8468459. [DOI] [PubMed] [Google Scholar]
- 29.Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 30.Houbre D., Duportail G., Deloulme J. C., Baudier J. The interactions of the brain-specific calmodulin-binding protein kinase C substrate, neuromodulin (GAP 43), with membrane phospholipids. J. Biol. Chem. 1991;266:7121–7131. [PubMed] [Google Scholar]
- 31.Huang K. P., Huang F. L., Chen H. C. Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch. Biochem. Biophys. 1993;305:570–580. doi: 10.1006/abbi.1993.1463. [DOI] [PubMed] [Google Scholar]
- 32.Prichard L., Deloulme J. C., Storm D. R. Interactions between neurogranin and calmodulin in vivo. J. Biol. Chem. 1999;274:7689–7694. doi: 10.1074/jbc.274.12.7689. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Sanchez P., Tejero-Diez P., Diez-Guerra F. J. Glutamate stimulates neurogranin phosphorylation in cultured rat hippocampal neurons. Neurosci. Lett. 1997;221:137–140. doi: 10.1016/s0304-3940(96)13309-7. [DOI] [PubMed] [Google Scholar]
- 34.Du G., Altshuller Y. M., Vitale N., Huang P., Chasserot-Golaz S., Morris A. J., Bader M. F., Frohman M. A. Regulation of phospholipase D1 subcellular cycling through coordination of multiple membrane association motifs. J. Cell Biol. 2003;162:305–315. doi: 10.1083/jcb.200302033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freyberg Z., Sweeney D., Siddhanta A., Bourgoin S., Frohman M., Shields D. Intracellular localization of phospholipase D1 in mammalian cells. Mol. Biol. Cell. 2001;12:943–955. doi: 10.1091/mbc.12.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda A., Nogami M., Yokozeki T., Yamazaki M., Nakamura H., Watanabe H., Kawamoto K., Nakayama K., Morris A. J., Frohman M. A., Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 37.Colley W. C., Sung T. C., Roll R., Jenco J., Hammond S. M., Altshuller Y., Bar-Sagi D., Morris A. J., Frohman M. A. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 38.Divecha N., Roefs M., Halstead J. R., D'Andrea S., Fernandez-Borga M., Oomen L., Saqib K. M., Wakelam M. J., D'Santos C. Interaction of the type Iα PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4,5-bisphosphate in the regulation of PLD2 activity. EMBO J. 2000;19:5440–5449. doi: 10.1093/emboj/19.20.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerendasy D. D., Sutcliffe J. G. RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes. Mol. Neurobiol. 1997;15:131–163. doi: 10.1007/BF02740632. [DOI] [PubMed] [Google Scholar]
- 40.Huang K. P., Huang F. L., Li J., Schuck P., McPhie P. Calcium-sensitive interaction between calmodulin and modified forms of rat brain neurogranin/RC3. Biochemistry. 2000;39:7291–7299. doi: 10.1021/bi000336l. [DOI] [PubMed] [Google Scholar]
- 41.Ramakers G. M., Heinen K., Gispen W. H., de Graan P. N. Long term depression in the CA1 field is associated with a transient decrease in pre- and postsynaptic PKC substrate phosphorylation. J. Biol. Chem. 2000;275:28682–28687. doi: 10.1074/jbc.M003068200. [DOI] [PubMed] [Google Scholar]
- 42.Sheu F. S., Mahoney C. W., Seki K., Huang K. P. Nitric oxide modification of rat brain neurogranin affects its phosphorylation by protein kinase C and affinity for calmodulin. J. Biol. Chem. 1996;271:22407–22413. doi: 10.1074/jbc.271.37.22407. [DOI] [PubMed] [Google Scholar]
- 43.Watson J. B., Margulies J. E., Coulter P. M., 2nd, Gerendasy D. D., Sutcliffe J. G., Cohen R. W. Functional studies of single-site variants in the calmodulin-binding domain of RC3/neurogranin in Xenopus oocytes. Neurosci. Lett. 1996;219:183–186. doi: 10.1016/s0304-3940(96)13203-1. [DOI] [PubMed] [Google Scholar]
- 44.Zhabotinsky A. M., Camp R. N., Epstein I. R., Lisman J. E. Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J. Neurosci. 2006;26:7337–7347. doi: 10.1523/JNEUROSCI.0729-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stace C. L., Ktistakis N. T. Phosphatidic acid- and phosphatidylserine- binding proteins. Biochim. Biophys. Acta. 2006;1761:913–926. doi: 10.1016/j.bbalip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Jones J. A., Rawles R., Hannun Y. A. Identification of a novel phosphatidic acid binding domain in protein phosphatase-1. Biochemistry. 2005;44:13235–13245. doi: 10.1021/bi0505159. [DOI] [PubMed] [Google Scholar]
- 47.Mettlen M., Platek A., Van Der Smissen P., Carpentier S., Amyere M., Lanzetti L., de Diesbach P., Tyteca D., Courtoy P. J. Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic. 2006;7:589–603. doi: 10.1111/j.1600-0854.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 48.Corrotte M., Chasserot-Golaz S., Huang P., Du G., Ktistakis N., Frohman M. A., Vitale N., Bader M.-F., Grant N. J. Stepwise requirements for phospholipase D isoforms during phagocytosis in macrophages. Traffic. 2006;7:365–377. doi: 10.1111/j.1600-0854.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 49.Gaertner T. R., Putkey J. A., Waxham M. N. RC3/Neurogranin and Ca2+/calmodulin-dependent protein kinase II produce opposing effects on the affinity of calmodulin for calcium. J. Biol. Chem. 2004;279:39374–39382. doi: 10.1074/jbc.M405352200. [DOI] [PubMed] [Google Scholar]
- 50.Gerendasy D. Homeostatic tuning of Ca2+ signal transduction by members of the calpacitin protein family. J. Neurosci. Res. 1999;58:107–119. [PubMed] [Google Scholar]
- 51.Jouvenceau A., Hedou G., Potier B., Kollen M., Dutar P., Mansuy I. M. Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur. J. Neurosci. 2006;24:564–572. doi: 10.1111/j.1460-9568.2006.04938.x. [DOI] [PubMed] [Google Scholar]
- 52.Munton R. P., Vizi S., Mansuy I. M. The role of protein phosphatase-1 in the modulation of synaptic and structural plasticity. FEBS Lett. 2004;567:121–128. doi: 10.1016/j.febslet.2004.03.121. [DOI] [PubMed] [Google Scholar]
- 53.Strack S., Kini S., Ebner F. F., Wadzinski B. E., Colbran R. J. Differential cellular and subcellular localization of protein phosphatase 1 isoforms in brain. J. Comp. Neurol. 1999;413:373–384. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.