Abstract
Type 1 diabetes (T1D) is often considered the prototype organ-specific autoimmune disease in clinical immunology circles. The key disease features − precise destruction of a single endocrine cell type occurring on a distinct genetic and autoimmune background − have been unravelled in recent years to such an extent that there is a growing expectation that the disease should be curable. T1D is something of an orphan disease, currently managed by endocrinologists yet dependent upon the wit of immunologists, both basic and clinical, to find the best approaches to prevention and cure. Type 1 diabetes thus represents one of the most active arenas for translational research, as novel immune-based interventions find their way to the clinic. The first serious attempt at immune-based treatment for T1D was in 1984, the first at prevention in 1993; current and planned trials will take us into the next decade before reporting their results. This paper represents the first attempt at a comprehensive review of this quarter century of endeavour, documenting all the strategies that have emerged into clinical studies. Importantly, the intense clinical activity has established robust infrastructures for future T1D trials and frameworks for their design. The evident success of the monoclonal anti-CD3 antibody trials in established T1D demonstrate that modulation of islet autoimmunity in humans after the onset of overt disease can be achieved, and give some reason to be cautiously optimistic for the ability of these and other agents, alone and in combination, to provide an effective immunotherapy for the disease.
Keywords: T1D, immunotherapy, autoimmune disease
The clinical problem
Type 1 diabetes (T1D) is a chronic autoimmune disorder precipitated in genetically susceptible individuals by environmental factors [1]. The preclinical period is marked by the presence of autoantibodies to beta cell antigens, including insulin, glutamic acid decarboxylase-65 (GAD65) and the insulinoma-associated tyrosine phosphatase, IA-2. The detection of these in the serum is highly predictive of the development of T1D [2,3]. In addition to autoantibodies; the preclinical disease stage is also characterized by the generation of activated, self-reactive lymphocytes that infiltrate the pancreas and selectively destroy the insulin-producing beta cells present in the islets [4]. This persistent, targeted destruction occurs ‘silently’ and may go undetected for many years. By the time the first clinical symptoms (most notably those associated with hyperglycaemia) become apparent, nearly 80% of the patient's beta cells have been destroyed, rendering the individual dependent on insulin injections for their survival. The administration of insulin, however, is not a cure − even when used to maintain tight glycaemic control, it does not halt the persistent autoimmune response. Nor does it prevent, in all patients, the devastating long-term complications, such as kidney failure, blindness, nerve and cardiovascular damage. These severe degrees of morbidity, coupled with the increasing incidence of the disease and a current lack of tried and tested alternative therapeutic approaches to treatment, bring a new sense of urgency to the search for tolerance-inducing therapies that could halt T1D progression following diagnosis, or prevent the disease altogether.
Immune-based intervention in autoimmune diabetes has been attempted at two main stages of the disease process − prior to clinical onset but after the appearance of islet autoantibodies (secondary prevention) and immediately after diagnosis (intervention). Although prevention is preferable to intervention the challenges of identifying sufficient at-risk patients among the general population, coupled with safety concerns with many of the currently available agents, have placed a greater practical emphasis on new-onset therapies as the major ‘test bed’. Immunotherapy at this stage, when there is still some residual beta cell mass (about 15–20%), aims to provide evidence of safety and proof-of-principle of efficacy in prolonging or enhancing endogenous beta cell function, leading to improved blood glucose homeostasis. The Diabetes Control and Complications Trial (DCCT) [5] and the Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study [6] have shown that maintaining tight blood glucose control after onset can prevent or delay the long-term complications of diabetes. Thus, maintaining and enhancing endogenous beta cell function as a result of immune intervention after diagnosis could have a significant impact on the long-term disease outcome, as well as potentially identifying agents that would be effective for secondary prevention.
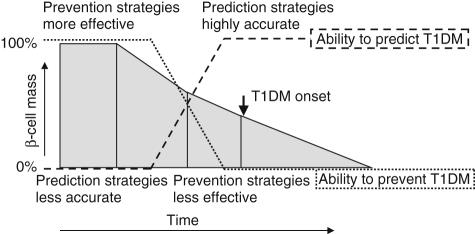
Decades of intense investigations of animal models of T1D, especially the non-obese diabetic (NOD) mouse, have led to the evaluation of a large number of potential interventions [7], many of which have shown promising results. However, translating these findings successfully to humans is proving to be significantly more challenging − both at the prevention and intervention stages. A number of therapeutic candidates have shown promise in animal models but mainly at the early stages of disease progression, i.e. during the preclinical phase. Unfortunately, this is also the stage at which the accuracy of disease prediction is the lowest (Fig. 1), making it difficult to justify ethically the application of many early treatments to individuals who may never develop T1D and thus face unwarranted potential side effects [8]. At the stage at which the disease can be predicted accurately or is already clinically manifest, the loss of beta cell mass is substantial, making potential therapies less effective in reversing or halting the autoimmune assault. Thus, as ever, the optimal therapeutic approach will be one that strikes the best risk–benefit balance between side effects and efficacy.
Fig. 1.
Model of prediction and intervention/prevention strategies in type 1 diabetes, as they relate to the timing of loss of beta cell mass (adapted from [8]).
Several additional factors underlie the chance of success of T1D clinical trials aimed at preventing or halting the disease. These include: the accurate translation of the dosing and scheduling regimen from animal models to human prevention/intervention trials; the notion that animal models are sufficiently good indicators of therapeutic success in a patient; and that the course of the autoimmune process is still amenable to modulation, especially after overt disease has been established. These factors add significant complexity to the quest for developing a successful prevention/intervention therapy for T1D.
Immunotherapeutic approaches for preventing or halting autoimmune diabetes have involved both antigen-specific and antigen non-specific approaches. Because T1D results from a failure to maintain immune tolerance to islet autoantigens, targeting these autoantigens should provide not only an effective means of controlling the autoimmune response but should also avoid the harmful effects associated with non-specific immunosuppression. Thus, antigen-specific approaches have been favoured over globally immunosuppressive therapies. Tables 1–4 summarize past and current T1D prevention and intervention trials based on antigen-specific versus non-specific agents.
Table 1.
Completed, ongoing and planned prevention trials in type 1 diabetes (T1D) using antigen-specific approaches
| Agent | Route | Stage of development | Details | References and links |
|---|---|---|---|---|
| Insulin | Parenteral | Pilot, completed 1993 | Small, pilot study, suggestive of efficacy | [88] |
| Insulin | Parenteral | Pilot, completed 1998 | Small, pilot study, suggestive of efficacy | [89] |
| Insulin (DPT-1) | Parenteral | Large efficacy study, completed 2002 | No effect seen on disease progression | [23] and http://www.diabetestrialnet.org |
| Insulin (DPT-1) | Oral | Large efficacy study, completed 2005 | No effect seen on disease progression; however, strong evidence from subanalysis of significant treatment effect on subjects with strong evidence of insulin autoimmunity. Repeat study planned | [22] and http://www.diabetestrialnet.org |
| Insulin (INIT I) | Intranasal | Phase I, completed 2004 | No acceleration of loss of beta cell function in individuals at risk for T1D Immune changes consistent with mucosal tolerance to insulin detected | [26] |
| Insulin (DIPP) | Intranasal | Phase I (ongoing) | [27] and http://research.utu.fi/dipp | |
| Insulin (INIT II) | Intranasal | Phase II, started December 2006 | Randomized, double blind, placebo-controlled trial of ntranasal insulin (1·6 mg or 16 mg) | https://studies.thegeorgeinstitute.org/init |
| Insulin | Oral | Efficacy study, planned | Repeat of oral arm of DPT-1 | http://www.diabetestrialnet.org |
| Insulin | Oral and intranasal | Pilot, planned | Pre-POINT study: dose finding in children with high genetic risk for T1D | [65] |
Table 4.
Completed, ongoing and planned intervention trials in type 1 diabetes (T1D) using non-antigen-specific approaches
| Agent | Stage of development | Details | References and links |
|---|---|---|---|
| Cyclosporine | Various trials completed 1984–96 | Remission induced successfully in recent onset patients, but therapy typically suspended due to unacceptable side-effects | [31,97] |
| Nicotinamide | Pilot | No effect | [98] |
| Anti-thymocyte globulin plus prednisolone | Pilot | Reduced insulin requirements more than 100 days after therapy; complicated by severe, transient thrombocytopenia | [99] |
| Bacille Calmette–Guerin (BCG) | Pilot | No effect | [62–64] |
| Diazoxide | No effect | [100,101] | |
| IFN-γ | Phase I | Small pilot; possible effect | [102] |
| PRODIAB (oral protease) | Phase I | No effect | IMDIAB Study Group, Rome |
| Anti-CD3 MoAb hOKT3g1(Ala-Ala) | Phases I/II, completed 2002 | Remission out to 18 months | [52] |
| Anti-CD3 MoAb ChAglyCD3(TRX4) | Phase II, completed 2005 | Reduced insulin requirement out to 18 months | [44] |
| hOKT3g1(Ala-Ala) | Phase II | Ongoing. Two courses of the Ab administered 1 year apart. | [19] |
| hOKT3gl (Ala-Ala) | Phase II | Ongoing. Single course of the Ab administered in patients with T1D of 4–12 months duration since diagnosis. | NCT003785081 |
| hOKT3g1(Ala-Ala) | Phases II/III | Planned | [54] |
| ChAglyCD3(TRX4) | Phase II/III | Planned | [55] |
| anti-CD20 MoAb (Rituximab) | Phase II | Ongoing | [20] |
| Anti-thymocyte globulin (ATG) | Phase I | Ongoing | NCT001905021 |
| Anti-thymocyte globulin (ATG) | Phase II | Planned | [19,20] |
| anti-CD52 (Campath-1H) | Phase I | Planned | NCT002142141 |
| Autologous umbilical cord blood cells | Phase I | Ongoing | NCT003053441 |
| Autologous gene-engineered DCs | Phase I | Starts 2007 | http://www.chp.edu/research/03_diabetes_research_stud.php#safestudy |
Clinical trial identifier; see http://www.ClinicalTrials.gov
Prevention trials in T1D
Early recognition of disease development
For prevention of T1D and also other autoimmune disorders, the paradigm has emerged that intervening early during the development of disease bears greater promise for success. This is presumably the case because organ damage has not progressed as far as a critical point of ‘no return’ and perhaps the self-regenerative capacity of beta cells is still well preserved. It is also possible that there are early ‘premalignant’ phases at which the autoimmune response is present but lacks destructive intensity, as has been described in the NOD mouse [9]. In T1D, we now have the ability to identify individuals at risk of developing disease by screening for islet autoantibodies, with genetic markers in the HLA region an additional risk factor [3]. Metabolic assessments can also be included to identify late stages of disease development, at which there is already some loss of glycaemic control. Thus, it is now feasible to ‘stage’ individuals according to various modelled rates of progression to T1D. In assessing the risk–benefit analysis of a particular therapy, this staging process may be of great benefit: the greater the projected risk and rate of progression, the more appropriate it may be to use therapies with greater potential side effects but a greater chance of efficacy.
Antigen-specific approaches or not? The advantage of T regulatory cells (Treg) induction
In general, one has the choice between antigen-specific and antigen non-specific interventions (Tables 1 and 2). The former have the advantage that systemic, generalized immunosuppression can be circumvented and have therefore been of great interest for the prevention and treatment of T1D, as the major short- and long-term consequences of systemic immunosuppression should be avoided. The concept of antigen-specific tolerance has been apparent for some years. Tolerance can occur as a result of anergy or deletion of antigen-specific, autoreactive T cells, although it seemsprobable that these mechanisms predominate when high-dose antigen is used [10,11]. More recently it has become apparent that tolerance can also involve active, regulatory mechanisms in the form of Treg induction. Treg have the advantage of actively down-modulating immunity to other antigens by acting as bystander suppressors [12]. In addition they can promote tolerogenic, regulatory responses to other (auto)-antigens, a process termed ‘infectious tolerance’[13,14]. The key advantage of autoantigen-specific Treg over the use of systemic immunosuppressants is their antigen specificity. Treg specific for self-antigens will be activated only at sites of active inflammation, where their cognate autoantigens are presented. In theory, therefore, autoantigen-induced Treg can act as site-specific, highly selective immunosuppressants, without compromising systemic immunity to viruses or other pathogens, thus maintaining full immune competence. Furthermore, they should be able to exploit natural mechanisms for Treg maintenance and regeneration, offering the prospect of long-lived tolerance. Advances in epitope identification [15] should encourage peptide immunotherapy, which continues to show therapeutic promise in several clinical arenas [10].
Table 2.
Completed, ongoing and planned prevention trials in type 1 diabetes (T1D) using non-antigen-specific approaches
| Agent | Route | Stage of development | Details | References and links |
|---|---|---|---|---|
| Ketotifen (histamine antagonist) | Oral | Pilot, completed 1994 | No effect | [90] |
| Cyclosporin | Oral | Pilot, completed 1996 | Delay but not prevention in high-risk group | [91] |
| Nicotinamide (Deutsche Nicotinamide Intervention Study, DENIS) | Oral | Efficacy study, completed 1998 | No effect | [92] |
| Nicotinamide European Nicotinamide Diabetes Intervention Trial (ENDIT) | Oral | Efficacy study, completed 2004 | No effect | [35] |
| Various nicotinamide combinations (plus cyclosporin, intensive insulin therapy, vitamin E) | Oral | Pilots 1994–2005 | No additive effects | [93–95] |
| Bacille Calmette–Guerin (BCG) | i.d. | Various pilot studies | No effect | [32–34] |
| Dietary gluten elimination | Oral | Pilot, completed 2002 | No effect on autoantibodies or disease | [96] |
| Vitamin D3 | Oral | Phase I, ongoing | NCT001419861 | |
| Hydrolysed cow's milk (TRIGR) | Oral | Phase I, ongoing | [36,37] | |
| Docosahexaenoic acid (DHA) | Oral | Pilot, ongoing | http://www.diabetestrialnet.org |
Clinical trial identifier; see http://www.ClinicalTrials.gov
Tracking induced Treg responses successfully to monitor trial outcome
Treg can act as bystander suppressors through a variety of mechanisms, which are not mutually exclusive. One major mode of action is the induction of anti-inflammatory cytokines, such as interleukin (IL)-4, IL-10 or transforming growth factor (TGF)-β[12]. Such cytokines can dampen the function of antigen-presenting cells (APC), which is context-dependent and can be overridden by major inflammatory stimuli, such as Toll-like receptor (TLR) ligation or IL-6 production [16]. In addition, Treg might act directly on responder cells or APC using cell–cell contact-dependent mechanisms, which might not involve the action of regulatory cytokines [17]. Lastly, as some Treg express high levels of CD25 (IL-2 receptor α chain), they can lower the bioavailability of IL-2 via receptor binding and in this way inhibit responder cell proliferation, which might be particularly relevant in in-vitro assays. Treg are heterogeneous, may act through a multitude of mechanisms and probably depend on the presence of APC for their function, and for these reasons it is proving difficult to design and implement suitable in vitro assays that reflect accurately their activity in vivo[17].
Establishment of suitable in vitro assays to monitor Treg function is of paramount importance for the success and design of clinical prevention trials that involve the antigen-specific modes of action or polyclonal activation of Treg[18]. The goal is to develop these as secondary measures that can be evaluated early, obviating the need to conduct long and expensive prevention trials to end-points such as maintained or elevated C-peptide levels or overt clinical diabetes development. There is progress in this direction in several laboratories, with published reports awaited eagerly. Currently, assays that detect the balance of proinflammatory and putative regulatory autoimmune responses are being evaluated for sensitivity and specificity on a larger scale, an effort promoted by consortia within the Immune Tolerance Network (ITN) and Type 1 Diabetes TrialNet [19,20].
Prevention trials − antigen-specific
Despite there seeming to be more questions than answers, numerous prevention trials have been conducted in recent years, promoting stepwise advances in the clinical arena. Most notably, based on encouraging findings in murine models by Weiner and others [21], oral insulin has been used in large randomized, controlled, blinded prevention trials (Diabetes Prevention Trial-1; DPT-1) in which some 100 000 first- and second-degree relatives of T1D patients were screened for risk before enrolment ([22] and Table 1). At their conclusion, neither the parenteral [23] nor the oral [22] insulin therapies could claim to show evidence of protection from T1D development. However, a subgroup analysis was performed to test the possibility that oral insulin might have greater effect as an immune modulator when given to subjects with high-titre insulin autoantibodies (IAA), as a marker of dominant insulin autoimmunity [22]. Importantly, this demonstrated a significant treatment effect in the oral insulin-treated, high-titre IAA group, which has been used as the rationale for a repeat study conducted by Type 1 Diabetes TrialNet to address this specific hypothesis [20]. As has already been alluded to, a key issue in the design of such studies has been the selection of dose on the basis of rodent studies. Conversion of doses in mice to human equivalents is based usually on surface area. The optimal dose for efficacy in the murine model was 100 mg/kg (equivalent to 300 mg/m2 in man) and a slight effect was observed at 10 mg/kg (equivalent to 30 mg/m2 in man) [24,25]. Moreover, mice received treatment twice per week giving a human equivalent of 600 mg/m2/week at the most efficacious dose. The DPT-1 dose of 7·5 mg/day is equivalent to 10·7–17·7 mg/m2/day, which is five- to eightfold less than the optimal weekly dose equivalent in the mouse. Unfortunately, the sheer numbers of subjects required for prevention studies of this nature preclude any possibility of a multiple-arm approach in which escalating doses are evaluated. Instead, these issues may need to be addressed in smaller studies which rely upon the use of surrogate immunological markers to evaluate dose effects, such as the planned ‘pre-POINT’ initiative as a dose-finding phase of POINT (Primary Oral/Intranasal Insulin Trial) (Table 1).
Additional trials of intranasal insulin are under way [Diabetes Prediction and Prevention Project (DIPP) and Intranasal Insulin Trial (INIT); Table 1][26,27]. Intranasal use of insulin might be of advantage, because lower dosages can be used and intranasal uptake could possibly be assessed and controlled more easily than the oral route. It is noteworthy that there is recent strong evidence from mouse studies that insulin is a primary target antigen for the anti-islet effector response in the NOD mouse [28], while several human studies, although less clear-cut, also argue for the primacy of this autoantigen [29,30,103]. It is therefore even more important to define precisely the class of response that is elicited after mucosal insulin administration in humans. Obviously, T cells producing regulatory cytokines are desirable and might be favoured by mucosal antigen presentation, whereas augmentation of pre-existing effector memory responses has to be avoided. In this context it is important to consider that antigen-specific induction of Treg in mouse models is typically only effective early during diabetes pathogenesis, and not once disease is established, unless combined with systemic immune modulators (see discussion of combination trials below). One explanation might be that later, during pathogenesis, augmentation of aggressive effector cells specific for insulin outweighs the induction of existing or de novo Treg.
Prevention trials − systemic immune modulators
From the discussion in the previous section, two issues that inhibit deployment of antigen-specific suppression of T1D in the clinic are evident: we do not yet have reliable markers to distinguish and quantify Treg from effector cells and we are uncertain which antigen(s) might constitute the primary targets in human T1D. The lesson from DPT-1 may even be that the disease is heterogeneous, and that different autoantigens have primacy in different subgroups. In light of this, considerable efforts have been devoted to non-antigen-specific prevention of T1D (Table 2). These ‘systemic’ immune modulatory interventions carry the obvious risk inherent to any immune suppressive regimen: weakening of host defence and opportunistic infections and long-term complications such as the development of cancer. Therefore, sufficiently low dosages of these immune modulators have to be applied over relatively brief periods, which may well compromise their efficacy. Indeed, it has been known since the late 1980s that powerful immune suppressants such as cyclosporin can have beneficial effects, yet at too high a price [31]. Other systemic immune modulators that are safe, such as bacille Calmette–Guerin (BCG) and nicotinamide, are at the opposite extreme, showing no promise of efficacy [32–35]. Finally, there is the distinct possibility that primary prevention (i.e. interventions in high risk groups before evidence of islet autoimmunity is manifest) will be effective and have an acceptable risk profile. In this regard, the results of the Trial to Reduce IDDM in the Genetically At-Risk (TRIGR) intervention [36,37], testing the hypothesis that early exposure to cow's milk protein is a risk factor, are eagerly awaited. Overall, it will be difficult to walk the line between tolerable immune suppression and long-term benefit, and therefore combination approaches using drugs that act at different checkpoints in the disease process, or that exhibit effects on the Treg compartment beyond their half-life in blood (such as monoclonal anti-CD3 antibody therapy, see discussion in next section) will have to be utilized. In addition, the lessons learned from treating cancer with multiple cytotoxic agents should be considered, namely that toxicity profiles for various agents are different and therefore combination therapy in which each drug is used at a relatively suboptimal dose can be very synergistic in terms of efficacy without being additive in terms of toxicity.
Prevention trials in T1D − conclusions
The negative results of the major prevention trials using insulin and nicotinamide (DPT-1 and the European Nicotinamide Diabetes Intervention Trial (ENDIT) [35]), leaving aside the encouraging subanalysis in oral DPT-1, reveal the complex nature of the disease and the difficulties in designing a preventive strategy. However, these studies also led to the establishment of important clinical infrastructures for the future. As an example, the Natural History Study being conducted by Type 1 Diabetes TrialNet [20] may provide more clues in relation to pathogenesis and offer cues for enhancing therapeutic approaches. Others, such as the clinical trial arms of Type 1 Diabetes TrialNet and the Immune Tolerance Network, offer a future in which ‘best practice’ for prevention studies can be developed, as well as a robust infrastructure to face the challenge of screening and recruitment [19,20]. Additional consortia, such as The Environmental Determinants of Diabetes in the Young study group (TEDDY [38]) and the newly developed Diabetes Research Clinical Network in the United Kingdom [39] are further examples of the spread of such initiatives. New impetus has been added to the study of the early phases of this condition by the organized acquisition of pancreata from prediabetic individuals [40], which has been conducted hitherto on an opportunistic basis. Indeed, if one considers the major therapeutic successes of immunotherapy, these have all been made in diseases such as rheumatoid arthritis (RA) and psoriasis, in which access to, and monitoring of the affected organ are much easier. Lastly, suitable T cell assays to identify the correct class of autoreactive T cell response after successful trials can be expected to emerge and will greatly aid trial design and interpretation.
New-onset T1D trials
Recent-onset T1D and beta cell regeneration/replication
Immunomodulation to reverse recent-onset diabetes is a clinical approach of considerable interest, for two major reasons. First, it is ethically easier to justify risk, because the disease is present and spontaneous long-lasting remission is unheard of. Secondly, intervention studies at this stage can be of much shorter duration (12–24 months), making them more affordable and more rapid in providing results.
How can clinically established diabetes be reversed? It is important to realize that loss of beta cells is a gradual process and that onset of hyperglycaemia does not signify the loss of all insulin reserve, but rather the presence of an insufficient beta cell mass or insulin production. Therefore, reversal of hyperglycaemia (which is also recognized to occur spontaneously for a brief period in some patients and is known as the ‘honeymoon’ period) could arise as a result of several putative physiological mechanisms, although their existence awaits firm confirmation. The first is the possible endogenous regeneration and replication of remaining beta cells, perhaps enabled by the initiation of insulin replacement therapy. This process may occur naturally in islets that have undergone immune damage, but present therapeutic efforts are focused on attempts to promote more generalized beta cell regeneration or replication throughout the pancreas. The second is the reduction of ‘metabolic stress’ on the remaining beta-cells, which has been found beneficial in several animal models [41]. In support, it is known that early and intensive insulin therapy in patients with recent-onset T1D can clearly decelerate the loss of remaining beta cell capacity (i.e. C-peptide decline [42]). A third, provocative possibility is that there is a transient change in immune phenotype after diagnosis and towards the honeymoon, from proinflammatory [interferon (IFN)-γ] to anti-inflammatory (IL-10) cytokine production, as proposed by Roep [43]. From these considerations it becomes clear that interventions in recent-onset T1D have to be operational within a small and clearly defined window, which might also vary on an individual basis. So far, most trials have included patients within 3 months of first diagnosis and with remaining beta cell mass as assessed by C-peptide measurements. It has also become evident that those patients with a greater remaining beta cell mass (i.e. higher C-peptide levels) may be more responsive to immune modulation [44].
Intervention trials in recent-onset T1D − antigen-specific
None of the antigen-specific trials have shown convincing promise in recent-onset T1D thus far (Table 3). Ongoing endeavours include the administration of insulin B chain with incomplete Freund's adjuvant, which has been highly effective in preventing diabetes in animal models [45] but has not so far been fully evaluated in recent-onset T1D studies. In addition, an altered peptide ligand based on putative major autoantigenic sites in the insulin B chain, which had induced strong T helper 2 (Th2) responses in animal models, has been administered without any evidence of success [46,47]. Of promise may be a DNA vaccine (BHT-3021) developed by Bayhill Therapeutics [48], which has shown striking efficacy in recent-onset diabetes in the NOD mouse, and early clinical trials should begin soon [49]. Lastly, Diamyd's GAD-65 protein has shown promising results in their first safety study in subjects with late-onset autoimmune diabetes of adulthood (LADA) [50] and preliminary, unpublished efficacy studies in children with T1D [51]. It is too early to judge whether these studies signify a trend in which GAD65 emerges as a primary autoantigen in human T1D.
Table 3.
Completed, ongoing and planned intervention trials in type 1 diabetes (T1D) using antigen-specific approaches
| Agent | Route | Stage of development | Details | References and links |
|---|---|---|---|---|
| NBI-6024 (APL of insulin) | s.c. completed | Phase I, | [47] | |
| Insulin B chain plus incomplete Freund's adjuvant | s.c. | Phase I, completed | http://www.immunetolerance.org/research/autoimmune/trials/orban1.html | |
| DiaPep277 (hsp60 peptide) | s.c | Phase II, completed | Phase II in adults reports preservation of C-peptide at 12–18 months | [58] |
| Phase II in children reports no treatment effect | [60] | |||
| Proinsulin peptide vaccine | i.d. | Phase I (ongoing) | [85] | |
| GAD65 | s.c | Phase I, completed in LADA | Phase II completed in children with T1D, report awaited | [50] http://www.diamyd.com |
| Proinsulin-based DNA vaccine (BHT-3021) | i.m. | Phase I planned | http://www.bayhilltherapeutics.com |
Intervention trials in recent-onset T1D − antigen non-specific
As listed in Table 4, several antigen non-specific intervention studies have been completed in recent-onset T1D. The most noteworthy and encouraging outcome has been seen after short-term courses of monoclonal antibodies directed against CD3 [44,52,53] and designed to lack Fc-binding capacity, thus removing any propensity of the therapy to lead to major T cell depletion. These antibodies lead to a short-term cytokine release syndrome and a transient loss or redistribution of T cells, but after the treatment course, full immunocompetence is re-established and, interestingly, there is an enhancement of CD25+ forkhead box P3 (FoxP3)+ populations. This long-term polyclonal increase of putative Treg could be responsible for the long-term protection (18–24 months) obtained in both of the published studies. Current and planned phase II/III trials being pursued by MacroGenics and TolerRx will hopefully build upon these encouraging observations [54,55]. It is, however, important to consider that in all probability the drug doses used thus far cannot be increased any further (current treatment levels for one of the anti-CD3 antibodies induced reactivation of latent Epstein–Barr virus infection [44], while the other treatment reported a high proportion of patients developing antibodies to the drug, which may preclude repeated use [52]). As an alternative, anti-CD3 monoclonal antibody could be given at its current dose regimen before the onset of clinical T1D or in combination with other interventions. This combination approach would ideally incorporate antigen-specific interventions (a combination that works well in the murine model [56]), and/or compounds that foster islet cell regeneration (e.g. exenatide [57]).
Another agent which showed early promise was the administration of a heat shock protein peptide, hsp277, designed initially as an antigen-specific approach on the basis of evidence that the peptide is targeted as an autoantigen in T1D and murine models [58,59]. The encouraging results derive from several, somewhat underpowered investigations which offer contrasting results [60], but not yet from any large-scale clinical trials. The current putative mechanism of action is a systemic immune modulation through activating TLR2 and thus induction of Treg[61]. Additional intervention trials with systemic agents are currently planned using anti-thymocyte globulin (ATG), which has been re-engineered by Genzyme, is efficacious in animal models, and has been used widely with success in clinical transplantation. Most interestingly, monoclonal anti-CD20 antibody (Rituximab), which depletes B cells and could act to reduce their antigen-presenting capacity or the proinflammatory effect of islet autoantibodies, is currently on trial in recent-onset T1D [20]. This compound, currently Food and Drug Administration (FDA)-approved for the treatment of RA and non-Hodgkin's lymphoma (NHL), has a relatively good safety profile and might provide an indirect pathway for interfering with the APC–T cell axis.
On the negative side, immunization with the BCG agent, or the use of IFN-α as systemic immune modulators have failed to show any effect despite, in both cases, promising results in animal models [62–64].
Intervention trials in recent-onset T1D − conclusions
Recent onset T1D is undoubtedly an ideal starting point for testing and refining immune-based intervention strategies, due to the shorter trial duration, lesser cost and more favourable risk–benefit analysis. We have learned that systemically dampening the immune system can be more effective than antigen-specific immune-based interventions at this stage, although the latter approaches remain in the very early stages of development where safety has been the emphasis. At diagnosis, it is possible that the anti-islet effector response has sufficient impetus that antigen-specific manipulations alone cannot work, and that suitable therapeutic windows need to be created for beta cell regeneration and the emergence of Treg to occur. It is therefore reasonable to assume that a systemically acting immunosuppressant will probably have to be part of any immune intervention in recent-onset T1D (see combinatorial trails in the last section of this review). There is no doubt that the success of intervention at T1D diagnosis that has been achieved, for example with anti-CD3, opens the door for these approaches to be deployed at earlier phases of the disease.
The crystal ball approach: surveying the horizon for new therapies
Agencies such as the Juvenile Diabetes Research Foundation (JDRF [65]) and other funding bodies have undertaken considerable efforts in recent years to promote drugs and therapeutic concepts from other autoimmune diseases that might potentially be of benefit in T1D. The most noteworthy candidates and ongoing endeavours are listed in Table 5 and will be discussed briefly.
Table 5.
Completed, ongoing and planned intervention therapies for autoimmune diseases other than type 1 diabetes (T1D)
| Agent | Stage of development | Details | Investigator/developer | Links |
|---|---|---|---|---|
| Targeting T cells, including antigen-specific therapies | ||||
| NI-0401 (anti-CD3) | Phase IIa | CD | NovImmune/Serono | http://www.novimmune.com |
| Visilizumab (Nuvion) (anti-CD3) | Phase II | UC, psoriasis, GvHD | PDL | http://www.pdl.com |
| BHT-3009 (antigen-specific) | Phase II | MS | Bayhill Therapeutic | http://www.bayhilltherapeutics.com |
| Abatecept (CTLA4-Ig) | Marketed | RA, MS | Bristol-Myers Squibb | http://www.bristolmyerssquibb.com |
| Belatacept (LEA29Y) | Phase III | Transplantation | Bristol-Myers Squibb | http://www.bristolmyerssquibb.com |
| TRX1 (anti-CD4) | Phase Ib | Cutaneous lupus | TolerRx/Genentech | http://www.tolerrx.comhttp://www.genentech.com |
| HuMax-CD4 (Zanolimumab) | Phase III | CTCL | Genmab/Serono | http://www.genmab.com |
| Phase II | Non-cutaneous T cell lymphoma | http://www.serono.com | ||
| Campath® (alemtuzumab) (anti-CD52) | Marketed | B-CLL | Genzyme | http://www.genzyme.com |
| Fodosine (transition-state analogue inhibitor of PNP) | Phase II | CTCL, CLL | BioCryst Pharmaceuticals | http://www.biocryst.com |
| AT-001 (orally available peptide derived from hsp dnaJ) | Phase II | RA | Androclus Therapeutics | http://www.androclus.com |
| Anti-CD40L | Currently discontinued | Transplantation, SLE | Biogen Idec, Novartis Bristol-Myers Squibb | |
| Treg cell therapy | Proof-of-concept | HSCT | Maria-Grazia Roncarolo | [106] |
| Targeting antigen presentation and B cells | ||||
| HCD122/CHIR-12·12 (fully human anti-CD40 antibody) | Phase I | CLL, ML | Xoma/Chiron | http://www.xoma.comhttp://www.chiron.com |
| SGN-40 (humanized Anti-CD40 MoAb) | Phase I | MM, NHL, CLL | SeattleGenetics | http://www.SeattleGenetics.com |
| Anti-CD40 | Phase I | CD | PanGenetics | http://www.PanGenetics.com |
| 2nd-generation anti-CD20 | Phases I/II | RA | Genentech/Hoffmann-LaRoche/Biogen Idec | http://www.genentech.com |
| IMMU-106 (anti-CD20) | Phase I | NHL | Immunomedics | http://www.immunomedics.com |
| TRU-015 (CD20 antagonist) | Phase II | RA | Trubion Pharmaceuticals | http://www.trubion.com |
| Epratuzumab (anti-CD22) | Phase III | SLE | Immunomedics | http://www.immunomedics.com |
| Phase II | Sjögren's syndrome | |||
| BR3-Fc | Phases I/II | RA | Genentech/Biogen Idec/Roche | http://www.genentech.comhttp://www.biogenidec.com |
| Atacicept (TACI extracellular domain fused to human IgG1) | Phase Ib B-CLL, NHL | SLE, RA, MM, | ZymoGenetics/Serono | http://www.zymogenetics.comhttp://www.serono.com |
| LymphoStat-B | Phase II | SLE, RA | Human Genome Sciences | http://www.hgsi.com |
| CEP-701 (Flt-3 inhibitor) | Phase II | AML | Cephalon Inc | http://www.cephalon.com |
| ‘Personalized DCs’ | Phase I/II | CLL | Argos Therapeutics | http://www.argostherapeutics.com |
| Targeting cytokines/chemokines | ||||
| Anakinra (Kineret) (hrIL-1Ra) | Marketed | RA | Amgen | http://www.amgen.com |
| AMG108 (anti-IL-1 MoAb) | Phase II | RA | Amgen | http://www.amgen.com |
| IL-1 Trap (IL-1 neutralizer) | Pivotal trial | CAPS | Regeneron Pharmaceuticals | http://www.regeneron.com |
| Phase II | Inflammation, FMF | http://www.clinicaltrials.gov | ||
| Tenovil (rhIL-10) | Currently discontinued | MS, psoriasis, IBD, RA | Schering-Plough | http://www.schering-plough.com |
| Eternacept (Enbrel) (TNF-α antagonist) | Marketed | RA and plaque psoriasis | Amgen | http://www.amgen.com |
| Infliximab (Remicade) (anti-TNF-α) | Marketed | CD, RA, UC, psoriatic arthritis | Centocor/J & J | http://www.centocor.com |
| Adalimumab (Humira) (anti-TNF-α) | Marketed | RA, CD (off label) | Abbott Laboratories | http://www.abbott.com |
| CNTO 1275 (anti-IL-12/IL-23 p40) | Phase II | RRMS | Centocor | http://www.centocor.com |
| STA-5326 (IL-12/IL-23 inhibitor) | Phase IIb | CD | Synta Pharmaceuticals | http://www.syntapharma.com |
| SMART anti-IL-12 antibody | Phase I | AID | PDL/Hoffmann-La Roche | |
| AMG 714 (anti-IL-15) | Phase II | Inflammatory and AID | Amgen/Genmab | http://www.amgen.com |
| HuZAF (anti-IFN-γ) | Phase II | RA | PDL/Biogen Idec | http://www.pdl.com |
| Targeting adhesion molecules | ||||
| Amevive (Alefacept) | Marketed | Psoriasis | Biogen Idec | http://www.biogenidec.com |
| Efalizumab (Raptiva) | Marketed | Chronic plaque psoriasis | Genentech | http://www.genentech.com |
| Natalizumab (Tysabri) | Marketed | MS | Biogen Idec | http://www.biogenidec.com |
| FTY720 | Phase II | MS | Novartis | http://www.novartis.com |
| S1P1 agonist | Phase I | AID | Actelion/Roche | http://www.actelion.com |
| Other approaches | ||||
| Atorvastatin (Lipitor) | Marketed; Phase II | Cholesterol lowering; MS | Pfizer | http://www.pfizer.com |
| Gleevec | Marketed | CML | Novartis | http://www.novartis.com |
| AAT (alpha-1 anti-trypsin) (Aralast) | Approved | AAT deficiency | Baxter Healthcare | http://www.baxter.com |
| AT-1001 | Phase II | Coeliac disease | Alba Therapeutics | http://www.albatherapeutics.com |
| EGCG (epigallocatechin gallate) green tea extract | Phase I | MS | PI: Frauke Zipp | Zipp F et al. unpulished observations |
| CP-690, 550 (a JAK 3 inhibitor) | Phase II | RA | Pfizer | http://www.clinicaltrials.gov |
CD: Crohn's disease; UC, ulcerative colitis; GvHD, graft-versus-host disease; MS, multiple sclerosis; RA, rheumatoid arthritis; LADA, latent autoimmune diabetes in adults; CTCL, coetaneous T-cell lymphoma; B-CLL, B-chronic lympocytic leukaemia; PNP, purine nucleoside phosphorylase; MM, multiple myeloma; NHL, non-Hodgkin's lymphoma; AID, autoimmune disease; SLE, systemic lupus erythematosus; AML, acute myeloid leukaemia; IBD, inflammatory bowel disease; RRMS, relapsing-remitting MS; Flt-3, FMS-like tyrosine kinase 3; CAPS, CIAS1-associated periodic syndromes; HSCT, haematopoietic stem cell transplantation, PNP, purine nucleoside phosphorylase.
Targeting T cells
Bristol-Myers Squibb has potentially interesting compounds that are licensed for RA and multiple sclerosis (MS) (CTLA4-Ig) or are under investigation in solid organ transplantation (the engineered high-affinity variant, LEA29Y) [66]. These agents will be systemically immunosuppressive and it will be important to assess whether their modulatory activity can reach beyond the time the drug is present in the body, as has been shown for the Treg compartment following anti-CD3 administration. If this proves not to be the case, then the safety profile of the drug will be under greater scrutiny than before in the context of T1D. The dilemma in relation to CTLA4-Ig and T1D is that, to date, rodent studies have shown a mixed level of efficacy, ranging from impressive disease protection to disease acceleration [66].
Novel anti-T cell monoclonal antibodies (e.g. anti-CD4 [55], anti-CD45 [67,107]) may also prove of interest. In both cases, preclinical data look encouraging and also indicate beneficial effects on the Treg compartment. Long term, it will be interesting to see whether affecting Treg indirectly by systemic drug therapy (see above) or by direct polyclonal amplification ex vivo (autologous cell therapy as currently being developed, for example by the JDRF Collaborative Center for Cell Therapy [68]) will be the most efficient way to re-establish immune regulation in T1D.
Targeting APC
CD40 is a key molecule required for ‘licensing’ of APC by T cells expressing CD40L in order to induce strong effector responses. Agonist antibodies for therapy in cancer are under development by Kirin Pharmaceuticals [104]. Agents that interfere with this pathway have been of high interest for preventing autoimmunity and, indeed, blockers for CD40L have been strikingly effective in animal models for various autoimmune diseases such as systemic lupus erythematosus, T1D and the animal model of MS, experimental allergic encephalomyelitis [69,70]. Clinical investigations with antibodies to CD40L have run into major difficulties, because CD40L is expressed on human platelets and therefore these agents lead to platelet aggregation through a hitherto undefined pathway; current trials are therefore on hold. Directly blocking CD40 on APC might circumvent such problems and antibodies to achieve this are under development by others.
B cell activation factor from the TNF family (BAFF) blockers might constitute an alternative to anti-CD20 treatments to target the antigen-presenting capacity of B lymphocytes, but one should probably best await the outcome of current clinical trials with anti-CD20 in T1D (see previous section). Lastly, the family of genes originally isolated from the Susan McDonough strain of FMS-like tyrosine kinase 3 (FLT3) inhibitors might interfere globally with the expansion of dendritic cells. Here, the issue of whether expanding DCs is helpful in preventing T1D, as has been suggested by some studies in the NOD model [71,105], or whether dampening DC function will be more effective, is not yet resolved. This controversy centres on the finding by some groups that NOD mice have a DC defect, which has not been established by all investigators in similar studies in humans afflicted with T1D. Therefore, it might be prudent to await further data on the function of DCs in patients with T1D and prediabetic individuals to determine how DC function should be influenced and which subset of DCs should be targeted.
Targeting natural killer (NK) T cells
The role of NK T cells in T1D has been discussed for many years. Strong evidence for their potential protective function has been based on the beneficial outcome of immunization with alpha-galactosyl-ceramide (Kirin compound 7000) in animal models [72–74], which is a good mimic of one of the cognate ligands recognized by NK T cells in the context of CD1. However, NK T cells produce both IL-4 and IFN-γ following their activation in mice and humans [75], and it is unclear whether there is a functional defect in NK T cells in humans with T1D, as individual NK T cell numbers vary considerably [76]. From recent studies in experimental autoimmune encephalomyelitis (EAE) models it is known that the precise ligand recognized by NK T cells defines the protective function and it is therefore reasonable to view α-galactosyl-ceramide (α-Gal-Cer) as some type of altered lipid ligand, which tends to skew immunity by NK T cells in a protective direction [77,78]. Protection, based on findings in animal models, is likely to be based on the ability of NK T cells to condition APC to induce Treg. A preclinical trial is under way to explore the potential of NK T cell activation by α-Gal-Cer in humans with T1D.
Targeting cytokines/chemokines
The role of IL-1β in beta cell destruction is well established, based on data from rodent models and from human islet studies [79]. Anakinra, a clinically licensed IL-1β receptor antagonist developed by Amgen for RA, might therefore be beneficial in preserving beta cell function in T1D, especially in light of encouraging data from a recent T2D trial showing improved beta cell secretory function and reduced markers of systemic inflammation (Mandrup-Poulsen and coworkers, unpublished observations). A potentially better alternative in terms of administration might be AMG108 (an anti-IL-1 monoclonal antibody), which is in phase II trials for RA. Direct administration of cytokines with known regulatory function such as IL-10 is fraught with the risk of inflammatory systemic side-effects, and dogged by the problem of a short half-life. Suitable delivery vectors might have to be used, but in this case the duration of expression might be hard to control. It might be better to ‘deliver’ cytokines directly to the site of islet inflammation and the pancreatic lymph node by induction of Treg. Short-term administration of cytokines might be useful to enhance induction of Treg in combination therapy regimens (see last section of this review).
Targeting adhesion molecules
The sequential entry of lymphocytes into single islets is believed to be a repetitive and key event on the road to T1D development. Therefore, interfering with lymphocyte rolling and extravasation/tissue entry is a highly attractive area for interventions, even during late phases of the disease. To date, limited preclinical and no clinical data are available for T1D. However, there are several compounds that have shown success in other diseases and are therefore of high potential interest for testing in T1D. Alefacept (Amevive) is a fusion protein between human leucocyte function-associated antigen-3 (LFA-3) and the human IgG1 antibody Fc region. By binding to the CD2 antigen on T cells, Alefacept prevents T cell activation and triggers apoptosis preferentially in memory-effector T cells and has shown good efficacy in psoriasis [80]. Efalizumab targets LFA-1 and also shows promise in a significant proportion of patients with psoriasis [81] and could therefore be tested in recent-onset T1D. Alefacept and Efalizumab exhibited an excellent safety profile and no marked enhancement of opportunistic infections was noted. On the negative side, such compounds might also restrict access of Treg to the islets and pancreatic lymph node, but no data to support this potential drawback are available today; perhaps it is an issue best addressed in preclinical studies. FTY720, a compound developed by Novartis, might also be very well suited to inhibit T cell extravasation and trafficking to inflamed areas.
Other targeting strategies
Interestingly, there are several compounds that one would not necessarily associate with the pathogenesis of a lymphocyte-mediated disease such as T1D, but which have shown promise in animal models. Among them are alpha-1 antitrypsin [82] which appears to affect trafficking and Treg function. Statins appear to exhibit beneficial effects in MS and RA models but not in the NOD mouse [83] and might therefore not be well suited for the treatment of T1D. Overall, mining for novel compounds by examining which interventions show promise in other autoimmune diseases is an important and valid approach to developing new strategies to prevent or revert T1D. However, one has to be cautious with systemic immune modulators, as such drugs might not be suited for prolonged application in humans, especially not in the young. Due to these ethical considerations, a combination therapy ‘cocktail’ of more than one effective compound will probably be needed to reverse T1D. In the final section we will introduce some promising endeavours along these lines.
Combination trials in T1D
Clearly, a single effective immune-based therapeutic approach has not been identified thus far. Among the best results is the halt in C-peptide decline following administration of non Fc-binding anti-CD3 in recent-onset T1D [44,52]. Therefore, it seems probable that combination approaches that involve autoantigen-specific immune modulation and induction of Treg that can mediate long-term tolerance will be needed to tackle this multi-faceted disease successfully. In addition to blocking the autoimmune response, we should also be thinking about enhancing beta cell function, for example through the use of exanatide and future drugs that can induce beta cell regeneration or replication (e.g. epidermal growth factor and gastrin, which are under investigation by Transition Therapeutics, Toronto, Canada, and NovoNordisk, Denmark [84]).
Table 6 lists some of the ongoing efforts to establish combinatorial therapies. It is noteworthy that combination of non-depleting anti-CD3 (Fab′)2 with a nasally delivered proinsulin peptide or nasal or oral insulin has exhibited strong synergy in two mouse models for T1D by enhancing induction of CD25+ FoxP3+ Treg and their secretion of immune modulatory cytokine such as TGF-β and IL-10 [56]. This strategy is likely to be pursued by the Diabetes Vaccine Development Centre in the future [85].
Table 6.
Completed, ongoing and planned prevention/intervention trials in type 1 diabetes (T1D) using combination approaches
| Agent | Stage of development | Details | References and links |
|---|---|---|---|
| Anti-CD3 and intranasal insulin | Phase II | Planned, recent onset T1D | [85] |
| Mycophenolate mofetil and anti-CD25 MoAb (Daclizumab) | Phase II | Ongoing, recent onset T1D | [20] |
| Anti-CD3 and Exenatide | Phase II | Various trials planned in recent onset T1D and in at-risk individuals (prevention) | [20] |
| IL-2 and Rapamycin | Phase II | Planned | [19,20] |
| Exenatide and Gastrin | Phase II | Planned | http://www.transitiontherapeutics.com, http://www.jdrf.org |
The combination of mycophenolate mofetil and anti-CD25 monoclonal antibody (Daclizumab) is currently under evaluation in an efficacy study in new onset T1D patients [20]. It combines two immune suppressive agents which have proved utility in other autoimmune disease settings where inhibition of T cell activation and proliferation is required, and also in the context of transplantation, including human islet grafting. The combination of IL-2 and rapamycin has impressive results from preclinical studies to support its rationale, which is that IL-2 will promote the expansion of CD4+ CD25+ Treg in preference to activating CD25+ effector T cells if the expansion of the latter can be simultaneously blocked with rapamycin [86]. One stark reality that should be considered is that, in general, drug companies are reticent to explore combination therapies, at least in the early stages of drug development.
Conclusions
Since the discovery of ‘insulin replacement’ in the early 1920s and its first life-saving application in a patient with juvenile diabetes, the hope has lingered that a cure for the disease is within reach. Today, despite the many remaining challenges in the field of T1D immunotherapy, hope is steadfastly approaching reality. A number of ongoing long-term prospective studies should provide important insights into disease pathogenesis and may reveal potential environmental triggers. Although the results of the completed prevention trials have thus far not met with success, they have examined and ruled out some antigen-specific and non-specific approaches that will inform the design of new studies. Importantly, the large prevention trials have established infrastructure for future T1D trials. The promise of the anti-CD3 trials in the new onset setting demonstrates the ability to successfully modulate the autoimmune response in humans after the onset of overt disease. Moreover, it provides a reason to be cautiously optimistic for the ability of this or other agents, especially if combined with antigen-specific approaches, to provide an effective immunotherapy for T1D. However, optimism must be tempered by the potential side-effects of the agents in the clinical pipeline, and trials will need to proceed with caution until the safety concerns have been adequately addressed.
A viable therapeutic approach must be not only effective but it must also be safer and less burdensome to administer than the standard therapy for T1D. To develop suitable assays (for example T cell assays [87]) to monitor and predict the success of a given immune-based intervention, we should expand our knowledge to all the epitopes [15], targeted by Treg and effector T cells over the development of T1D. Although there are many candidates, we do not understand the role of single antigens during disease progression and response to therapy, but deployment of smarter assays in therapeutic trials may shed light on this.
Acknowledgments
We would like to acknowledge Dr Robert Goldstein at JDRF for seeding the idea for this analysis and providing guidance in its development. M von Herrath is a consultant for NovoNordisk.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Bingley PJ, Williams AJ, Gale EA. Optimized autoantibody-based risk assessment in family members. Implications for future intervention trials. Diabetes Care. 1999;22:1796–801. doi: 10.2337/diacare.22.11.1796. [DOI] [PubMed] [Google Scholar]
- 3.Achenbach P, Bonifacio E, Ziegler AG. Predicting type 1 diabetes. Curr Diabetes Rep. 2005;5:98–103. doi: 10.1007/s11892-005-0035-y. [DOI] [PubMed] [Google Scholar]
- 4.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313:353–60. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 5.Anon. Retinopathy and nephropaty in patients with type 1 diabetes four years after a trial of intensive therapy. The diabetes control and complications. Research group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–26. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson MA. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can't we prevent or reverse this disease? ADA Outstanding Scientific Achievement Lecture 2004. Diabetes. 2005;54:1253–63. doi: 10.2337/diabetes.54.5.1253. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D. Genetic control of diabetes progression. Immunity. 1997;7:873–83. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- 10.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11(Suppl 4):S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 11.Peakman M, Dayan CM. Peptide immunotherapy: fighting fire with fire? Immunology. 2001;104:361–6. doi: 10.1046/j.1365-2567.2001.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells − they're back and critical for regulation of autoimmunity! Immunol Rev. 2001;182:149–63. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 13.Waldmann H, Cobbold S. How do monoclonal antibodies induce tolerance? A role for infectious tolerance? Annu Rev Immunol. 1998;16:619–44. doi: 10.1146/annurev.immunol.16.1.619. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann H, Cobbold S. Regulating the immune response to transplants. a role for CD4+ regulatory cells? Immunity. 2001;14:399–406. doi: 10.1016/s1074-7613(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 15.Di Lorenzo TP, Roep BO, Peakman M. Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007 doi: 10.1111/j.1365-2249.2006.03244.x. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 17.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Peakman M, Roep BO. Secondary measures of immunologic efficacy in clinical trials. Curr Opin Endocrinol Diabetes. 2006;13:325–31. [Google Scholar]
- 19. http://www.immunetolerance.org.
- 20. http://www.diabetestrialnet.org.
- 21.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes. The Diabetes Prevention Trial − Type 1. Diabetes Care. 2005;28:1068–76. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 23.DPT-1 Diabetes study group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–91. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 24.Homann D, Dyrberg T, Petersen J, Oldstone MB, von Herrath MG. Insulin in oral immune ‘tolerance’: a one-amino acid change in the B chain makes the difference. J Immunol. 1999;163:1833–8. [PubMed] [Google Scholar]
- 25.Petersen JS, Bregenholt S, Apostolopolous V, et al. Coupling of oral human or porcine insulin to the B subunit of cholera toxin (CTB) overcomes critical antigenic differences for prevention of type I diabetes. Clin Exp Immunol. 2003;134:38–45. doi: 10.1046/j.1365-2249.2003.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison LC, Honeyman MC, Steele CE, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27:2348–55. doi: 10.2337/diacare.27.10.2348. [DOI] [PubMed] [Google Scholar]
- 27.Kupila A, Sipila J, Keskinen P, et al. Intranasally administered insulin intended for prevention of type 1 diabetes − a safety study in healthy adults. Diabetes Metab Res Rev. 2003;19:415–20. doi: 10.1002/dmrr.397. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–3. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent SC, Chen Y, Bregoli L, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–8. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 31.Bougneres PF, Landais P, Boisson C, et al. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–72. doi: 10.2337/diab.39.10.1264. [DOI] [PubMed] [Google Scholar]
- 32.Dahlquist G, Gothefors L. The cumulative incidence of childhood diabetes mellitus in Sweden unaffected by BCG-vaccination. Diabetologia. 1995;38:873–4. doi: 10.1007/BF03035306. [DOI] [PubMed] [Google Scholar]
- 33.Huppmann M, Baumgarten A, Ziegler AG, Bonifacio E. Neonatal bacille Calmette–Guerin vaccination and type 1 diabetes. Diabetes Care. 2005;28:1204–6. doi: 10.2337/diacare.28.5.1204. [DOI] [PubMed] [Google Scholar]
- 34.Parent ME, Siemiatycki J, Menzies R, Fritschi L, Colle E. Bacille Calmette–Guerin vaccination and incidence of IDDM in Montreal. Can Diabetes Care. 1997;20:767–72. doi: 10.2337/diacare.20.5.767. [DOI] [PubMed] [Google Scholar]
- 35.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363:925–31. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 36.Akerblom HK, Virtanen SM, Ilonen J, et al. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005;48:829–37. doi: 10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]
- 37. http://www.trigr.org.
- 38. http://teddy.epi.usf.edu/.
- 39. http://www.ukdrn.org/.
- 40.Gianani R, Putnam A, Still T, et al. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab. 2006;91:1855–61. doi: 10.1210/jc.2005-1171. [DOI] [PubMed] [Google Scholar]
- 41.von Herrath MG, Wolfe T, Mohrle U, Coon B, Hughes A. Protection from type 1 diabetes in the face of high levels of activated autoaggressive lymphocytes in a viral transgenic mouse model crossed to the SV129 strain. Diabetes. 2001;50:2700–8. doi: 10.2337/diabetes.50.12.2700. [DOI] [PubMed] [Google Scholar]
- 42.Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320:550–4. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- 43.Alizadeh BZ, Hanifi-Moghaddam P, Eerligh P, et al. Association of interferon-gamma and interleukin 10 genotypes and serum levels with partial clinical remission in type 1 diabetes. Clin Exp Immunol. 2006;145:480–4. doi: 10.1111/j.1365-2249.2006.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 45.Muir A, Peck A, Clare-Salzler M, et al. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-gamma transcription. J Clin Invest. 1995;95:628–34. doi: 10.1172/JCI117707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alleva DG, Gaur A, Jin L, et al. Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9–23) peptide. Diabetes. 2002;51:2126–34. doi: 10.2337/diabetes.51.7.2126. [DOI] [PubMed] [Google Scholar]
- 47.Alleva DG, Maki RA, Putnam AL, et al. Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol. 2006;63:59–69. doi: 10.1111/j.1365-3083.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 48. http://www.bayhilltherapeutics.com/index.html.
- 49. http://www.uchsc.edu/misc/diabetes/Keystone06/Gottlieb_etal.ppt (slides N°49–50).
- 50.Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005;19:238–46. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 51. http://www.diamyd.com.
- 52.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 53.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1 (Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. http://www.macrogenics.com.
- 55. http://www.tolerrx.com.
- 56.Bresson D, Togher L, Rodrigo E, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Treg. J Clin Invest. 2006;116:1371–81. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–6. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 58.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–53. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 59.Elias D, Reshef T, Birk OS, van der Zee R, Walker MD, Cohen IR. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci USA. 1991;88:3088–91. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazar L, Ofan R, Weintrob N, et al. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab Res Rev. 2006. (Published online: 24 November 2006 DOI: 10.1002/dmrr.711) [DOI] [PubMed]
- 61.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–32. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Allen HF, Klingensmith GJ, Jensen P, Simoes E, Hayward A, Chase HP. Effect of bacillus Calmette–Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care. 1999;22:1703–7. doi: 10.2337/diacare.22.10.1703. [DOI] [PubMed] [Google Scholar]
- 63.Elliott JF, Marlin KL, Couch RM. Effect of bacille Calmette–Guerin vaccination on C-peptide secretion in children newly diagnosed with IDDM. Diabetes Care. 1998;21:1691–3. doi: 10.2337/diacare.21.10.1691. [DOI] [PubMed] [Google Scholar]
- 64.Shehadeh N, Calcinaro F, Bradley BJ, Bruchim I, Vardi P, Lafferty KJ. Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet. 1994;343:706–7. doi: 10.1016/s0140-6736(94)91583-0. [DOI] [PubMed] [Google Scholar]
- 65. http://onlineapps.Jdfcure.org/AbstractReport.cfm?grant=id/26014/abs_type=LAY.
- 66.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24(3):233–8. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 67. http://www.novartis.com.
- 68. http://jdrfcelltherapy.diabetes.ucsf.edu.
- 69.Homann D, Jahreis A, Wolfe T, et al. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity. 2002;16:403–15. doi: 10.1016/s1074-7613(02)00290-x. [DOI] [PubMed] [Google Scholar]
- 70.Howard LM, Miller SD. Immunotherapy targeting the CD40/CD154 costimulatory pathway for treatment of autoimmune disease. Autoimmunity. 2004;37:411–18. doi: 10.1080/08916930410001716095. [DOI] [PubMed] [Google Scholar]
- 71.Kared H, Masson A, Adle-Biassette H, Bach JF, Chatenoud L, Zavala F. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4(+) CD25(+) regulatory T-cells. Diabetes. 2005;54:78–84. doi: 10.2337/diabetes.54.1.78. [DOI] [PubMed] [Google Scholar]
- 72.Chen YG, Choisy-Rossi CM, Holl TM, et al. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J Immunol. 2005;174:1196–204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]
- 73.Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–6. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 74.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 75.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 76.Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zajonc DM, Maricic I, Wu D, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–26. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2006;25 doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 79.Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986;29:63–7. doi: 10.1007/BF02427283. [DOI] [PubMed] [Google Scholar]
- 80.Krueger GG, Ellis CN. Alefacept therapy produces remission for patients with chronic plaque psoriasis. Br J Dermatol. 2003;148:784–8. doi: 10.1046/j.1365-2133.2003.05239.x. [DOI] [PubMed] [Google Scholar]
- 81.Gottlieb AB, Krueger JG, Wittkowski K, Dedrick R, Walicke PA, Garovoy M. Psoriasis as a model for T-cell-mediated disease: immunobiologic and clinical effects of treatment with multiple doses of efalizumab, an anti-CD11a antibody. Arch Dermatol. 2002;138:591–600. doi: 10.1001/archderm.138.5.591. [DOI] [PubMed] [Google Scholar]
- 82.Song S, Goudy K, Campbell-Thompson M, et al. Recombinant adeno-associated virus-mediated alpha-1 antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther. 2004;11:181–6. doi: 10.1038/sj.gt.3302156. [DOI] [PubMed] [Google Scholar]
- 83.Lozanoska-Ochser B, Barone F, Pitzalis C, Peakman M. Atorvastatin fails to prevent the development of autoimmune diabetes despite inhibition of pathogenic {beta}-cell-specific CD8 T-cells. Diabetes. 2006;55:1004–10. doi: 10.2337/diabetes.55.04.06.db05-1261. [DOI] [PubMed] [Google Scholar]
- 84.von Herrath M. E1-INT (Transition Therapeutics/Novo Nordisk) Curr Opin Invest Drugs. 2005;6:1037–42. [PubMed] [Google Scholar]
- 85. http://www.dvdc.org.au.
- 86.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes. 2002;51:638–45. doi: 10.2337/diabetes.51.3.638. [DOI] [PubMed] [Google Scholar]
- 87.Peakman M, Roep B. Secondary measures of immunologic efficacy in clinical trials. Curr Opin Endocrinol Diabetes. 2006;13:325–31. [Google Scholar]
- 88.Keller RJ, Eisenbarth GS, Jackson RA. Insulin prophylaxis in individuals at high risk of type I diabetes. Lancet. 1993;341:927–8. doi: 10.1016/0140-6736(93)91215-8. [DOI] [PubMed] [Google Scholar]
- 89.Fuchtenbusch M, Rabl W, Grassl B, Bachmann W, Standl E, Ziegler AG. Delay of type I diabetes in high risk, first degree relatives by parenteral antigen administration: the Schwabing Insulin Prophylaxis Pilot Trial. Diabetologia. 1998;41:536–41. doi: 10.1007/s001250050943. [DOI] [PubMed] [Google Scholar]
- 90.Bohmer KP, Kolb H, Kuglin B, et al. Linear loss of insulin secretory capacity during the last six months preceding IDDM. No effect of antiedematous therapy with ketotifen. Diabetes Care. 1994;17:138–41. doi: 10.2337/diacare.17.2.138. [DOI] [PubMed] [Google Scholar]
- 91.Carel JC, Boitard C, Eisenbarth G, Bach JF, Bougneres PF. Cyclosporine delays but does not prevent clinical onset in glucose intolerant pre-type 1 diabetic children. J Autoimmun. 1996;9:739–45. doi: 10.1006/jaut.1996.0096. [DOI] [PubMed] [Google Scholar]
- 92.Lampeter EF, Klinghammer A, Scherbaum WA, et al. The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS Group. Diabetes. 1998;47:980–4. doi: 10.2337/diabetes.47.6.980. [DOI] [PubMed] [Google Scholar]
- 93.Crino A, Schiaffini R, Ciampalini P, et al. A two year observational study of nicotinamide and intensive insulin therapy in patients with recent onset type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:749–54. doi: 10.1515/jpem.2005.18.8.749. [DOI] [PubMed] [Google Scholar]
- 94.Crino A, Schiaffini R, Manfrini S, et al. A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (IMDIAB IX) Eur J Endocrinol. 2004;150:719–24. doi: 10.1530/eje.0.1500719. [DOI] [PubMed] [Google Scholar]
- 95.Pozzilli P, Visalli N, Boccuni ML, et al. Randomized trial comparing nicotinamide and nicotinamide plus cyclosporin in recent onset insulin-dependent diabetes (IMDIAB 1). The IMDIAB Study Group. Diabet Med. 1994;11:98–104. doi: 10.1111/j.1464-5491.1994.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 96.Hummel M, Bonifacio E, Naserke HE, Ziegler AG. Elimination of dietary gluten does not reduce titers of type 1 diabetes-associated autoantibodies in high-risk subjects. Diabetes Care. 2002;25:1111–16. doi: 10.2337/diacare.25.7.1111. [DOI] [PubMed] [Google Scholar]
- 97.Jenner M, Bradish G, Stiller C, Atkison P. Cyclosporin A treatment of young children with newly-diagnosed type 1 (insulin-dependent) diabetes mellitus. London Diabetes Study Group. Diabetologia. 1992;35:884–8. doi: 10.1007/BF00399937. [DOI] [PubMed] [Google Scholar]
- 98.Elliott RB, Chase HP. Prevention or delay of type 1 (insulin-dependent) diabetes mellitus in children using nicotinamide. Diabetologia. 1991;34:362–5. doi: 10.1007/BF00405010. [DOI] [PubMed] [Google Scholar]
- 99.Eisenbarth GS, Srikanta S, Jackson R, et al. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res. 1985;2:271–6. [PubMed] [Google Scholar]
- 100.Bjork E, Berne C, Kampe O, Wibell L, Oskarsson P, Karlsson FA. Diazoxide treatment at onset preserves residual insulin secretion in adults with autoimmune diabetes. Diabetes. 1996;45:1427–30. doi: 10.2337/diab.45.10.1427. [DOI] [PubMed] [Google Scholar]
- 101.Ortqvist E, Bjork E, Wallensteen M, et al. Temporary preservation of beta-cell function by diazoxide treatment in childhood type 1 diabetes. Diabetes Care. 2004;27:2191–7. doi: 10.2337/diacare.27.9.2191. [DOI] [PubMed] [Google Scholar]
- 102.Brod SA, Atkinson M, Lavis VR, et al. Ingested IFN-alpha preserves residual beta cell function in type 1 diabetes. J Interferon Cytokine Res. 2001;21:1021–30. doi: 10.1089/107999001317205141. [DOI] [PubMed] [Google Scholar]
- 103.Mannering SI, Harrison LC, Williamson NA, et al. The insulin A-chain epitope recognized by human T cells is post transnationally modified. J Exp Med. 2005;202:1191–7. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. http://www.tcmouse.com.
- 105.O'Keeffe M, Brodnicki TC, Fancke B, et al. FMS-like tyrosine kinase 3 ligand administration overcomes a genetically determined dendritic cell deficiency in NOD mice and protects against diabetes development. Int Immunol. 2005;17:307–14. doi: 10.1093/intimm/dxh210. [DOI] [PubMed] [Google Scholar]
- 106.Battaglia M, Gianfrani C, Gregory S, et al. Trl cells: from discovery to their clinical application. Seminars in Immunology. 2006;18:120–27. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 107.Gregori S, Mangia P, Bacchetta R, et al. An anti-CD45RO/RB monoclonal antibody modulates T cell responses via induction of apoptosis and generation of regulatory T cells. J Exp Med. 2005;201:1293–305. doi: 10.1084/jem.20040912. [DOI] [PMC free article] [PubMed] [Google Scholar]