Abstract
UVB irradiation modulates immune responses in the skin and is a major cause of sunburn, during which neutrophils accumulate in the skin. Because of their abundance in skin and ability to produce a variety of proinflammatory mediators, we propose that mast cells may play a key role in ultraviolet B (UVB)-induced skin inflammation. Cord blood-derived human mast cells were treated in vitro with varying doses of UVB and production of multiple cytokines was measured in culture supernatants. UVB exposure significantly increased the release of interleukin (IL)-8 and modestly increased IL-1α production, but cytokines such as IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, tumour necrosis factor (TNF)-α and interferon (IFN)-γ were unaffected. Cycloheximide reduced the UVB-mediated induction of IL-8 by 30–40%, suggesting that new protein synthesis contributed to IL-8 production. In line with this, UVB treatment of mast cells significantly increased IL-8 mRNA. In contrast to its effect on IL-8 production, optimal doses of UVB did not provoke histamine or tryptase release, indicating little effect on degranulation. Our data suggest that mast cells may play a major role during UVB-induced acute inflammation by selectively inducing cytokines involved in neutrophil recruitment.
Keywords: human mast cells, inflammation, interleukin-8, ultraviolet radiation
Introduction
Ultraviolet B (UVB) (280–320 nm) exposure is a major cause of sunburn characterized by an acute inflammatory response whereby erythema, changes in vascular permeability [1], dilatation of dermal blood vessels and epithelial hyperplasia are hallmark features. The acute inflammatory changes in skin following short-term irradiation by excessive UVB are well established. They are characterized by accumulation of neutrophils and mononuclear cells within the dermis [2]. Vascular endothelial cells show signs of activation and express E-selectin [3] and anti-intercellular adhesion molecule (ICAM)-1 [4] 6–24 h after UVB irradiation. These adhesion molecules promote leucocyte binding to the endothelial surface and a leucocyte transmigration to affected sites [5]. Accumulation of inflammatory cells peaks at 12 h [2], although the mechanism(s) of neutrophil recruitment after sunburn are currently unclear.
There is strong evidence linking UVB with functional deficiencies in cellular immunity, resulting in changes in epidermal pro- and anti-inflammatory cytokine profiles, suppression of phagocytosis [6] and increased reactive oxygen species (ROS) production by keratinocytes [7], reduced antigen presentation by Langerhans cells [8] and induction of early lymphocyte depletion and late T cell proliferation [9]. UVB can also provoke apoptosis [10], immunosuppression [11], and ROS production can initiate cell damage and induce apoptosis [10,12] and cause direct DNA damage [13]. Although there is clear evidence for involvement of cytokines such as interleukin (IL)-1, -8, -10, -15 and tumour necrosis factor (TNF)-α in UVB-induced dermal inflammation [14–17], cellular sources and the kinetics of production of these cytokines have not been elucidated fully.
Mast cells derived from the bone marrow reside in peripheral tissue as mature or committed progenitor cells. Their functions as early effector cells of allergic diseases [18,19] and in innate immunity [18] have been described extensively. One important role of mast cells is their ability to promote rapid recruitment of neutrophils to sites of inflammation [20]. Although UVB affects predominantly the epidermis, studies indicate that a proportion of UVB light (∼ 15%) penetrates into the reticular dermis [21] where mast cells are located [22]. There is sufficient evidence implicating mast cells in UVB-induced acute inflammation and immune modulation [23] but their exact roles are not understood fully.
This study aims to investigate the effects of UVB on mast cells and determine the profile of proinflammatory and immune-modulatory mediators. Here we show that UVB selectively induced the release of IL-8 and IL-1β in a dose- and time-dependent manner without causing significant mast cell degranulation. IL-8 production was due partially to de novo synthesis and mediated through the p38-mitogen-activated protein kinase (MAPK) signalling pathway. This study suggests that mast cells may contribute to recruitment of neutrophils during UVB-induced acute inflammation.
Materials and methods
Reagents
IL-6 and IL-10 were purchased from R&D Systems (Minneapolis, MN, USA), and stem cell factor was a generous gift from Amgen (Thousand Oaks, CA, USA). Mouse anti-human α and β tryptase antibody (clone AA1) was purchased from Dako (Glostrup, Denmark), mouse anti-human chymase antibody was from Chemicon International (Temecula, CA, USA) and goat anti-mouse-horseradish peroxidase (HRP) was from Bio-Rad (Hercules, CA, USA). Cycloheximide was from Sigma (St Louis, MO, USA), histamine enzyme-linked immunosorbent assay (ELISA) was from Immuno Biological Laboratories (Hamburg, Germany) and the human IL-8 ELISA kit was from R&D systems. Mouse anti-human c-kit [CD117-fluorescein isothioyanate (FITC)-conjugated] antibody was purchased from BD Bioscience Pharmingen (San Diego, CA, USA) and the Bio-Plex cytokine assay system was from Bio-Rad. The p38 MAPK inhibitors, SB202190, SB203580 and the extracellular signal-regulated kinase (ERK) MAPK inhibitor PD98059 were purchased from Calbiochem (La Jolla, CA, USA).
Human mast cell culture
Highly purified cord blood-derived human mast cells were generated by long-term culture of progenitor cells as described previously [24,25]. In brief, heparinized cord blood, obtained after routine caesarian section from the Australian Cord Blood Bank at the Royal Women's Hospital (Sydney, Australia), was depleted of erythrocytes by sedimentation on 4·5% dextran solution for 1 h and centrifugation over Ficoll-Paque Plus (Amersham Biosciences, Uppsala, Sweden). Cells from the mononuclear interface were collected after centrifugation and washed twice with phosphate-buffered saline (PBS) and once with PBS containing 5 mM ethylenediamine tetraacetic acid (EDTA). Mononuclear cells were then cultured at 37°C in a humidified atmosphere containing 5% CO2 at a starting density of 2 × 106/ml in high glucose RPMI-1640 (Gibco, Auckland, NZ) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 0·1 mM non-essential amino acids, 2 µg/ml gentamycin (all from Sigma), 100 U/ml penicillin, 100 µg/ml streptomycin, 0·2 µM 2-mercaptoethanol (all from Gibco), 100 ng/ml stem cell factor (SCF), 50 ng/ml IL-6 and 10 ng/ml IL-10. The medium was replenished weekly. The mast cells were assessed morphologically by metachromatic toluidine blue staining and immunohistochemical staining of cytospin preparations with anti-chymase and anti-tryptase antibodies [25] and by flow cytometric analysis of CD117-stained cell suspensions [24,25]. Within 6–8 weeks of culture greater than 95% of cells were metachromatic and positive for c-kit, tryptase and chymase.
UVB irradiation of mast cells
Mature cord blood-derived mast cells (106/ml) were placed in wells of a six-well Nunc plate (Nunc, Roskilde, Denmark) containing 10% FBS (Sigma) in phenol red-free RPMI-1640 (Gibco) containing SCF (100 ng/ml). Cells were irradiated with 5–30 mJ/cm2 of UVB light (FL20SE bulbs; Philips, Sydney, Australia), as described previously [26–29]. The intensity of the UVB light was monitored and calibrated before each experiment using a radiometer-photometer (model IL1400A; International Light, Newburyport, MA, USA). Exposure of cells for 45 s provides 5 mJ/cm2 and 3 min irradiation delivers 20 mJ/cm2 of UVB. Following exposure, plates were incubated in a humidified atmosphere of air/CO2 (5%) at 37°C, for the times specified. Mock-irradiated cells served as controls and were resuspended in the same media and handled in the same way as UVB-treated cells. After initial UVB dose–response studies for cytokine production and cell viability assay (annexin V apoptosis detection kit; BD Bioscience Pharmingen), 20 mJ/cm2 of UVB provided optimal production of cytokines with minimal levels of cell death (apoptosis). Hence, this dose was used for subsequent experiments.
Mast cell degranulation after UVB treatment
To determine the immediate effect of various doses UVB on mast cells, histamine and tryptase levels were used as a measure of degranulation 1 h after irradiation. Histamine levels in supernatants and in the corresponding cell pellets lysed were measured by ELISA according to the manufacturer's protocol (Immuno Biological Laboratory). The percentage of histamine release was quantified as: (histamine in supernatant/histamine in supernatant + histamine in cell pellets) × 100.
Supernatants from the above experiments were used to determine tryptase released from mast cell granules using Western blotting [30]. In brief, 20 µl supernatants were loaded onto 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and separated under reducing conditions. After electrophoresis, proteins were transferred onto PolyScreen™ polyvinylidene difluoride (PVDF) transfer membranes (Perkin Elmer Life Sciences, Boston, MA, USA), membranes were blocked with 5% skimmed milk powder in Tris-buffered saline, pH 7·5 containing 0·1% Tween 20 (TBST) for 1 h, then incubated with 2 µg/ml of mouse anti-human α/β tryptase antibody for 2 h at room temperature. After washing with TBST (4 × 5 min), membranes were incubated with goat anti-mouse HRP (100 ng/ml) for 2 h at room temperature. After washing with TBST (4 × 5 min), membranes were incubated with HRP-chemiluminescence substrate (PerkinElmer) and blots analysed using LAS-3000 (FUJI Photo Film Ltd, Tokyo, Japan).
Cytokine production by UVB irradiated mast cells
Mast cells (106/ml) irradiated with various doses of UVB (5–30 mJ/cm2) were then incubated for 1–48 h; 200 µl culture supernatants were then collected at 1, 8, 24 and 36 h, residual cell debris removed by centrifugation at 14 000 g for 2 min and stored at −80°C. Concentrations of IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, TNF-α and IFN in supernatants were measured using the Bio-Plex system, according to the manufacturer's instructions (Bio-Rad). In brief, 50 µl aliquots were added to 50 µl antibody-conjugated beads in a 96-well filter plate, incubated for 30 min at room temperature, and washed three times with Bio-Plex washing buffer. Plates were subsequently incubated for 30 min with 25 µl biotinylated anti-cytokine antibody/well, then washed with Bio-Plex washing buffer and 50 µl streptavidin-conjugated phycoerythrin added to each well. Following a final wash with Bio-Plex washing buffer A, 125 µl Bio-Plex assay buffer was added, fluorescence measured using the Luminex 100 system (Bio-Rad), and results analysed by Bio-Plex Manager™ software (Bio-Rad). The lower and upper levels of cytokine detection for this assay were 1 pg/ml to 32 ng/ml (Bio-Rad). After initial screening to identify cytokines modulated by UVB, levels of IL-8 in culture supernatants were determined using by ELISA according to the manufacturer's instructions, the sensitivity was ≥ 32 pg/ml.
Inhibition of IL-8 production by cycloheximide
To determine whether IL-8 was newly synthesized or released from pre-formed stores, mast cells were treated with cycloheximide. Cycloheximide is a glutarimide antibiotic which inhibits protein synthesis of eukaryotic cells by blocking the translation of messenger RNA [31]. In brief, immediately after UVB irradiation (5 or 20 mJ/cm2), cells were centrifuged at 1200 g at room temperature for 10 min, resuspended in phenol red-free RPMI-1640 with stem cell factor (100 ng/ml) and cycloheximide (0–0·5 µg/ml) added. Cells (1·5 × 105/ml) were transferred to 96-well Nunc plates and incubated for 24 h, then the supernatants were harvested and IL-8 levels quantified.
Quantitative analysis of IL-8 mRNA by real-time polymerase chain reaction (PCR)
Mock-irradiated or UVB-irradiated (20 mJ/cm2) mast cells (5 × 106) were incubated in 10% FBS phenol red-free RPMI-1640 containing SCF (100 ng/ml) for 4 h, washed with PBS and total RNA extracted using RNAgents™ Total RNA Isolation System (Promega, Madison, WI, USA). cDNA was synthesized by reverse transcription of 1 µg total RNA, using a cDNA synthesis kit (Life Technologies, Inc., Gaithersburg, MD, USA). IL-8 mRNA was quantified with ABI prism Sequence Detection System, using Assays-on-Demand gene expression product purchased from Applied Biosystems (Foster City, CA, USA). Real-time PCR was performed according to the manufacturer's instructions. Thermal conditions used were 50°C for 2 min, followed by an initial denaturation step at 95°C for 2 min, then 35 cycles at 95°C for 15 s and 85°C for 5 min.
Inhibition of IL-8 production by pharmacological MAPK inhibitors
To determine whether the MAPK pathway mediated IL-8 induction by UVB, mast cells (1 × 106/ml) were pretreated with pharmacological inhibitors of ERK or p38; PD98059 (10 µM) [26], SB202190 (1 µM) [26], SB203580 (1 µM) [32] or vehicle control [dimethylsulphoxide (DMSO), 0·05%]. Mast cells were incubated with the respective agent for 1 h prior to irradiation with 20 mJ/cm2 UVB. Culture supernatants were collected 24 h post-irradiation and IL-8 quantified.
Statistical analysis
Statistical analyses were performed using one-way analysis of variation (anova) followed by Kruskal–Wallis test. P-values less than 0·05 (P < 0·05) were considered to be statistically significant.
Results
Immediate responses of cord blood-derived mast cells to UVB irradiation
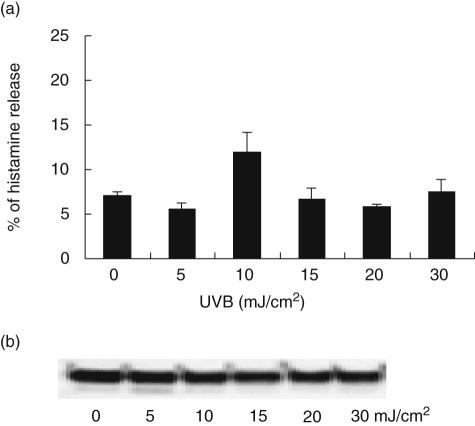
To determine whether UVB caused immediate degranulation of mast cells, levels of mediators known to be stored in granules (histamine and tryptase) were analysed in culture supernatants harvested 1 h after exposure. Although there was significant degranulation in response to 10 µM calcium ionophore (80 ± 5·6% of total histamine, P < 0·01), irradiation with various doses of UVB (5–30 mJ/cm2) did not provoke significant degranulation (P > 0·05 for all doses, one-way anova). Maximum histamine levels reached 12·0 ± 2·2% from cells irradiated with 10 mJ/cm2 UVB compared to 7·1 ± 0·3% in mock-irradiated controls (Fig. 1a). Similarly, tryptase (Fig. 1b) or other pre-stored cytokines such as TNF-α and IL-6 (data not shown) did not increase above basal/constitutive levels.
Fig. 1.
Mast cell degranulatory products after ultraviolet B (UVB) irradiation. Degranulation of mast cells 1 h after UVB irradiation determined by histamine enzyme-linked immunosorbent assay (ELISA) (a) and Western blotting for tryptase (b) indicated no significant degranulation in response to various doses of UVB. (a) Each bar represents the mean ± standard error from three independent experiments.
IL-8 is selectively released by mast cells after UVB irradiation
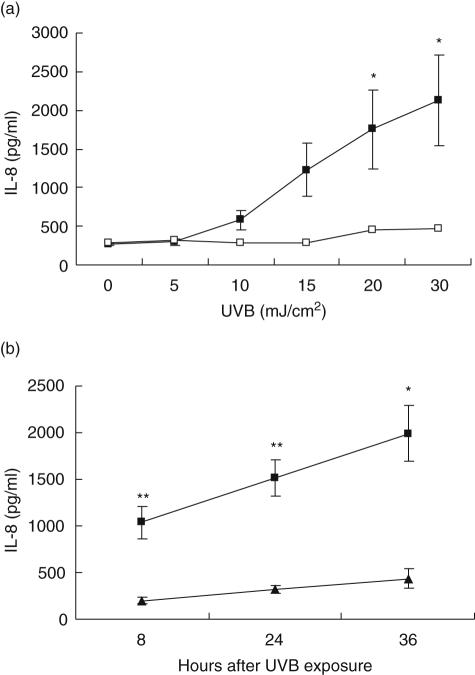
Treatment of cells with various doses of UVB for 8 h caused a dose-dependent increase in the release of IL-8 from 271 ± 21 pg/ml in non-irradiated cells to 1757 ± 511 pg/ml in cells irradiated with 20 mJ/cm2 UVB (P < 0·05; Fig. 2a). Although cells irradiated with 30 mJ/cm2 also showed significant increase in IL-8 production (P < 0·05), there were substantial levels of cell death at this dose (data not shown), therefore 20 mJ/cm2 was selected for subsequent studies. Irradiation of cells with optimal dose (20 mJ/cm2) of UVB caused a significant time-dependent increase (five- to eightfold) in IL-8 production measured at 8, 24 and 36 h (P < 0·05 for all time-points, Fig. 2b).
Fig. 2.
Induction of interleukin (IL)-8 by ultraviolet B (UVB) irradiation in mast cells. (a) Dose-dependent IL-8 production by mast cells after UVB irradiation showing significant increases at 8 h (solid squares) (*P < 0·05) (n = 3) but not at 1 h (open squares) (n = 2). (b) Time-dependent increase in IL-8 in response to 20 mJ/cm2 UVB; significant increases in IL-8 occurred at each time-point (solid squares) compared to mock-irradiated control cells (solid trianges) (n = 7) (**P < 0·01, *P < 0·05).
Less dramatic increases in IL-1α production were also evident 24 h after UVB irradiation (Table 1). IL-1α production showed a similar trend, albeit at low levels (0·8–4·3 pg/ml). Interestingly, no UVB dose altered IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, TNF-α or IFN-γ levels (Table 1).
Table 1.
Ultravioler B (UVB) induces interleukin (IL)-8 and IL-1β production by mast cells after UVB irradiation
| UVB (mJ/cm2) | ||
|---|---|---|
| Cytokines (pg/ml)* | 0 | 20 |
| IL-1β | 21·9 ± 6·1 | 52·1 ± 3·2 |
| IL-2 | 43·3 ± 20·6 | 48·7 ± 26·0 |
| IL-4 | 24·0 ± 3·2 | 40·6 ± 15·5 |
| IL-6† | 30·7 ± 5·1 | 29·4 ± 6·2 |
| IL-8 | 367·3 ± 89·8 | 1846·4 ± 167·6 |
| IL-10 | 314·6 ± 30·1 | 291·9 ± 7·1 |
| IL-12 | 12·4 ± 5·8 | 20·9 ± 11·3 |
| IL-13 | 12·7 ± 5·7 | 13·5 ± 6·8 |
| TNF-α | 26·5 ± 7·3 | 26·6 ± 6·6 |
| IFN-γ | 154·1 ± 80·4 | 158·3 ± 76·3 |
Values represent means ± s.e. from two independent experiments using supernatants harvested at 24 h.
IL-6 = ng/ml. IFN: interferon; TNF: tumour necrosis factor.
UVB caused release of newly synthesized and preformed IL-8
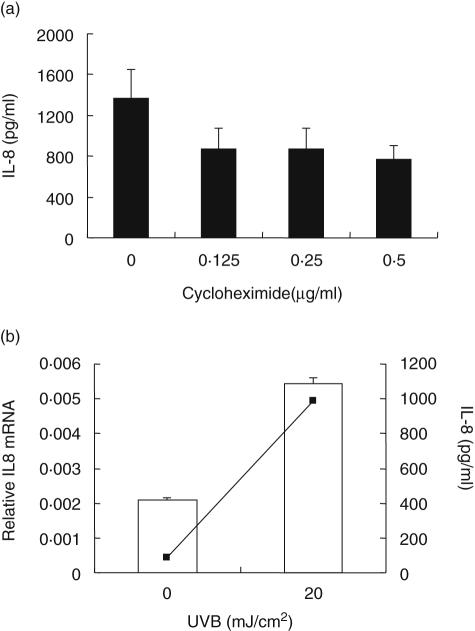
To determine whether IL-8 produced by mast cells in response to UVB irradiation was due to de novo protein synthesis or to release of the preformed stores cytokine, cells irradiated with 20 mJ/cm2 UVB were treated immediately with cycloheximide (range, 0·125–0·5 µg/ml) and supernatants harvested 24 h later. Cycloheximide at 0·5 µg/ml substantially reduced IL-8 levels (by 44%); however, this was not statistically significant (P < 0·17; Fig. 3a). These results indicated that a significant proportion of IL-8 released in response to UVB irradiation was newly synthesized. Because IL-8 mRNA levels in mast cells collected 4 h after irradiation increased approximately threefold (Fig. 3b), these data would indicate a direct effect on transcriptional activity. Moreover, IL-8 in culture supernatants collected from the same cells used to detect mRNA increased 10–12-fold (990 pg/ml in cells treated with UVB compared to 85 pg/ml in controls).
Fig. 3.
Ultraviolet B (UVB)-induced new synthesis of interleukin (IL)-8 by mast cells. (a) Partial inhibition of IL-8 synthesis by UVB-irradiated mast cells after treatment with various doses of cycloheximide (n = 3). (b) Expression of IL-8 mRNA and protein by mast cells 4 h after UVB-irradiation (n = 2). Histogram indicates real-time reverse transcription–polymerase chain reaction (RT–PCR) for IL-8 mRNA and line graph shows levels of IL-8 protein in supernatants of the same samples showing 10-fold increase in IL8 levels compared to control cells.
UVB-induced IL-8 synthesis is mediated via the MAPK p38 signalling pathway
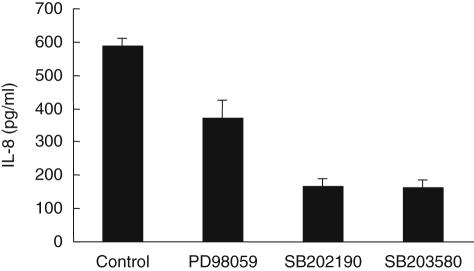
The downstream signalling likely to mediate IL-8 production following UV exposure was investigated by pretreating mast cells with pre-optimized doses of p38 inhibitors (SB202190 and SB203580) and an ERK inhibitor (PD98059). The p38 inhibitors reduced IL-8 production by irradiated cells by 70–80% (Fig. 4). In contrast, the ERK inhibitor PD98059 caused only 25–35% inhibition (Fig. 4), suggesting that signalling via the p38 MAPK pathway may play a critical role in UVB-induced IL-8 production by mast cells.
Fig. 4.
Inhibition of ultraviolet B (UVB)-induced interleukin (IL)-8 production by mitogen-activated protein kinase (MAPK) inhibitors. Mast cells were pretreated with preoptimized dose of extracellular signal-regulated kinase (ERK) inhibitor (PD98059, 10 µM) or two p38 inhibitors (SB202190, 1 µM or SB203580, 1 µM) in duplicate for 1 h, then irradiated with 20 mJ/cm2 of UVB. Levels of IL-8 in culture supernatants collected 24 h after irradiation were measured by enzyme-linked immunosorbent assay (ELISA) (n = 2). Data expressed as mean ± standard error of two independent experiments.
Discussion
Human mast cells modulate immunological responses by releasing a range of preformed and newly synthesized cytokines following stimulation with mediators such as SCF or upon cross-linking of their high-affinity IgE receptor (FcεRI) [33]. Mast cells may contribute to UVB-induced acute inflammation of skin [34], but their exact role in the pathogenesis of dermal inflammation is not understood fully. One important role of mast cells is to promote rapid accumulation of neutrophils to sites of inflammation through rapid generation of stored mediators such as histamine, TNF-α and IL-8 [20].
In this study, mast cells were treated with a range of UVB doses equivalent to sun exposures that cause changes in human skin, ranging from mild erythema (10–15 mJ/cm2) to moderate sunburn (20–30 mJ/cm2), which is characterized by acute inflammation and accumulation of neutrophils [2,21]. For the first time, we show that UVB-irradiated mastcells are a major source of the primary neutrophil chemoattractant cytokine, IL-8. We demonstrate significant dose- and time-dependent increases in production of IL-8 and an early up-regulation of IL-8 mRNA. Furthermore, pretreatment of mast cells with the protein synthesis inhibitor, cycloheximide decreased UVB-mediated IL-8 production by ∼40%, indicating that secreted levels were partially derived from newly synthesized protein.
Interestingly, mast cells irradiated with UVB did not release significant amounts of other preformed or newly synthesized mediators, indicating that the release of IL-8 and IL-1β by these cells was highly selective. Our data with pharmacological inhibitors of p38 showing > 70% reduction in IL-8 production suggests involvement of the p38 signalling pathway in UVB-mediated mast cell activation. This is consistent with previous studies that demonstrated up-regulation of the MAPK signalling pathway via p38 by UVB [35,36].
The kinetics of IL-8 production by mast cells coincided with the time-course of neutrophil accumulation in human skin after UVB irradiation [2]. Thus UVB may induce selective production of IL-8 in mast cells that could contribute to histological changes in the dermis typical of ‘sunburn’[2,21]. Our observations are consistent with previous results showing attenuated dermal inflammation and absence of neutrophils in UVB-treated mast cell-deficient mice [37]. In contrast to the dosed used here, irradiation with high-dose UVB (75 mJ/cm2) substantially decreased IL-8 secretion by skin mast cells [38]. We found that such high doses of UVB caused significant apoptosis (∼40%) of mast cells (unpublished observation) that might have been responsible for the reduction in IL-8 production observed by Guhl et al. [38].
Cord blood-derived mast cells used in this study show a phenotype that closely resembles human skin mast cells that are known to degranulate and release preformed mediators such as TNF-α in response to UVB irradiation in vivo[1,38]. However, in vitro irradiation of cord blood-derived mast cells with various doses of UVB did not induce significant degranulation. Thus UVB-induced mast cell degranulation in vivo might be due to indirect mechanisms involving release of mast cell activators such as SCF by other cells [39]. Trans-urocanic acid derived from UVB-irradiated keratinocytes promotes formation of cis-urocanic acid that in turn stimulates production of neuropeptide that could also contribute to mast cell degranulation [40,41].
Although the underlying mechanisms are not well established, dermal mast cells are proposed to play a role in UVB-induced suppression of cell-mediated immunity [42]. We postulated that UVB-irradiated mast cells might produce cytokines that regulate immune responses. However, we were unable to demonstrate production of immune regulatory cytokines such as IL-10 or IL-12 by UVB treatment. It is plausible that neutrophils recruited by mast cell-derived IL-8 might produce immunosuppressive cytokines such IL-10 [43], thereby indirectly regulating immune responses.
In conclusion, we demonstrate an early and selective production of preformed and newly synthesized IL-8 by cord blood-derived mast cells in response to UVB treatment via the MAPK p38 signalling pathway. Mast cells, as a major source of the primary neutrophil chemoattractant (IL-8) and the proinflammatory cytokine (IL-1β), may play a key role in initiating UVB-induced acute inflammation.
Acknowledgments
This study was supported by grants from the National Health and Medical Research Council of Australia and University of New South Wales Faculty Research.
References
- 1.Walsh LJ, Ultraviolet B. irradiation of skin induces mast cell degranulation and release of tumour necrosis factor-alpha. Immunol Cell Biol. 1995;73:226–33. doi: 10.1038/icb.1995.37. [DOI] [PubMed] [Google Scholar]
- 2.Hawk JL, Murphy GM, Holden CA. The presence of neutrophils in human cutaneous ultraviolet-B inflammation. Br J Dermatol. 1988;118:27–30. doi: 10.1111/j.1365-2133.1988.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 3.Norris P, Poston RN, Thomas DS, Thornhill M, Hawk J, Haskard DO. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991;96:763–70. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes LE, Joyce M, West DC, Strickland I, Friedmann PS. Comparison of changes in endothelial adhesion molecule expression following UVB irradiation of skin and a human dermal microvascular cell line (HMEC-1) Photodermatol Photoimmunol Photomed. 1996;12:114–21. doi: 10.1111/j.1600-0781.1996.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 5.Dosquet C, Weill D, Wautier JL. Molecular mechanism of blood monocyte adhesion to vascular endothelial cells. Nouv Rev Fr Hematol. 1992;34(Suppl.):S55–9. [PubMed] [Google Scholar]
- 6.Kasahara S, Aizawa K, Okamiya M, et al. UVB irradiation suppresses cytokine production and innate cellular immune functions in mice. Cytokine. 2001;14:104–11. doi: 10.1006/cyto.2001.0849. [DOI] [PubMed] [Google Scholar]
- 7.Heck DE, Vetrano AM, Mariano TM, Laskin JD. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem. 2003;278:22432–6. doi: 10.1074/jbc.C300048200. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz T. Photoimmunosuppression. Photodermatol Photoimmunol Photomed. 2002;18:141–5. doi: 10.1034/j.1600-0781.2002.180307.x. [DOI] [PubMed] [Google Scholar]
- 9.Di Nuzzo S, de Rie MA, van der Loos CM, Bos JD, Teunissen M B. Solar-simulated ultraviolet irradiation induces selective influx of CD4+ T lymphocytes in normal human skin. Photochem Photobiol. 1996;64:988–93. doi: 10.1111/j.1751-1097.1996.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 10.Kulms D, Schwarz T. Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem Pharmacol. 2002;64:837–41. doi: 10.1016/s0006-2952(02)01146-2. [DOI] [PubMed] [Google Scholar]
- 11.Duthie MS, Kimber I, Norval M. The effects of ultraviolet radiation on the human immune system. Br J Dermatol. 1999;140:995–1009. doi: 10.1046/j.1365-2133.1999.02898.x. [DOI] [PubMed] [Google Scholar]
- 12.Nicolo C, Tomassini B, Rippo MR, Testi R. UVB-induced apoptosis of human dendritic cells: contribution by caspase-dependent and caspase-independent pathways. Blood. 2001;97:1803–8. doi: 10.1182/blood.v97.6.1803. [DOI] [PubMed] [Google Scholar]
- 13.Horio T, Miyauchi-Hashimoto H, Okamoto H. DNA damage initiates photobiologic reactions in the skin. Photochem Photobiol Sci. 2005;4:709–14. doi: 10.1039/b417759m. [DOI] [PubMed] [Google Scholar]
- 14.Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108:763–8. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- 15.Saade NE, Nasr IW, Massaad CA, Safieh-Garabedian B, Jabbur SJ, Kanaan SA. Modulation of ultraviolet-induced hyperalgesia and cytokine upregulation by interleukins 10 and 13. Br J Pharmacol. 2000;131:1317–24. doi: 10.1038/sj.bjp.0703699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding W, Beissert S, Deng L, et al. Altered cutaneous immune parameters in transgenic mice overexpressing viral IL-10 in the epidermis. J Clin Invest. 2003;111:1923–31. doi: 10.1172/JCI15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamadzadeh M, Takashima A, Dougherty I, Knop J, Bergstresser PR, Cruz PD., Jr Ultraviolet B radiation up-regulates the expression of IL-15 in human skin. J Immunol. 1995;155:4492–6. [PubMed] [Google Scholar]
- 18.Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990;11:458–64. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- 19.Parikh SA, Cho SH, Oh CK. Preformed enzymes in mast cell granules and their potential role in allergic rhinitis. Curr Allergy Asthma Rep. 2003;3:266–72. doi: 10.1007/s11882-003-0049-y. [DOI] [PubMed] [Google Scholar]
- 20.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 21.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–68. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 22.Grimbaldeston MA, Finlay-Jones JJ, Hart PH. Mast cells in photodamaged skin: what is their role in skin cancer? Photochem Photobiol Sci. 2006;5:177–83. doi: 10.1039/b504344a. [DOI] [PubMed] [Google Scholar]
- 23.Hart PH, Grimbaldeston MA, Jaksic A, et al. Ultraviolet B-induced suppression of immune responses in interleukin-4-/- mice: relationship to dermal mast cells. J Invest Dermatol. 2000;114:508–13. doi: 10.1046/j.1523-1747.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 24.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–80. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C(4) synthase expression by interleukin 4. J Exp Med. 2001;193:123–33. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Girolamo N, Coroneo MT, Wakefield D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK-dependent pathway. Invest Ophthalmol Vis Sci. 2003;44:4705–14. doi: 10.1167/iovs.03-0356. [DOI] [PubMed] [Google Scholar]
- 27.Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3430–7. [PubMed] [Google Scholar]
- 28.Nolan TM, DiGirolamo N, Sachdev NH, Hampartzoumian T, Coroneo MT, Wakefield D. The role of ultraviolet irradiation and heparin-binding epidermal growth factor-like growth factor in the pathogenesis of pterygium. Am J Pathol. 2003;162:567–74. doi: 10.1016/S0002-9440(10)63850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy M, Kim KH, Harten B, et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci. 1997;38:2483–91. [PubMed] [Google Scholar]
- 30.Tedla N, Wang HW, McNeil HP, et al. Regulation of T lymphocyte trafficking into lymph nodes during an immune response by the chemokines macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta. J Immunol. 1998;161:5663–72. [PubMed] [Google Scholar]
- 31.Setkov NA, Kazakov VN, Rosenwald IB, Makarova GF, Epifanova OI. Protein synthesis inhibitors, like growth factors, may render resting 3T3 cells competent for DNA synthesis. a radioautographic and cell fusion study. Cell Prolif. 1992;25:181–91. doi: 10.1111/j.1365-2184.1992.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 32.Cao J, Cetrulo CL, Theoharides TC. Corticotropin-releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen-activated protein kinase pathway. Mol Pharmacol. 2006;69:998–1006. doi: 10.1124/mol.105.019539. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–20. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- 34.Ikai K, Danno K, Horio T, Narumiya S. Effect of ultraviolet irradiation on mast cell-deficient W/Wv mice. J Invest Dermatol. 1985;85:82–4. doi: 10.1111/1523-1747.ep12275365. [DOI] [PubMed] [Google Scholar]
- 35.Kim AL, Labasi JM, Zhu Y, et al. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J Invest Dermatol. 2005;124:1318–25. doi: 10.1111/j.0022-202X.2005.23747.x. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa S, Ohtani T, Mizuashi M, et al. p38 Mitogen-activated protein kinase mediates dual role of ultraviolet B radiation in induction of maturation and apoptosis of monocyte-derived dendritic cells. J Invest Dermatol. 2004;123:361–70. doi: 10.1111/j.0022-202X.2004.23238.x. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez S, Moran M, Kochevar IE. Chronic photodamage in skin of mast cell-deficient mice. Photochem Photobiol. 1999;70:248–53. [PubMed] [Google Scholar]
- 38.Guhl S, Stefaniak R, Strathmann M, et al. Bivalent effect of UV light on human skin mast cells-low-level mediator release at baseline but potent suppression upon mast cell triggering. J Invest Dermatol. 2005;124:453–6. doi: 10.1111/j.0022-202X.2004.23523.x. [DOI] [PubMed] [Google Scholar]
- 39.Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol. 2004;165:2099–109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalil Z, Townley SL, Grimbaldeston MA, Finlay-Jones JJ, Hart PH. cis-Urocanic acid stimulates neuropeptide release from peripheral sensory nerves. J Invest Dermatol. 2001;117:886–91. doi: 10.1046/j.0022-202x.2001.01466.x. [DOI] [PubMed] [Google Scholar]
- 41.Townley SL, Grimbaldeston MA, Ferguson I, et al. Nerve growth factor, neuropeptides, and mast cells in ultraviolet-B-induced systemic suppression of contact hypersensitivity responses in mice. J Invest Dermatol. 2002;118:396–401. doi: 10.1046/j.0022-202x.2001.01679.x. [DOI] [PubMed] [Google Scholar]
- 42.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–53. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piskin G, Bos JD, Teunissen MB. Neutrophils infiltrating ultraviolet B-irradiated normal human skin display high IL-10 expression. Arch Dermatol Res. 2005;296:339–42. doi: 10.1007/s00403-004-0522-z. [DOI] [PubMed] [Google Scholar]