Abstract
β-Glucans are glucose polymers with a variety of stimulatory effects on the immune system. The objective of this study was to determine the effect of prophylactic oral administration of soluble Saccharomyces cerevisiae-derived β-1,3/1,6-glucan (SBG) on the outcome of experimental endotoxaemia and shock-associated organ injury. Male Wistar rats were pretreated with SBG orally (SBGpo, 20 mg/kg/day) for 14 days, subcutaneously (SBGsc, 2 mg/kg/day) for 3 days, or vehicle (placebo). Rats were anaesthetized and subjected to endotoxaemia by intravenous infusion of Escherichia coli lipopolysaccharide (LPS) (6 mg/kg) or saline infusion (sham). We observed significant levels of plasma β-glucan in the SBGpo group (P < 0·5), although the SBGsc group had levels approximately 40-fold higher despite a 10-fold lower dose. SBG prophylaxis caused enhanced blood pressure recovery following LPS-induced blood pressure collapse. Oral treatment with SBG attenuated the LPS-induced rise in plasma creatinine levels (P < 0·05), indicating protection against renal injury. SBG also attenuated the plasma levels of aspartate aminotransferase and alanine aminotransferase (SBGpo, P < 0·01; SBGsc, P < 0·01), indicating protection against LPS-induced hepatic injury. A moderate increase in baseline interleukin (IL)-1β levels was observed in the SBGsc group (P < 0·05). In the LPS-challenged rats, plasma levels of proinflammatory cytokines was moderately reduced in both SBG-treated groups compared to placebo. SBG treatment, particularly oral administration, had a striking effect on the haemodynamics of LPS-treated rats, although only a minute fraction of the orally administered β-glucan translocated to the circulation. Enhanced organ perfusion may thus be responsible for the attenuated levels of indicators of kidney and liver injury seen in SBG-treated rats.
Keywords: β-glucan, endotoxaemia, shock, multiple organ dysfunction, syndrome (MODS), immunomodulation
Introduction
Sepsis, a systemic host response to infection, with multiple organ dysfunction syndrome (MODS), continues to be the main cause of morbidity and mortality in intensive care units [1]. Systemic administration of β-glucans has been shown to mediate protection against sepsis and MODS [2,3], modulate cytokine profiles [4,5] and prolong survival [5–8] in experimental animal models. β-Glucans have also been shown to possess an array of beneficial properties, including enhancing protection against infections [6,8,9], tumour development [10,11] and radiation injury [12,13], lowering plasma lipids [14,15], increasing salivary IgA secretion [16], promoting wound healing [17], mediating protection against myocardial ischaemia and reperfusion injury [18] as well as restoring haematopoiesis following bone marrow injury [19]. These heterogeneous glucose polymers consist of a backbone of β-(1→3)-linked β-D-glucopyranosyl units with β-(1→6)-linked side chains of varying length and distribution. They are major cell wall structural components in fungi and are also found in some bacteria and plants (reviewed in [20]).
Recognition of bacterial components such as Gram-negative lipopolysaccharide (LPS) by the innate immune system trigger the release of potent mediators of inflammation that result in an exaggerated pathogenic inflammatory response in the circulation and vital organs. The proinflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-1α and IL-6 are thought to be important mediators during the early stage of sepsis and MODS. Synergistic effects between these cytokines, secondary inflammatory mediators and reactive oxygen intermediates have been shown to be involved in the pathogenesis of sepsis and associated organ injury (reviewed in [21,22]).
The immunomodulating potential of β-glucans has been attributed to their ability to prime and activate leucocytes. Several receptors, expressed both by immune and non-immune cells, have been implicated in recognition of β-glucans, including the type 3 complement receptor, scavenger receptors, lactocylceramide and dectin-1 (reviewed in [23]). Several reports indicate that orally administered β-glucans may exert biological effects [2,10,11,24–26]. However, no reports on the absorption and pharmacokinetics of orally administered soluble glucans have been available until recently [8].
We designed this study to investigate the protective capacity of orally administered soluble β-glucan in an experimental rat model of LPS-induced shock and shock-associated organ injury. The specific objectives of the investigation were: (1) to establish whether an orally administered Saccharomyces cerevisiae-derived water-soluble β-glucan was absorbed from the gastrointestinal tract and translocated to systemic circulation, (2) to examine the effect of this β-glucan on haemodynamic parameters during the progression of endotoxin-induced shock, (3) to investigate whether β-glucan administration mediate protection against development of organ injury/dysfunction and (4) to study the effect of β-glucan on systemic inflammation.
Materials and methods
Materials
Endotoxin free (< 0·5 EU/ml) SBG, a S. cerevisiae-derived water-soluble β-1,3/1,6-glucan was provided by Biotec Pharmacon ASA (Tromsø, Norway). LPS from Escerichia coli (B6:026, chromatography purified) was from Sigma (St Louis, MO, USA).
Animals
Male Wistar rats (Taconic Europe, Denmark) were maintained in the minimal disease unit at the Centre for Comparative Medicine at Rikshospitalet-Radiumhospitalet University Hospital, Oslo, Norway for at least 1 week before they were entered into experiments. Animals were supplied with water (reversed osmosis and ionic-exchange-treated) and fed conventionally (Rat and Mouse no. 3 Breeding, Special Diets Services, Witham, Essex, UK) ad libitum. Cages were kept at 21 ± 1°C and 55 ± 10% relative humidity. Light conditions consisted of alternating 12-h light/dark cycles with 1 h dusk and dawn with gradual decrease or increase of light intensity. The present investigation was approved by the national ethics committee for animal experiments.
Surgical procedure and sample collection
Rats [275 (244–306) g; mean and (range)] were anaesthetized with thiopental sodium [intraval sodium, 120 mg/kg, intraperitoneally (i.p.)]. Anaesthesia was maintained by supplementary injections of thiopental sodium as required and the rectal temperature was maintained at 37°C using a homeothermic blanket (Harvard Apparatus, Holliston, MA, USA). Surgery was carried out essentially as described previously [27]. Briefly, the trachea was cannulated to facilitate respiration. The right carotid artery was cannulated to facilitate repeated blood sampling and connected to a pressure transducer (Harvard Apparatus) for monitoring of mean arterial blood pressure. The jugular vein was cannulated for the administration of LPS or vehicle (saline). The urine bladder was also cannulated to facilitate urine flow. Cardiovascular parameters were allowed to stabilize for approximately 30 min before animals were subjected to endotoxaemia. Rats were given saline 1 ml/kg/h intravenously (i.v.) throughout the experiment for fluid resuscitation. Blood samples were collected from the cannulated artery immediately prior to LPS or saline (sham) infusion and at 1, 3 and 6 h thereafter. Blood samples were collected in heparinized microcentrifuge tubes and plasma was obtained by immediate centrifugation at 2200 g for 3 min at room temperature. Plasma samples were stored at − 70°C for subsequent analysis.
Experimental design
Rats were divided into the following experimental groups:
SBGpo group: SBG was administered orally to rats by tube feeding [20 mg/kg body weight (bw), 400–600 µl of a 10-mg/ml solution] daily for 14 days prior to surgery. Following surgery, LPS (6 mg/kg) (n = 8) or vehicle (sham, n = 5) was administered via the jugular vein over a 10-min period.
SBGsc group: SBG was administered in three subcutaneous injections (2 mg/kg bw, 100–120 µl of a 5-mg/ml solution) 48 h, 24 h and immediately prior to surgery. Following surgery, LPS (6 mg/kg) (n = 8) or vehicle (sham, n = 5) was administered via the jugular vein over a 10-min period.
Placebo group: rats received subcutaneous injections or oral instillation of equal volumes of vehicle [phosphate buffered saline (PBS)] as described above for SBG. Following surgery, LPS (6 mg/kg) (n = 8) or vehicle (sham, n = 10) was administered via the jugular vein over a 10-min period.
Measurement of biochemical indicators of organ dysfunction and injury
Liver injury was assessed by measuring rise in plasma levels of alanine aminotransferase (ALAT, a specific marker for parenchymal injury), aspartate aminotransferase (ASAT, a non-specific marker for parenchymal injury), bilirubin (a marker of hepatic secretory dysfunction) and γ-glutamyl-transferase (γ-GT, and indicator of liver dysfunction and liver injury). Renal dysfunction was assessed by measuring the rises in serum levels of urea and creatinine (an indicator of reduced glomerular filtration rate), whereas pancreatic injury was assessed by measuring amylase. All the above-mentioned organ function markers were measured by enzymatic photometric assays (Roche Automated Clinical Chemistry Analyser; Roche Diagnostics, Indianapolis, USA).
Measurement of plasma cytokines
Plasma cytokine levels were measured with a rat-specific Bio-Plex multiplex suspension array assay (Bio-Rad Laboratories, Hercules, CA, USA). Measurements and data analysis were performed on a Bio-Plex system, powered by xMAP technology by Luminex, operated with Bio-Plex Manager 4·0 software (Bio-Rad Laboratories). The instrument was calibrated with the CAL2 settings (LOW RP1 target value) using Bio-Plex calibration beads (Bio-Rad Laboratories). All samples were diluted 1 : 4 in Bio-Plex rat serum sample diluent buffer (Bio-Rad Laboratories) and the assays carried out according to manufacturer's instructions. Plasma samples were analysed as single samples, whereas standards were analysed in duplicate.
Measurement of plasma β-glucan
Plasma β-glucan levels were determined with FungitellTM, a 1,3-β-D-glucan specific protease zymogen-based colorimetric assay according to the manufacturer's instructions (Associates of Cape Cod Inc., East Falmouth, MA, USA). Samples and standards were analysed in duplicate.
Statistical analysis
Plasma β-glucan levels are expressed as mean ± standard error of the mean (s.e.m.) values. These data failed the D'Angostino and Pearson omnibus normality test and were analysed by non-parametric analysis of variance (anova) (Kruskall–Wallis test) with Dunn's multiple comparison test. Mean arterial blood pressure and cytokine measurements are expressed as mean ± s.e.m. values and analysed by two-way anova with Bonferroni's multiple comparison test. Data representing plasma levels of indicators of organ dysfunction and injury are analysed by anova with Bonferroni's multiple comparison test. Highly suspect outlier values were identified by Grubb's outlier detection test and excluded from further analysis. All statistical analysis was carried out using GraphPad Prism, version 4 (GraphPad Software, San Diego, CA, USA). Differences at P = 0·05 were considered statistically significant.
Results
Absorption and translocation of orally administered SBG
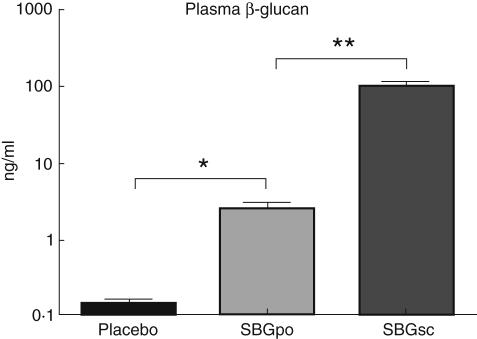
Male Wistar rats were divided into arbitrarily three groups: group 1 received 20 mg SBG/kg bw daily via feeding tube for 14 days prior to surgery (SBGpo); group 2 received 2 mg SBG/kg bw subcutaneously 48 h, 24 h and immediately prior to surgery (SBGsc); and group 3 received PBS orally or subcutaneously prior to surgery (placebo). To determine the extent of β-glucan absorption into circulation in the SBGpo group, plasma concentration of β-glucan was examined by means of the FungitellTM method. We found significant levels of plasma β-glucan in the SBGpo group compared with placebo (P < 0·05), although the SBGsc group had levels approximately 40-fold higher despite a 10-fold lower dose (Fig. 1).
Fig. 1.
Plasma β-glucan concentration following oral and subcutaneous soluble Saccharomyces cerevisiae-derived β-1,3/1,6-glucan (SBG) administration. SBG, a S. cerevisiae-derived water-soluble β-glucan, was administered by oral gavage [20 mg/kg body weight (bw)] daily for 14 days (SBGpo, n = 13) or as subcutaneous injections (2 mg/kg bw) on 3 consecutive days (SBGsc, n = 13) to male Wistar rats. Placebo control animals received corresponding volumes of phosphate-buffered saline (n = 17). Blood samples were collected from the cannulated carotid artery. Plasma β-glucan levels were measured with a 1,3-β-D-glucan specific, protease zymogen-based, colorimetric assay. Data are presented as mean ± s.e.m. on a log scale. *P < 0·05, **P < 0·01 as determined by non-parametric analysis of variance with Dunn's multiple Comparison Test.
Effect of SBG on mean arterial blood pressure in endotoxaemia
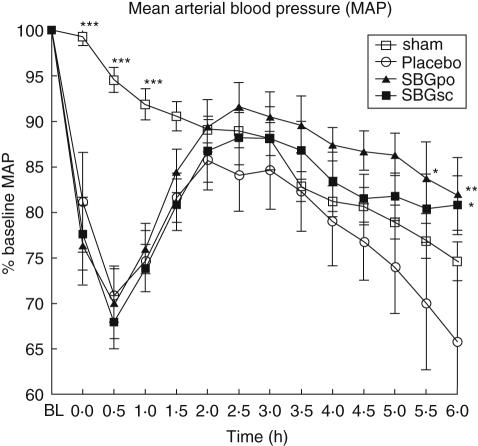
To investigate the effect of prophylactic SBG-treatment on haemodynamics during LPS-induced shock, mean arterial blood pressure (MAP) was monitored. Baseline levels of MAP for all groups of animals ranged from 136 ± 5 to 147 ± 7 mmHg, and did not differ significantly between groups. MAP in sham-control animals decreased gradually throughout the experiment (Fig. 2). SBG administration, mucosal or systemic, did not affect MAP of the sham-control animals significantly (data not shown). Systemic administration of LPS caused an immediate and dramatic decrease in MAP. Approximately 40 min after initiation of the LPS infusion, MAP levels started to increase and continued to do so until the 2·5 h time-point at which the blood pressure again started to decrease slowly (Fig. 2). Oral administration of SBG enhanced the recovery of MAP compared with placebo treatment and MAP remained higher in this group throughout the experiment (P < 0·05 at 5·5 h and P < 0·01 at 6 h versus placebo). Subcutaneous injection of SBG also resulted in enhanced MAP recovery (P < 0·05 at 6 h versus placebo), although to a lesser extent than did oral SBG prophylaxis.
Fig. 2.
Effect of soluble Saccharomyces cerevisiae-derived β-1,3/1,6-glucan (SBG) administration on mean arterial blood pressure (MAP) in endotoxaemia. Rats were pretreated with SBG or placebo as described, followed by anaesthesia and general surgery to catheterize the jugular vein and carotid artery. MAP was allowed to stabilize for approximately 30 min before intravenous infusion of lipopolysaccharide (LPS) (SBGpo n = 8, SBGsc n = 8 and placebo n = 8) or vehicle (sham n = 20) over a 10-min period. The end of the infusion defined the 0 h time-point. MAP was recorded at baseline (BL), 0 h and every 30 min until the 6 h time-point at which the rats were sacrificed. Overall mean MAP at baseline was 142 ± 2 mmHg, with variation between all groups of animals ranging from 136 ± 5 to 147 ± 7 mmHg. No statistically significant differences were observed between the sham animals, regardless of SBG or placebo pretreatment (SBGpo n = 5, SBGsc n = 5 and placebo n = 10) (data not shown), thus the data were combined to one sham group. MAP dynamics are presented as percentage of BL, mean ± s.e.m. *P < 0·05, **P < 0·01, ***P < 0·001 versus placebo as determined by two-way analysis of variance with Bonferroni's multiple comparison test.
Effect of SBG on indicators of organ injury
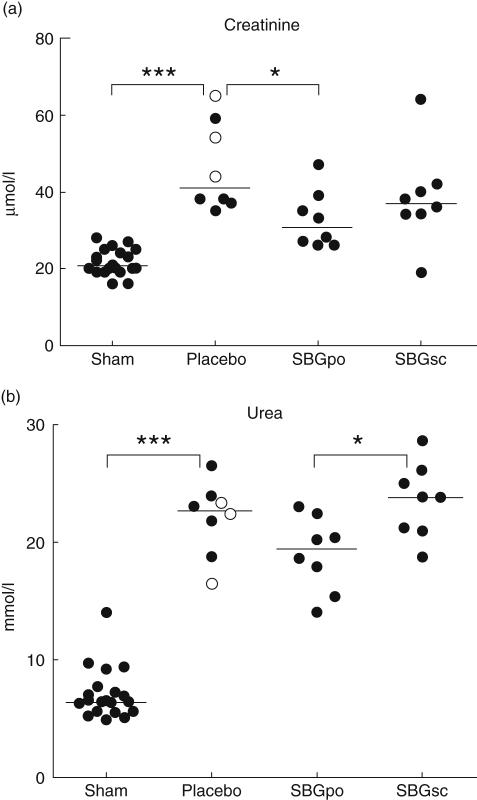
Organ injury was assessed by quantification of plasma level of various biochemical markers 6 h following intravenous LPS administration. LPS infusion caused a twofold increase in levels of plasma creatinine compared to sham (P < 0·001). The creatinine level in the SBGpo group was reduced by approximately 25% compared to the placebo group P < 0·05). This effect was not observed in the SBGsc group (Fig. 3a). LPS infusion also caused a 3·5-fold increase in the plasma urea level compared to sham (P < 0·001). Although not statistically significant, the SBGpo group had a minor reduction in plasma urea compared to placebo control animals after LPS administration (P= 0·07). The plasma urea level in the SBGsc group remained at the placebo level and was significantly higher than in the SBGpo group (P < 0·05) (Fig. 3b).
Fig. 3.
Effect of soluble Saccharomyces cerevisiae-derived β-1,3/1,6-glucan (SBG) prophylaxis on indicators of kidney injury and renal dysfunction in the endotoxic rat. Rats were pretreated with SBG or placebo and underwent surgery as described. At the experimental end-point, 6 h after intravenous infusion of lipopolysaccharide (LPS) (SBGpo n = 8, SBGsc n = 8 and placebo n = 8) or vehicle (sham n = 20), plasma samples were collected and examined for levels of creatinine (a) and urea (b) by automated enzymatic photometric assays. No statistically significant differences were observed between the sham animals, regardless of SBG or placebo pretreatment (SBGpo n = 5, SBGsc n = 5 and placebo n = 10) (data not shown), thus the data were combined to one sham group. In the placebo group oral and subcutaneous pretreatment is indicated by open (○) and closed circles (•), respectively. Each data point represents one animal, bars represent median values. *P < 0·05, ***P < 0·001 as determined by analysis of variance with Bonferroni's multiple comparison test.
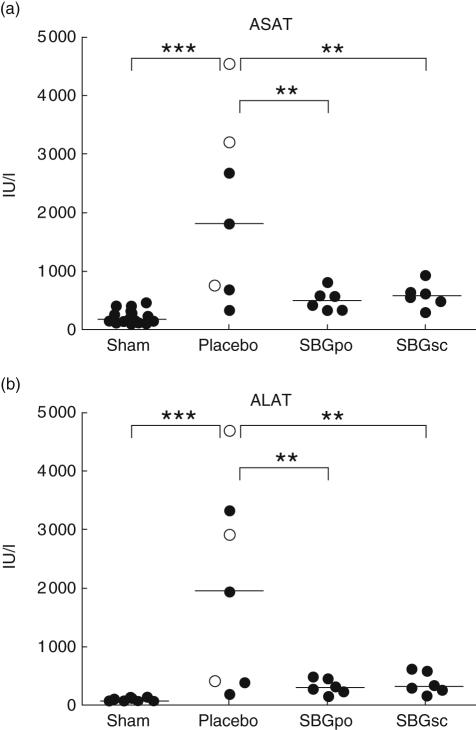
LPS-infusion to placebo-treated rats caused an approximately 10-fold elevation in ASAT level compared to sham (P < 0·001). Both the SBGpo group (P= 0·01) and the SBGsc group (P= 0·01) had significantly attenuated plasma ASAT levels compared with placebo following LPS infusion (Fig. 4a). Intravenous LPS administration caused an approximately 25-fold increase in mean plasma ALAT level compared to sham animals (P < 0·001). Mean ALAT values in both SBG-treated groups were significantly reduced compared to the placebo-treated animals (SBGpo; P < 0·01, SBGsc; P < 0·01) (Fig. 4b).
Fig. 4.
Effect of soluble Saccharomyces cerevisiae-derived β-1,3/1,6-glucan (SBG) prophylaxis on indicators of liver injury and hepatic dysfunction in the endotoxic rat. Rats were pretreated with SBG or placebo and underwent surgery as described. At the experimental end-point, 6 h after intravenous infusion of lipopolysaccharide (LPS) (SBGpo n = 8, SBGsc n = 8 and placebo n = 8) or vehicle (sham 20), plasma samples were collected and examined for levels of aspartate aminotransferase (ASAT) (a) and alanine aminotransferase (ALAT) (b) by automated enzymatic photometric assays. No statistically significant differences were observed between the sham animals, regardless of SBG or placebo pretreatment (SBGpo n = 5, SBGsc n = 5 and placebo n = 10) (data not shown), thus the data were combined to one sham group. In the placebo group oral and subcutaneous pretreatment is indicated by open (○) and closed circles (•), respectively. Each data point represents one animal, bars represent median values. **P < 0·01, ***P < 0·001 as determined by analysis of variance with Bonferroni's multiple comparison test.
Administration of LPS also caused increased plasma levels of γ-GT, bilirubin and pancreatic amylase compared to sham animals. However, these variables were not altered by either oral or subcutaneous prophylaxis with SBG (data not shown).
Effect of SBG on baseline plasma cytokine levels
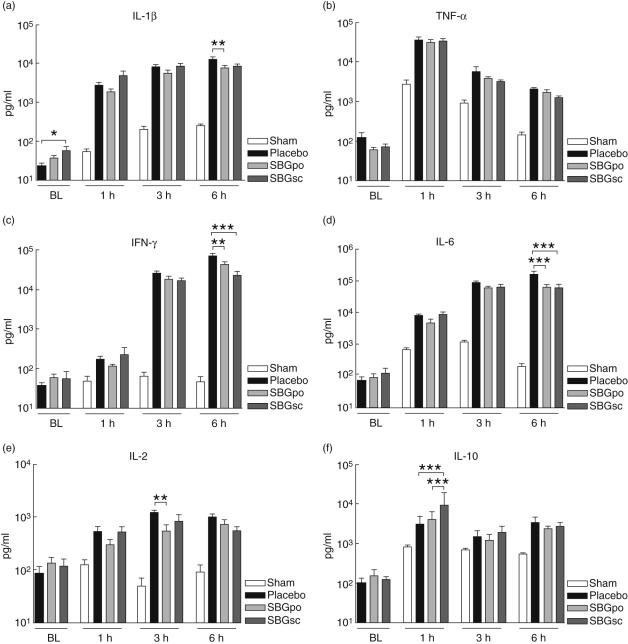
Prophylactic treatment with SBG resulted in a statistically significant increase in plasma level of IL-1α in the SBGsc group (P < 0·05) compared to placebo-treated animals prior to LPS infusion (Fig. 5a). A slight elevation in IL-2, IL-6, IL-10 and IFN-γ levels was also observed, whereas TNF-α levels were modestly reduced following SBG treatment. These changes were, however, not statistically significant (Fig. 5).
Fig. 5.
Effect of soluble Saccharomyces cerevisiae-derived β-1,3/1,6-glucan (SBG) administration on plasma cytokine levels. Rats were pretreated with SBG or placebo and underwent surgery as described. Blood samples were collected from the cannulated carotid artery prior to administration of lipopolysaccharide (LPS) or vehicle, BL, and 1, 3 and 6 h after infusion. Plasma levels of tumour necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6, IL-1-α, IL-2, and IL-10 were analysed using a multiplex bead-array assay. Samples collected prior to LPS injection included SBGpo (n = 13), SBGsc (n = 10) and placebo (n = 14) experimental groups. Samples collected 1, 3 and 6 h after LPS challenge included SBGpo (n = 8), SBGsc (n = 8) and placebo (n = 8) experimental groups. No statistically significant differences were observed between the animals receiving vehicle (sham n = 20), regardless of SBG or placebo pretreatment (SBGpo n = 5, SBGsc n = 5 and placebo n = 10) (data not shown), thus the data were combined to one sham group. Data are presented as mean ± s. e.m. *P < 0·05, **P < 0·01, ***P < 0·001 as determined by two-way analysis of variance with Bonferroni's multiple comparison test.
Effect of SBG on plasma cytokine levels following LPS infusion
Blood samples were collected 1, 3 and 6 h after intravenous infusion of LPS, and plasma levels of cytokines were analysed. Administration of LPS caused an immediate and substantial rise in plasma level of TNF-α (Fig. 5b). Subsequently, levels decreased gradually but remained considerably higher than baseline values even after 6 h. Conversely, the plasma level of IFN-γ increased gradually and remained high at the 6 h time-point (Fig. 5c). Following LPS infusion, plasma levels of IL-1α, IL-2, IL-6 and IL-10 increased rapidly, levelled off and remained at an elevated level throughout the experiment (Fig. 5).
In the SBGsc group, the LPS-induced rise in IFN-γ and IL-6 levels were significantly attenuated (IFN-γ; P < 0·001,IL-6; P < 0·001) compared to placebo-treated animals at 6 h (Fig. 5c,d). SBGsc-treated animals also had significantly higher plasma levels of IL-10 compared to both placebo (P < 0·001) and SBGpo (0·001) at 1 h (Fig. 5f).
In the SBGpo group, levels of IFN-γ (P < 0·01) and IL-6 (P < 0·001) were also significantly attenuated compared to placebo control rats at 6 h (Fig. 5c,d). Furthermore, levels of IL-1α (P < 0·01) and IL-2 (P < 0·01) were significantly attenuated compared to placebo control rats at 6 and 3 h, respectively (Fig. 5a,e).
Neither SBGsc nor SBGpo treatment reduced the elevated levels of TNF-α significantly, although a tendency towards reduction in TNF-α level was observed at 3 and 6 h (Fig. 5b). In the sham animals, no significant differences in plasma levels between placebo and SBG treatment were observed for any of the studied cytokines at 1, 3 and 6 h (data not shown).
Discussion
In this report we demonstrate for the first time that prophylactic treatment with orally administered water-soluble β-1,3/1,6-glucan produces a beneficial effect on haemodynamics and attenuates critical organ injury in the LPS-challenged rats.
Several animal sepsis models exist, but none fully resemble the timing of disease onset, progression and the use of supportive therapeutic intervention in clinical human sepsis. Systemic injection of LPS, a key mediator in Gram-negative sepsis, has been demonstrated to produce pathophysiological alterations, including systemic inflammation, haemodynamic disturbance and organ dysfunction similar to those reported for septic patients. We chose to investigate the outcome of oral prophylactic β-glucan treatment in the applied endotoxin-based model because the time of onset, the amount of circulating endotoxin and the severity of the sepsis-like reaction is tightly controlled [28].
Whether β-glucans are absorbed from the gastrointestinal tract has been a matter of dispute. Recently, Rice et al. [8] administered fluorescently labelled β-glucans orally to rats and detected fluorescence in plasma shortly after administration, suggesting rapid uptake. However, they did not address whether the detected fluorescence originated from fluorochromes associated with β-glucan in plasma or from detached fluorochromes. Conversely, in a recent phase I clinical trial Lehne et al. [16] reported lack of systemic absorption of orally administered soluble β-glucan. We found that oral administration of SBG to rats produced plasma levels of β-glucan 17-fold higher than that observed by us in the placebo control animals. The total amount of β-glucan in plasma was estimated to be approximately 30 ng following 14 consecutive days of oral administration of 5–6 mg per day. Thus only a minute fraction of a single oral dose of SBG was translocated to plasma and the biological relevance of intestinal absorption remains uncertain. Pharmacokinetic analysis and further comparisons between the two chosen routes of delivery were beyond the scope of this work. Tissue levels of SBG were not quantified due to methodological limitations.
Dendritic cells (DCs) sample constitutively the intestinal mucosa for food- and environmental antigens, commensal microbes and their products. These DCs migrate from the intestinal epithelium and Peyer's patches to the mesenteric lymph nodes (MLNs), where they are involved in the development of oral tolerance and systemic immunity [29]. We speculate that mucosal DCs sample or interact with soluble β-glucan locally via projections across the epithelium and then migrate via afferent lymphatics to the MLNs, where immune modulation is initiated. In support of this hypothesis, Rice et al. [8] reported recently that orally administered β-glucan was bound and internalized by intestinal epithelial cells and gut-associated lymphoid tissue leading to increased dectin-1 and Toll-like receptor 2 expression associated with increased survival in experimental sepsis. Furthermore, Hong et al. [11] demonstrated that fluorescent β-glucan particles were taken up by gastrointestinal macrophages and shuttled to the spleen, lymph nodes and the bone marrow.
In severe sepsis and septic shock the release of proinflammatory mediators leads to haemodynamic disturbances. The present investigation demonstrated clearly that oral, as well as subcutaneous, administration of SBG had beneficial effects on the mean arterial blood pressure in rats after experimental induction of endotoxaemia. The protective effect became pronounced as the shock response progressed and at the 5·5- and 6-h time-points, MAP in the orally treated group was significantly higher than in the placebo control group. MAP in the subcutaneously treated group was significantly elevated at 6 h compared to placebo.
We observed a gradual decrease in blood pressure in the sham animals, similar to what has been described in this model previously [30]. We find it plausible that the gradual fall in MAP is caused by the stress of the surgery in a non-sterile environment: cannulation, repeated blood sampling and the fact that the animals are under anaesthesia for more than 6 h. Reduced blood pressure in sham animals over time resulted in no statistically significant difference in MAP between placebo and sham-treated animals at the late time-points, although a clear trend was observed. Furthermore, a single animal with vastly improved MAP dynamics was observed in the placebo group, explaining the large s.e.m. value. Nevertheless, indicators of organ injury and cytokine levels for this animal indicated development of a significant shock reaction with organ injury.
The cellular and molecular mechanisms behind the observed haemodynamic protection effect of orally administered SBG on MAP remain unknown. Although controversial, β-glucans have been demonstrated to hold anti-oxidant properties [31]. Accordingly, SBG treatment may affect the oxidative status, and hence mediate indirect effects on myocardial- and smooth muscle contraction and consequently enhance blood pressure.
Infusion of LPS to rats resulted in increased plasma levels of creatinine and urea, indicating impaired glomerular filtration due to renal injury. Here we demonstrated that oral administration of SBG significantly attenuated plasma creatinine levels and also resulted in a minor reduction of plasma urea in the endotoxic rats. However, subcutaneous delivery of the soluble β-glucan did not mediate the same level of protection against renal injury.
To monitor liver injury we measured plasma levels of ALAT and ASAT, caused by hepatocyte leakage, although ASAT may also be released from skeletal and heart muscle cells (reviewed in [32]). Accordingly, our observation that SBG significantly attenuated the plasma levels of ASAT and ALAT indicated strongly that SBG had a protective effect against LPS-induced hepatic injury. Both subcutaneous and oral administration of soluble β-glucan mediated protection against liver injury and dysfunction in contrast to the better efficacy of oral β-glucan in reducing kidney injury. The mechanism behind the organ-protective capacity of β-glucan, however, remains elusive. We hypothesize that the superior blood pressure in the SBG-treated rats may contribute to the reduced kidney and liver injury, reflecting the benefit of enhanced organ perfusion.
Our observations on the organ-protective effect of SBG are in agreement with recent papers. Sener et al. [2] and Toklu et al. [26] demonstrated reduced TNF-α levels following administration of β-glucan in animal models of sepsis, suggesting that the organ-protective capacity of SBG may be due to modulation of the cytokine profile in the endotoxic rat. We found that prophylactic subcutaneous treatment with SBG produced a moderate increase in baseline plasma levels of IL-1α, whereas the expression level for the other cytokines studied was not significantly changed, regardless of delivery route. The literature on the effects of β-glucan on cytokine expression is inconsistent, probably reflecting a complex biological interplay as well as the use of different experimental systems and a variety of β-glucan preparations. In agreement with our findings, Rasmussen and coworkers [7] demonstrated that treatment with aminated soluble β-glucan and β-glucan-derivatized microbeads resulted in increased levels of IL-1 but no change in TNF-α in a murine model. Although Engstad et al. [33] found that soluble β-glucan induced production of IL-8 and monocyte tissue factor as well as minor amounts of TNF-α, IL-6 and IL-10 in human whole blood cultures, Wakshull and coworkers [34] found no production of inflammatory cytokines following glucan exposure in a similar assay. β-Glucan treatment of isolated leucocytes and monocytic cell lines has, to a variable degree, induced or had no effect on production of inflammatory cytokines [4,35]. Furthermore, conflicting data exist regarding the activation or inhibition of the transcription factors nuclear factor-kappa B (NF-κB) and NF-interleukin-6 (IL-6) transcription factor by β-glucan [5,35]. These inconsistencies contribute to the enigma associated with the mechanisms by which β-glucans work.
LPS activates an immense range of genes, including an array of inflammatory mediators with the capacity to cause injury to vital organs (reviewed in [36]). In contrast to Sener et al. [2] and Toklu et al. [26], we did not observe a statistically significant reduction in TNF-α levels following SBG treatment. Thus, the observed organo-protective effect of SBG appears not to be coupled solely to the modulation of this key mediator of inflammation. We found that LPS-induced increase in plasma IL-1α, IFN-γ, IL-6 and IL-2 levels were attenuated at later time-points following prophylactic treatment with SBG, suggesting that these mediators in the early phase of sepsis may return more rapidly to baseline levels in SBG-treated rats. In accordance with our data, Nakagawa et al. [37] reported that soluble β-glucan extracted from Candida albicans significantly suppressed endotoxin-induced IL-6, IL-2 and IFN-γ production in cultures of human monocytes or peripheral blood mononuclear cells. In contrast to our observations, Soltys and Quinn [4] reported a reduction in TNF-α production and described a substantial increase in IL-6 production from lymphocytes and monocytes isolated from β-glucan-treated mice following a subsequent challenge with LPS in vitro.
The subtle changes in the cytokine profile reported here are unlikely to explain solely the observed beneficial effect of SBG on organ function and haemodynamics in the endotoxaemic rat. Nevertheless, the cytokine data demonstrate that SBG prophylaxis does affect the systemic inflammation induced by LPS infusion. Notably, this is the case even after oral applications that produce low plasma levels of SBG. The contribution of this cytokine modulation on the observed organoprotective and haemodynamic effect remains unknown.
The present work identified a discrepancy between the effects of oral and subcutaneous administration of β-glucan. Plasma IL-10 and urea levels were significantly higher in the subcutaneously treated group at 1 and 6 h, respectively, compared to the orally treated animals. Interestingly, renal protection was seen only in the SBGpo group, despite the fact that the plasma level of SBG in the SBGsc group was much higher. Moreover, the higher plasma level of SBG in the SBGsc group did not lead to superior protection of the liver, recovery of MAP or modulation of cytokines compared to oral administration. Thus, it seems that the plasma level does not correlate with the observed effect of SBG for any of the parameters tested. This suggests that discrete cellular and molecular mechanisms may be involved in the mode of action, depending on the route of delivery. Further research is required to elucidate this discrepancy.
In conclusion, we demonstrated a striking positive effect on the haemodynamics during the progression of LPS-induced shock in rats that had received prophylactic SBG treatment. In spite of the fact that only a small fraction of orally administered β-glucan was absorbed from the gastrointestinal tract to the circulation, oral administration of SBG had a more pronounced effect than subcutaneous injection. We observed attenuated levels of indicators of kidney and liver injury following SBG treatment, which may be due to enhanced organ perfusion. Additionally, SBG prophylaxis caused subtle changes in the cytokine profile, including attenuated levels of mediators of inflammation subsequent to endotoxin challenge. Characterization of how β-glucans exert their biological effects may add to the understanding of the workings of the innate immune system and may be helpful in identifying new targets and applications for β-glucans in human therapy.
Acknowledgments
This work was supported financially by Biotec Pharmacon ASA, Tromsø, Norway and the Research Council of Norway. We thank Anne Pharo at the Institute of Immunology, Grethe Dyrhaug at the Institute for Surgical Research and the Department of Medical Biochemistry, Rikshospitalet-Radiumhospitalet Medical Center, Oslo, Norway for skilful technical assistance.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sener G, Toklu H, Ercan F, Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. Int Immunopharmacol. 2005;5:1387–96. doi: 10.1016/j.intimp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Babayigit H, Kucuk C, Sozuer E, Yazici C, Kose K, Akgun H. Protective effect of beta-glucan on lung injury after cecal ligation and puncture in rats. Intens Care Med. 2005;31:865–70. doi: 10.1007/s00134-005-2629-x. [DOI] [PubMed] [Google Scholar]
- 4.Soltys J, Quinn MT. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with beta-(1,6)-branched beta-(1,3)-glucan. Infect Immun. 1999;67:244–52. doi: 10.1128/iai.67.1.244-252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DL, Ha T, Li C, Kalbfleisch JH, Laffan JJ, Ferguson DA. Inhibiting early activation of tissue nuclear factor-kappa B and nuclear factor interleukin 6 with (1→3)-beta-D-glucan increases long-term survival in polymicrobial sepsis. Surgery. 1999;126:54–65. doi: 10.1067/msy.1999.99058. [DOI] [PubMed] [Google Scholar]
- 6.Onderdonk AB, Cisneros RL, Hinkson P, Ostroff G. Anti-infective effect of poly-beta 1-6-glucotriosyl-beta 1-3-glucopyranose glucan in vivo. Infect Immun. 1992;60:1642–7. doi: 10.1128/iai.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen LT, Fandrem J, Seljelid R. Dynamics of blood components and peritoneal fluid during treatment of murine E. coli sepsis with beta-1,3-D-polyglucose derivatives. II. Interleukin 1, tumour necrosis factor, prostaglandin E2, and leukotriene B4. Scand J Immunol. 1990;32:333–40. doi: 10.1111/j.1365-3083.1990.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 8.Rice PJ, Adams EL, Ozment-Skelton T, et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther. 2005;314:1079–86. doi: 10.1124/jpet.105.085415. [DOI] [PubMed] [Google Scholar]
- 9.Kernodle DS, Gates H, Kaiser AB. Prophylactic anti-infective activity of poly-[1–6]-beta-D-glucopyranosyl-[1–3]-beta-D-glucopryanose glucan in a guinea pig model of staphylococcal wound infection. Antimicrob Agents Chemother. 1998;42:545–9. doi: 10.1128/aac.42.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung NK, Modak S, Vickers A, Knuckles B. Orally administered beta-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol Immunother. 2002;51:557–64. doi: 10.1007/s00262-002-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong F, Yan J, Baran JT, et al. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 12.Patchen ML, MacVittie TJ. Comparative effects of soluble and particulate glucans on survival in irradiated mice. J Biol Resp Mod. 1986;5:45–60. [PubMed] [Google Scholar]
- 13.Gu YH, Takagi Y, Nakamura T, et al. Enhancement of radioprotection and anti-tumor immunity by yeast-derived beta-glucan in mice. J Med Food. 2005;8:154–8. doi: 10.1089/jmf.2005.8.154. [DOI] [PubMed] [Google Scholar]
- 14.Nicolosi R, Bell SJ, Bistrian BR, Greenberg I, Forse RA, Blackburn GL. Plasma lipid changes after supplementation with beta-glucan fiber from yeast. Am J Clin Nutr. 1999;70:208–12. doi: 10.1093/ajcn.70.2.208. [DOI] [PubMed] [Google Scholar]
- 15.Bell S, Goldman VM, Bistrian BR, Arnold AH, Ostroff G, Forse RA. Effect of beta-glucan from oats and yeast on serum lipids. Crit Rev Food Sci Nutr. 1999;39:189–202. doi: 10.1080/10408399908500493. [DOI] [PubMed] [Google Scholar]
- 16.Lehne G, Haneberg B, Gaustad P, Johansen PW, Preus H, Abrahamsen TG. Oral administration of a new soluble branched beta-1,3-D-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin Exp Immunol. 2006;143:65–9. doi: 10.1111/j.1365-2249.2005.02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delatte SJ, Evans J, Hebra A, Adamson W, Othersen HB, Tagge EP. Effectiveness of beta-glucan collagen for treatment of partial-thickness burns in children. J Pediatr Surg. 2001;36:113–8. doi: 10.1053/jpsu.2001.20024. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Ha T, Kelley J, et al. Modulating Toll-like receptor mediated signaling by (1→3)-beta-D-glucan rapidly induces cardioprotection. Cardiovasc Res. 2004;61:538–47. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Cramer DE, Allendorf DJ, Baran JT, et al. Beta-glucan enhances complement-mediated hematopoietic recovery after bone marrow injury. Blood. 2006;107:835–40. doi: 10.1182/blood-2005-07-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–5. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 21.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 22.Bochud PY, Calandra T. Science, medicine, and the future: pathogenesis of sepsis: new concepts and implications for future treatment. BMJ. 2003;326:262–6. doi: 10.1136/bmj.326.7383.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown GD, Gordon S. Immune recognition of fungal beta-glucans. Cell Microbiol. 2005;7:471–9. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 24.Dritz SS, Shi J, Kielian TL, et al. Influence of dietary beta-glucan on growth performance, nonspecific immunity, and resistance to Streptococcus suis infection in weanling pigs. J Anim Sci. 1995;73:3341–50. doi: 10.2527/1995.73113341x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki I, Tanaka H, Kinoshita A, Oikawa S, Osawa M, Yadomae T. Effect of orally administered beta-glucan on macrophage function in mice. Int J Immunopharmacol. 1990;12:675–84. doi: 10.1016/0192-0561(90)90105-v. [DOI] [PubMed] [Google Scholar]
- 26.Toklu HZ, Sener G, Jahovic N, Uslu B, Arbak S, Yegen BC. Beta-glucan protects against burn-induced oxidative organ damage in rats. Int Immunopharmacol. 2006;6:156–69. doi: 10.1016/j.intimp.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Wang JE, Pettersen S, Stuestol JF, et al. Peptidoglycan of S. aureus causes increased levels of matrix metalloproteinases in the rat. Shock. 2004;22:376–9. doi: 10.1097/01.shk.0000140299.48063.89. [DOI] [PubMed] [Google Scholar]
- 28.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–65. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 29.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JE, Dahle MK, Yndestad A, et al. Peptidoglycan of Staphylococcus aureus causes inflammation and organ injury in the rat. Crit Care Med. 2004;32:546–52. doi: 10.1097/01.CCM.0000109775.22138.8F. [DOI] [PubMed] [Google Scholar]
- 31.Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder IW, Williams DL. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic Biol Med. 2001;30:393–402. doi: 10.1016/s0891-5849(00)00485-8. [DOI] [PubMed] [Google Scholar]
- 32.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Can Med J. 2005;172:367–79. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engstad CS, Engstad RE, Olsen JO, Osterud B. The effect of soluble beta-1,3-glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int Immunopharmacol. 2002;2:1585–97. doi: 10.1016/s1567-5769(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 34.Wakshull E, Brunke-Reese D, Lindermuth J, et al. PGG-glucan, a soluble [beta]-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-[kappa]B-like factor in human PMN. Evidence for a glycosphingolipid [beta]-(1,3)-glucan receptor. Immunopharmacology. 1999;41:89–107. doi: 10.1016/s0162-3109(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 35.Adams DS, Pero SC, Petro JB, Nathans R, Mackin WM, Wakshull E. PGG-glucan activates NF-kappaB-like and NF-IL-6-like transcription factor complexes in a murine monocytic cell line. J Leukoc Biol. 1997;62:865–73. doi: 10.1002/jlb.62.6.865. [DOI] [PubMed] [Google Scholar]
- 36.Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med. 1999;5:123–32. doi: 10.1016/s1357-4310(98)01430-0. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa Y, Ohno N, Murai T. Suppression by Candida albicans and beta-glucan of cytokine release from activated human monocytes and from T cells in the presence of monocytes. J Infect Dis. 2003;187:710–3. doi: 10.1086/368334. [DOI] [PubMed] [Google Scholar]