Abstract
Opsonization of apoptotic cardiocytes by maternal anti-Ro/SSA and anti-La/SSB antibodies contributes to tissue injury in the neonatal lupus syndrome. The objective of the current study was to quantify the surface membrane expression of Ro/La components during different phases of apoptosis and map the Ro/La apotopes (epitopes expressed on apoptotic cells) bound by cognate antibodies. Multi-parameter flow cytometry was used to define early and late apoptotic populations and their respective binding by monospecific anti-Ro and anti-La IgGs. Anti-Ro60 bound specifically to early apoptotic Jurkat cells and remained accessible on the cell surface throughout early and late apoptosis. In contrast, anti-La bound exclusively to late apoptotic cells in experiments controlled for non-specific membrane leakage of IgG. Ro52 was not accessible for antibody binding on either apoptotic population. The immunodominant NH2-terminal and RNA recognition motif (RRM) epitopes of La were expressed as apotopes on late apoptotic cells, confirming recent in vivo findings. An immunodominant internal epitope of Ro60 that contains the RRM, and is recognized by a majority of sera from mothers of children with congenital heart block (CHB) and patients with primary Sjögren's syndrome, was also accessible as an apotope on early apoptotic cells. The distinct temporal expression of the immunodominant Ro60 and La apotopes indicates that these intracellular autoantigens translocate independently to the cell surface, and supports a model in which maternal antibody populations against both Ro60 and La apotopes act in an additive fashion to increase the risk of tissue damage in CHB.
Keywords: apotope, congenital heart block, immunodominant, La/SSB, Ro/SSA
Introduction
Translocation and cell surface expression of the intracellular Ro/SSA (comprising the structurally unrelated Ro52 and Ro60 proteins) and La/SSB ribonucleoprotein autoantigens during apoptosis and their subsequent recognition by cognate antibodies is thought to play a central role in causing selective cardiac injury in the neonatal lupus syndrome (NLS) [1–4]. Recent evidence has revealed that the binding of anti-Ro/La antibodies to their respective ‘apotopes’ (epitopes expressed on apoptotic cells) may inhibit the physiological clearance of apoptotic cardiocytes by resident cardiocytes, resulting in an exaggerated accumulation of opsonized apoptotic cells. It has been postulated that these cell-bound immune complexes are then diverted toward clearance by infiltrating macrophages, the consequence of which is inflammation and subsequent scarring [5]. These studies have identified a novel role for Ro and La antigens as potential natural ligands for apoptotic cell clearance and highlighted the importance of mapping specific apotopes by anti-Ro/La antibodies.
Although numerous studies have reported that Ro and La translocate to surface blebs during apoptosis [6–8], it is still controversial as to whether these autoantigens are externalized at the surface and available for binding by extracellular antibodies. One limitation has been that many studies have employed immunofluorescence, a semiquantitative technique in which interpretation can be ambiguous due to non-specific leakage of antibody and fixation artefacts. In addition, specificity of the anti-Ro52/Ro60/La reagents in some studies has been poorly defined. Accordingly, the current study was initiated to confirm and extend surface membrane expression of Ro/La components utilizing well-characterized reagents and quantitative multi-parameter flow cytometry controlled for membrane leakage of IgG. The overall goal was to identify apotopes on the Ro and La molecules which could form the basis of an improved diagnostic predictor of heart block. According to the apoptotic cell opsonization model of congenital heart block (CHB) [2,5], maternal autoantibodies reacting with such apotopes would be more likely to represent the pathogenic species.
Materials and methods
Patient and control sera
Sera from patients with primary Sjögren's syndrome (pSS) were characterized for anti-Ro52/Ro60/La/ribonucleoprotein (RNP) specificities by counterelectrophoresis, immunoblot (INNO-LIA™; Innogenetics, Ghent, Belgium) and enzyme-linked immunosorbent assay (ELISA) (RELISA™; Immunoconcepts, Sacramento, CA, USA). Sera defined as monospecific for anti-Ro52, anti-Ro60 or anti-RNP were used in flow cytometry studies. Control human sera were collected from healthy donors and a patient with high titre anti-RNP antibodies. Additional sera from mothers who gave birth to at least one child with CHB were obtained from the Research Registry for Neonatal Lupus (Hospital for Joint Diseases, New York University School of Medicine). This study was approved by the Clinical Ethics Committee of the Flinders Medical Centre.
Preparation of total IgG and affinity-purified IgG
Immunoglobulin G (IgG) was purified from monospecific anti-Ro52, anti-Ro60 and anti-RNP sera on protein A-Sepharose columns (Pharmacia, Uppsala, Sweden) according to the manufacturer's recommendations. Affinity-purified anti-Ro52 antibodies were prepared from two mothers whose children have neonatal lupus (one CHB, one rash) using a soluble recombinant maltose-binding protein (MBP) fusion protein of full-length human Ro52 coupled to cyanogen bromide-activated Sepharose 4B (Pharmacia). The specificity of monospecific and affinity-purified IgG preparations was confirmed by recombinant Ro52/Ro60/La ELISA, as described previously [9]. Soluble recombinant glutathione S-transferase (GST) fusion proteins of full-length human La and non-overlapping fragments encompassing the NH2-terminal (La A), RRM region (La C) and COOH-terminal (La L2/3) epitopes were prepared as described previously [10]. La fusion proteins were coupled to cyanogen bromide-activated Sepharose 4B (Pharmacia) according to the manufacturer's recommendations. Specific autoantibodies present in patient sera were bound to the appropriate recombinant protein columns, washed with 50 column volumes of phosphate-buffered saline (PBS) and eluted with 0·1 M glycine (pH 2·3). Eluted human autoantibodies were neutralized by collection in 2 M Tris (pH 8) and dialysed against PBS. The specificity of the anti-La A/La C/La L2/3 IgG was confirmed by ELISA, as reported previously [11].
Polyclonal antibodies
A rabbit anti-Ro60 anti-serum, which produced a single Ro60 precipitin on counter-immunoelectrophoresis (CIEP), was provided by Dr Tim Gross (Oklahoma Medical Research Foundation, Oklahoma City, OK, USA). A rabbit anti-Ro52 anti-serum prepared by immunization with full-length recombinant human Ro52 hexa-histidine fusion protein was provided by Dr Catherine Keech (University of Melbourne, Victoria, Australia).
Mapping of Ro60 epitopes by ELISA
Soluble overlapping fragments of mouse Ro60 amino acids (aa) 1–150, 82–244 and 393–538 expressed as MBP fusion proteins were prepared from pMAL cDNA vectors (provided by Dr Ken Kaufman, Oklahoma Medical Research Foundation, Oklahoma City, OK, USA). Fusion proteins were purified by maltose-affinity chromatography, as described previously [12]. Serum samples from 27 pSS patients and 35 mothers who had at least one child with CHB were diluted 1/100 and preabsorbed with Escherichia coli extract (Promega, Mountain View, CA, USA). Reactivity with recombinant MBP-Ro60 fragments was assessed using ELISA. A serum was considered to be reactive with Ro60 fragments if the value obtained was 2 standard deviations (s.d.) above the mean value obtained using a group of 25 healthy controls.
Cell culture and induction of apoptosis
Jurkat and HeLa cells (American Type Culture Collection, Manassas, VA USA) were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), glutamate, penicillin and streptomycin. Staurosporine-induced apoptosis of human Jurkat cells was used as a model to delineate early and late apoptotic populations, as described previously [13,14]. For induction of early apoptosis by an intrinsic death pathway, 5 × 105 cells/ml were transferred to serum-free media containing 0·5% bovine serum albumin (BSA) (Sigma-Aldrich, Sydney, Australia) and 1 µg/ml staurosporine for 3 h at 37°C. Cells were rendered late apoptotic by removing staurosporine and incubating for an additional 18 h in serum-free media supplemented with 0·5% BSA. To render cells apoptotic via an extrinsic pathway, anti-Fas monoclonal antibody (MoAb) (Upstate Biotech, Lake Placid, NY, USA) was used in accordance with the manufacturer's recommendations. Briefly, cells were transferred to RPMI-1640 supplemented with 2% FCS and 20 ng/ml anti-Fas MoAb for 24 h at 37°C. HeLa cells rendered apoptotic with staurosporine, as described above, were used in additional experiments. Apoptosis was confirmed by microscopic observation of cell size, morphology and flow cytometric analysis of phosphadylserine exposure (annexin V binding) and active caspase staining using the Apofluor® Green Caspase Activity Assay kit, which detects activated caspase-1, -3, -4, -5, -6, -7, -8 and -9 (ICN Biomedicals, Livermore, CA, USA), according to the manufacturer's recommendations.
Permeabilized cells were prepared with 10 mM HEPES, 0·1% saponin and 4% formaldehyde in PBS for 10 min at 4°C to validate the activity of the anti-RNP, anti-Ro52, anti-Ro60 and anti-La IgG preparations and rabbit anti-Ro60, anti-Ro52 anti-serum.
Multi-parameter flow cytometry for evaluating anti-Ro/La antibody binding to apoptotic cells
To exclude the possibility of active internalization of antibody all binding studies were performed at 4°C in the presence of 0·02% azide. Apoptotic or permeabilized cells were washed twice in fluorescence activated cell sorter (FACS) wash (PBS, 1% FCS, 0·02% sodium azide) and incubated with either 0·5 mg/ml human monospecific anti-RNP, anti-Ro52 or anti-Ro60 IgG, 20 µg/ml human affinity-purified anti-La or anti-Ro52 IgG, 2% rabbit anti-Ro60 or anti-Ro52 anti-serum for 30 min. Washing was repeated and cells were stained with 1 : 50 dilutions of secondary antibody, anti-human-IgG-fluorescein isothiocyanate (FITC) (Dako Cytomation, Glostrub, Denmark) or anti-rabbit-IgG-FITC (Jackson IR, West Grove, PA, USA) for 30 min. Early and late apoptotic cell populations were distinguished by annexin V and propidium iodide (PI) staining, as described previously [13]. Briefly, cells were washed twice with PBS and resuspended in annexin V binding buffer (10 mM Hepes/NaOH, pH 7·4, 140 mM NaCl, 2·5 mM CaCl2). Five μl of annexin V-allophycocyanin (APC) conjugate (BD Biosciences, North Ryde, Australia) and 5 µg/ml PI were added and incubated for 15 min in the dark at room temperature. Triple labelling of IgG, annexin V and PI was analysed on a FACS Canto equipped with 488 nm and 633 nm emission lasers (BD San Jose, CA, USA). IgG-FITC was detected at 530/30 nm, annexin V-APC at 660/20 nm and PI at 670 nm. Baseline fluorescence levels were obtained from cells that were stained with secondary antibodies only.
To confirm the specificity of the binding of human anti-Ro60 IgG and rabbit anti-Ro60 anti-serum, inhibition experiments were conducted. Human monospecific anti-Ro60 IgG at 0·5 mg/ml or 2% rabbit anti-Ro60 anti-serum were preincubated with 200 µg/ml of the soluble MBP-Ro60 fragments aa 82–244 and 393–538 or MBP for 1 h at room temperature prior to addition to apoptotic cells. Binding of human IgG was assessed by multi-parameter flow cytometry as described above. Results were expressed as percentage reduction in binding compared with the MBP control.
Results
Surface accessibility of Ro60 and La on early and late apoptotic cells
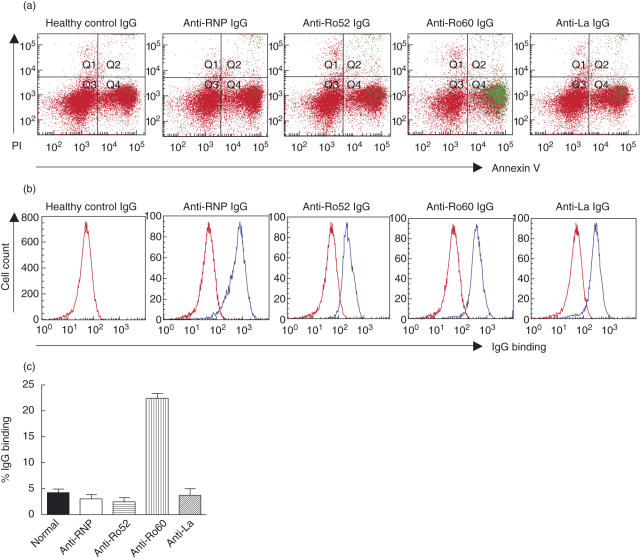
Multi-parameter flow cytometry was employed to evaluate anti-Ro52/Ro60/La IgG binding to different populations of apoptotic cells. Cells analysed for IgG binding were positive for annexin V and active caspase staining (data not shown). A high titre anti-RNP IgG was included to control for cell permeability to IgG and non-specific movement of IgG through the apoptotic cell membrane. Staurosporine-induced early apoptotic Jurkat cells (PI negative, annexin V positive) were bound by the anti-Ro60 IgG but not the anti-Ro52 or anti-La IgGs, indicating that Ro60 alone is exposed during early apoptosis (Fig. 1a,c). To confirm the activity of the IgGs, permeabilized cells were stained with monospecific anti-RNP, anti-Ro60 IgG, affinity-purified anti-Ro52 or anti-La IgG. All IgG preparations bound with their respective intracellular antigens in permeabilized cells (Fig. 1b).
Fig. 1.
Flow cytometric analysis of anti-Ro/La antibody binding to early apoptotic cells. Early apoptotic Jurkat cells were prepared by exposure to staurosporine (1 µg/ml, 3 h, 37°C). Early apoptotic and permeabilized cells were labelled with healthy control IgG, monospecific anti-Ro60 or anti-ribonucleoprotein (anti-RNP) IgG or affinity-purified anti-Ro52 or anti-La IgG followed by staining with annexin V and propidium iodide (PI). Representative flow cytometry data are shown in (a) and (b). (a) Anti-Ro60 IgG binding is shown in green and indicates that Ro60 is accessible for binding on the surface of the early apoptotic cells. (b) Permeabilized cells were prepared with 10 mM HEPES, 0·1% saponin, 4% formaldehyde in phosphate-buffered saline (PBS) (10 min, 4°C) to confirm reactivity of all IgG preparations for their native antigen. (c) Pooled data (n = 6) evaluating anti-Ro/La antibody binding to early apoptotic cells. Values are mean ± standard error of the mean.
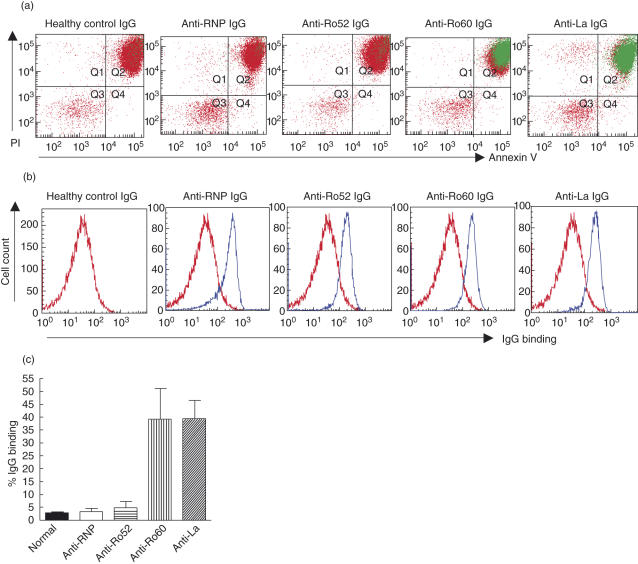
We then examined the binding of anti-Ro52/Ro60/La IgGs to a defined late apoptotic population that was annexin V positive, permeable to PI (MW 688·4) and impermeable to IgG (see anti-RNP IgG panel, Fig. 2). As observed with the early apoptotic cells, the anti-Ro52 IgG was unable to access its cognate antigen. The anti-Ro60 IgG remained bound to the late apoptotic cells, indicating that Ro60 remains accessible on the cell surface throughout early and late apoptosis. In contrast to the absence of affinity-purified anti-La binding of early apoptotic cells, anti-La IgG bound to late apoptotic cells, suggesting that the La antigen is translocated to the cell surface during late apoptosis (Fig. 2). Anti-RNP IgG did not bind to either apoptotic cell population, and therefore excluded the possibility of non-specific leakage of IgG and binding of intracellular antigen (Figs 1 and 2). These results therefore indicate that Ro60 and La are exposed on the external surface of apoptotic cells and that there are clear temporal differences in the surface expression of these antigens during apoptosis.
Fig. 2.
Flow cytometric analysis of anti-Ro/La antibody binding to late apoptotic cells. Late apoptotic Jurkat cells were prepared by exposure to staurosporine (1 µg/ml, 3 h, 37°C) followed by 1 h incubation in serum-free media. Late apoptotic and permeabilized cells were labelled with healthy control IgG, monospecific anti-RNP IgG (to exclude the possibility of non-specific movement of IgG through the apoptotic cell membrane), monospecific anti-Ro60 IgG or affinity purified anti-Ro52 or anti-La IgG followed by staining with annexin V and propidium iodide (PI) to evaluate surface exposure of the Ro/La autoantigens. Representative flow cytometry data is shown in (a) and (b). (a) Anti-Ro60 and anti-La IgG binding is shown in green and indicates that both Ro60 and La are accessible on the surface of the late apoptotic cells (PI/annexin V positive). (b) Permeabilized cells were prepared with 10 mM HEPES, 0·1% saponin, 4% formaldehyde in phosphate-buffered saline (PBS) (10 min, 4°C) to confirm reactivity of all IgG preparations for their native antigen. (c) Pooled data (n = 6) evaluating anti-Ro and anti-La antibody binding to the late apoptotic population of cells. Values are mean ± standard error of the mean.
To determine whether the observed differences in anti-Ro60 and anti-La binding during different stages of apoptosis were dependent on the apoptotic trigger, parallel experiments were performed following induction of an extrinsic apoptotic pathway with anti-Fas MoAb. Cells rendered apoptotic by anti-Fas MoAb revealed both early and late populations that showed equivalent anti-Ro/La IgG binding profiles as apoptotic cells induced with staurosporine. Healthy control IgG bound to 7% and 6% of early and late anti-fas MoAb-induced apoptotic cells, respectively, which was comparable to anti-RNP IgG binding to 5% of early and 7% of late apoptotic cells. Anti-Ro52 IgG was similar to the control IgG, binding 6% of early and 4% of late apoptotic cells. In contrast, anti-Ro60 IgG bound to 37% and 42% of early and late apoptotic cells and anti-La IgG bound to 6% of early apoptotic cells and 36% of late apoptotic cells.
As the above experiments did not demonstrate Ro52 exposure during either early or late apoptosis (Figs 1 and 2), additional flow cytometry studies were performed using a rabbit polyclonal anti-Ro52 anti-serum and affinity-purified anti-Ro52 IgGs from two mothers of children with separate manifestations of neonatal lupus. None of these anti-Ro52 antibodies bound to early or late apoptotic populations, providing further evidence that Ro52 is not exposed on the surface of apoptotic Jurkat cells. The absence of Ro52 surface accessibility was also confirmed on HeLa cells rendered apoptotic by staurosporine (data not shown).
Mapping of immunodominant La apotopes during late apoptosis
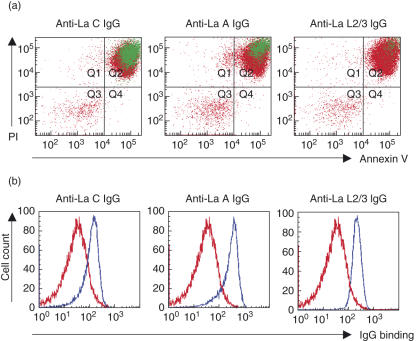
Having previously demonstrated that the NH2-terminal (La A) and RRM (La C) epitopes are accessible for antibody binding on apoptotic cardiocytes in vitro and matrigel implants in vivo[11], the accessibility of La A and La C on apoptotic Jurkat cells was evaluated. Anti-La A and anti-La C bound to late apoptotic (annexin V/PI positive) cells, while anti-La L2/3 was unable to access its cognate antigen (Fig. 3a). Reactivity of anti-La L2/3 for the native antigen was confirmed in permeabilized cells, which showed binding for all La epitopes (Fig. 3b). This finding confirms the cell surface topology of the La molecule during apoptosis, where the NH2-terminus and RRM are exposed and the COOH-terminus remains intracellular.
Fig. 3.
Immunodominant apotopes of La are accessible for binding on the surface of late apoptotic Jurkat cells. Late apoptotic cells were prepared by exposure to staurosporine (1 µg/ml, 3 h, 37°C) followed by 18 h incubation in serum-free media. Cells were labelled with non-overlapping affinity-purified fragments NH2-terminal (La A), RNA recognition motif (RRM) region (La C) and COOH-terminal (La L2/3) followed by staining with annexin V and propidium iodide (PI) to evaluate epitopes of La accessible for binding on the apoptotic cell surface. Representative flow cytometry data are shown in (a) and (b). (a) IgG binding is shown in green and indicates that the NH2-terminal (La A) and RRM region (La C) epitopes are translocated to the cell surface during late apoptosis. (b) Permeabilized cells were prepared with 10 mM HEPES, 0·1% saponin, 4% formaldehyde in phosphate-buffered saline (PBS) (10 min, 4°C) to confirm reactivity of the affinity-purified La fragments.
An immunodominant Ro60 apotope is exposed during early and late apoptosis
Having mapped La apotopes on late apoptotic cells, experiments were performed to determine whether immunodominant epitopes of Ro60 are exposed as apotopes on the surface of early and late apoptotic cells. In preliminary experiments to determine the immunodominance of different regions of Ro60, sera from pSS patients and mothers of CHB children were evaluated for reactivity with soluble overlapping MBP-Ro60 proteins. For the pSS group, 81·5% reacted with the aa 82–244 fragment; 33·3% with the COOH-terminus (aa 393–538); and 14·8% with the NH2-terminus (aa 1–150). Sera from mothers with CHB children showed 77·1% reactivity with the aa 82–244 fragment; 28·6% with the COOH-terminus (aa 393–538); and 8·6% with the NH2-terminus (aa 1–150). These results reveal that the Ro60 aa 82–244 domain contains an immunodominant epitope while minor epitopes are present in the COOH-terminal and NH2-terminal regions.
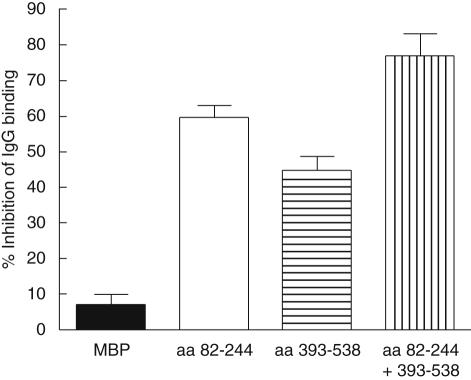
Inhibition experiments were performed to determine whether the aa 82–244 and aa 393–538 fragments are expressed as apotopes during apoptosis. Pre-incubation of a human monospecific anti-Ro60 IgG with these fragments individually and together versus an MBP control inhibited binding to early apoptotic cells by 62% for the aa 84–244 fragment, 43% for the aa 393–538 fragment and 82% with both fragments compared to 9% for MBP (Fig. 4). Inhibition experiments with late apoptotic cells were similar to early apoptotic cells, with Ro60 aa 82–244 and aa 393–538 inhibiting binding of monospecific anti-Ro60 IgG by 62% and 39% separately and 79% together, compared to 7% for MBP.
Fig. 4.
Major and minor apotopes of Ro60 are accessible for binding on the surface of early apoptotic cells. Inhibition experiments were preformed using maltose-binding protein (MBP)-Ro60 aa 82–244 and aa 393–538 to block the binding of a monospecific anti-Ro60 IgG which reacted specifically with these fragments. Monospecific anti-Ro60 IgG (0·5 mg/ml) was incubated with 200 µg/ml of soluble MBP-Ro60 fragments or MBP (1 h, room temperature) prior to the addition to staurosporine-induced (1 µg/ml, 3 h, 37°C) early apoptotic cells. Values are mean percentage of inhibition and standard error of the mean (n = 5).
Exposure of the Ro60 aa 82–244 domain on the surface of early apoptotic cells was confirmed by additional inhibition experiments with a rabbit polyclonal anti-Ro60 anti-serum, which was shown to react specifically with this fragment by ELISA. Pre-incubation of this anti-serum with the aa 82–244 fragment inhibited binding to early apoptotic cells by 58% versus 5% for MBP.
Discussion
The identification of Ro and La as natural ligands for efferocytosis and the inhibitory effect of anti-Ro/La binding on physiological clearance of apoptotic cardiocytes in vitro provides a logical explanation for the massive accumulation of apoptotic cells in septal tissue of CHB hearts compared with normal fetal hearts [15]. Anti-Ro/La antibody-mediated inhibition of apoptotic cell uptake may also contribute to the accumulation of apoptotic cells after photoprovocation in patients with cutaneous lupus erythematosus [16,17], and possibly contribute to glomerular apoptotic cell deposition in lupus glomerulonephritis [18]. Further evidence supporting a role for Ro60 in apoptotic cell clearance in vivo also comes from the observation of increased numbers of apoptotic keratinocytes after UVB irradiation in Ro60 knock-out mice [19]. Given their emerging roles as targets for opsonization as well as ligands for clearance of apoptotic cells, it is important to resolve controversies relating to the accessibility of the Ro and La intracellular autoantigens during different phases of apoptosis and to identify their immunodominant apotopes.
Based on quantitative multi-parameter flow cytometry, Ro60 and La are expressed on the apoptotic cell surface and opsonized by specific extracellular antibodies in the absence of non-specific membrane leakage of IgG. Moreover, the apotopes of Ro60 and La that are surface-bound by specific IgGs correspond to immunodominant epitopes recognized by sera from patients with pSS and mothers of children with CHB. With respect to La, the pattern of apotope expression paralleled previous findings in a murine xenograft model, where a COOH-terminal epitope on the molecule was masked with externalization of NH2-terminal and RNA recognition motif (RRM) domains. The potential importance of anti-La NH2-terminal and anti-La RRM specificity was confirmed by the detection of this reactivity using sera from mothers of children with CHB [11]. With respect to Ro60, the majority of pSS and maternal–CHB sera reacted on ELISA with an internal conformational epitope spanning aa 82–244 in Ro60 that contains the RRM. This region has been shown to encompass an immunodominant epitope in earlier studies (reviewed in [20]). The expression of this epitope on apoptotic cells (i.e. as an apotope) indicates its potential availability for opsonization by transplacental anti-Ro60 IgG. A previous study has demonstrated anti-Ro60 antibodies within the heart of a child who died with CHB [21] and a recent study has shown a higher incidence of anti-Ro60 in sera from mothers of children with CHB than appreciated previously, emphasizing the importance of this specificity as a factor in CHB [22].
A key finding of the current study was that Ro60 and La are exposed on the external surface of early and late apoptotic cell populations, respectively. The distinct temporal expression of the immunodominant Ro60 and La apotopes implies that these intracellular autoantigens translocate independently to the cell surface. The COOH-terminal epitope of La contains a predicted glycosaminoglycan (GAG) attachment site upstream of the putative caspase cleavage site. These GAG side chains target proteins to the extracellular matrix and cell surface by direct interactions with other molecules. We hypothesize that annexin I, a calcium-dependent GAG-binding protein, may serve as a vehicle for translocating La to the extracellular surface [11]. Analysis of the Ro60 sequence does not suggest a molecular mechanism for translocation and membrane tethering of this molecule during early apoptosis. However, the Ro60 inhibition experiments with different recombinant fragments have shown that the RRM and COOH-terminal regions are externalized fully on apoptotic cells, raising the possibility that the NH2-terminal domain may be involved in membrane attachment. The biological relevance of the differential temporal expression of Ro60 and La on the cell surface during apoptosis requires further investigation using human fetal cardiocytes to extend these findings to the antibody-mediated tissue injury hypothesis of CHB. Nevertheless, the observations reported here are consistent with the notion that maternal antibodies reactive with Ro60 and La apotopes may act in an additive fashion to increase the risk of tissue damage.
Surprisingly, we were unable to detect cell surface exposure of Ro52 on early or late apoptotic human Jurkat and HeLa cells using several monospecific anti-Ro52 reagents. This contrasts with earlier studies, in which surface binding of anti-Ro52 antibodies to apoptotic fetal cardiocytes and impairment of their clearance [1,5] was reported. These observations suggest that translocation of Ro52 to the cell membrane may be cell type-specific or caused by a mechanism operating in parallel with apoptosis such as oxidative stress [23]. Alternatively, Ro52 may be translocated to the surface of apoptotic HeLa and Jurkat cells, but have no attachment site to anchor it to the cell surface, therefore preventing detection by flow cytometry. Maternal anti-Ro52 with the p200 specificity may also contribute to CHB by binding to live cardiocytes and inducing calcium overload and apoptosis [24,25].
In considering the role of anti-Ro and La antibodies in the pathogenesis of CHB, potential mechanisms include direct effects on living cardiocytes and/or impairment of apoptotic cell clearance [5,25]. Priority should be given to defining the molecular pathways whereby Ro60 and La translocate to the surface membrane during apoptosis and identifying the functional domains on these autoantigens which mediate their physiological uptake by neighbouring cardiocytes.
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council grant and a NIH grant AR-42455 to J. P. Buyon and grants from the Sjögren's Syndrome Foundation to R. M. Clancy. The serum specimens described herein were obtained through the Research Registry for Neonatal Lupus, which is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (contract AR-4–2220 to J. P. Buyon). We thank Ms Sheree Bailey (flow cytometry facility, Department of Immunology, Flinders Medical Centre) for her technical assistance.
References
- 1.Miranda-Carus M, Askanase AD, Clancy RM, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind to the surface of apoptotic fetal cardiocytes and promote secretion of TNF-α by macrophages. J Immunol. 2000;165:5345–51. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 2.Buyon JP, Clancy RM. Maternal autoantibodies and congential heart block: mediators, markers, and therapeutic approach. Semin Arthritis Rheum. 2003;33:140–54. doi: 10.1016/j.semarthrit.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Tran HB, Macardle PJ, Hiscock J, et al. Anti-La/SSB antibodies transported across the placenta bind apoptotic cells in fetal organs targeted in neonatal lupus. Arthritis Rheum. 2002;46:1572–9. doi: 10.1002/art.10316. [DOI] [PubMed] [Google Scholar]
- 4.Tran H, Ohlsson M, Beroukas D, et al. Subcellular redistribution of La/SSB autoantigen during physiologic apoptosis in the fetal mouse heart and conduction system. Arthritis Rheum. 2002;46:202–8. doi: 10.1002/1529-0131(200201)46:1<202::AID-ART10062>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Clancy RM, Neufing PJ, Zheng P, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–22. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawley W, Doherty A, Denniss S, et al. Rapid lupus autoantigen relocalization and reactive oxygen species accumulation following ultraviolet irradiation of human keratinocytes. Rheumatology. 2000;39:253–61. doi: 10.1093/rheumatology/39.3.253. [DOI] [PubMed] [Google Scholar]
- 8.Ohlsson M, Jonsson R, Brokstad K. Subcellular redistribution and surface exposure of the Ro52, Ro60 and La48 autoantigens during apoptosis in human ductal epithelial cells: a possible mechanism in the pathogenesis of Sjögren's syndrome. Scand J Immunol. 2002;56:456–69. doi: 10.1046/j.1365-3083.2002.01072_79.x. [DOI] [PubMed] [Google Scholar]
- 9.Keech CL, Gordon TP, McCluskey J. The immune response to 52-kilodalton Ro and 60-kilodalton Ro is linked in experimental autoimmunity. J Immunol. 1996;157:3694–9. [PubMed] [Google Scholar]
- 10.Topfer F, Gordon TP, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A) Proc Natl Acad Sci USA. 1995;92:875–9. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neufing PJ, Clancy RM, Jackson MW, Tran HB, Buyon JP, Gordon TP. Exposure and binding of selected immunodominant La/SSB epitopes on human apoptotic cells. Arthritis Rheum. 2005;52:3934–42. doi: 10.1002/art.21486. [DOI] [PubMed] [Google Scholar]
- 12.Gordon T, Kinoshita G, Cavill D, et al. Restricted specificity of intermolecular spreading to endogenous La (SS-B) and 60 kDa Ro (SS-A) in experimental autoimmunity. Scand J Immunol. 2002;56:168–73. doi: 10.1046/j.1365-3083.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 13.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidlyserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Meth. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 14.Patel V, Longacre A, Hsiao K, et al. Apoptotic cells, at all stages of the death process, trigger characteristic signaling events that are divergent from and dominant to those triggered by necrotic cells: implications for the delayed clearance model of autoimmunity. J Biol Chem. 2006;281:4663–70. doi: 10.1074/jbc.M508342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy R, Kapur R, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn A, Herrmann M, Kleber S, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939–50. doi: 10.1002/art.21658. [DOI] [PubMed] [Google Scholar]
- 17.Huber L, Gay S, Distler O, Pietsky D. The effect of UVB on lupus skin: new light on the role of apoptosis in the pathogenesis of autoimmunity. Rheumatology. 2006;45:500–1. doi: 10.1093/rheumatology/kel036. [DOI] [PubMed] [Google Scholar]
- 18.Watson S, Cailhier J, Hughes J, Savill J. Apoptosis and glomerulonephritis. Curr Direct Autoimmun. 2006;9:188–204. doi: 10.1159/000090782. [DOI] [PubMed] [Google Scholar]
- 19.Xue D, Shi H, Smith J, et al. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen. Proc Natl Acad Sci USA. 2003;100:7503–8. doi: 10.1073/pnas.0832411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahren-Herlenius M, Muller S, Isenberg D. Analysis of B-cell epitopes of the Ro/SS-A autoantigen. Immunol Today. 1999;20:234–40. doi: 10.1016/s0167-5699(99)01458-9. [DOI] [PubMed] [Google Scholar]
- 21.Reichlin M, Brucato A, Frank M, et al. Concentration of autoantibodies to native 60-kd Ro/SS-A and denatured 52-kd Ro/SS-A in eluates from the heart of a child who died with congenital complete heart block. Arthritis Rheum. 1994;37:1698–703. doi: 10.1002/art.1780371120. [DOI] [PubMed] [Google Scholar]
- 22.Gordon P, Khamashta M, Rosenthal E, et al. Anti-52 kDa Ro, anti-60 kDa Ro, and anti-La antibody profiles in neonatal lupus. J Rheum. 2004;31:2480–7. [PubMed] [Google Scholar]
- 23.Saegusa J, Kawano S, Koshiba M, et al. Oxidative stress mediates cell surface expression of SS-A/Ro antigen on keratinocytes. Free Radic Biol Med. 2002;32:1006–16. doi: 10.1016/s0891-5849(02)00797-9. [DOI] [PubMed] [Google Scholar]
- 24.Salomonsson S, Sonesson S, Ottosson L, et al. Ro/SSA autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med. 2005;201:11–7. doi: 10.1084/jem.20041859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahren-Herlenius M, Sonesson S. Specificity and effector mechanisms of autoantibodies in congenital heart block. Curr Opin Immunol. 2006;18:1–7. doi: 10.1016/j.coi.2006.09.012. [DOI] [PubMed] [Google Scholar]