Abstract
Over past decades the 17DD yellow fever vaccine has proved to be effective in controlling yellow fever and promises to be a vaccine vector for other diseases, but the cellular and molecular mechanisms by which it elicits such broad-based immunity are still unclear. In this study we describe a detailed phenotypic investigation of major and minor peripheral blood lymphocyte subpopulations aimed at characterizing the kinetics of the adaptive immune response following primary 17DD vaccination. Our major finding is a decreased frequency of circulating CD19+ cells at day 7 followed by emerging activation/modulation phenotypic features (CD19+interleukin(IL)10R+/CD19+CD32+) at day 15. Increased frequency of CD4+human leucocyte antigen D-related(HLA-DR+) at day 7 and CD8+HLA-DR+ at day 30 suggest distinct kinetics of T cell activation, with CD4+ T cells being activated early and CD8+ T cells representing a later event following 17DD vaccination. Up-regulation of modulatory features on CD4+ and CD8+ cells at day 15 seems to be the key event leading to lower frequency of CD38+ T cells at day 30. Taken together, our findings demonstrate the co-existence of phenotypic features associated with activation events and modulatory pathways. Positive correlations between CD4+HLA-DR+ cells and CD4+CD25high regulatory T cells and the association between the type 0 chemokine receptor CCR2 and the activation status of CD4+ and CD8+ cells further support this hypothesis. We hypothesize that this controlled microenviroment seems to be the key to prevent the development of serious adverse events, and even deaths, associated with the 17DD vaccine reported in the literature.
Keywords: 17DD vaccination, flow cytometry, lymphocyte subsets, yellow fever
Introduction
Yellow fever (YF) is an acute viral disease of public health importance in Africa and South America, and of interest for travel agencies in other areas of the world. Recently, however, concern about the potential emergence of urban YF has increased, mainly because of the widespread dissemination of the urban vector, the increasing activity of sylvatic YF in the endemic region and the susceptible status of non-endemic urban populations [1]. The fear of YF urbanization has prompted some to advocate the immediate vaccination of urban populations [2].
In 1936, workers at the Rockefeller Foundation developed the 17D live attenuated vaccine by prolonged serial passage of wild-type YF virus in chicken embryo tissue [3]. This highly effective vaccine has been in routine use ever since, and wherever high vaccine coverage rates have been achieved there has been a dramatic decrease in the prevalence of disease in travellers and residents of countries where YF is endemic [4]. The vaccines currently available are made of the attenuated substrains of virus developed in the late 1930s that induce seroconversion in more than 99% of recipients and provides immunity for 30 years or longer [5]. Therefore, vaccination against YF constitutes the single most effective means for the control of YF. The vaccine is recommended for regular immunization in endemic and epizootic regions based on the high cost-effectiveness of the vaccine and on the severity of YF.
Despite the vaccine's efficacy in controlling YF over several decades and its promise as a vaccine vector for other diseases, the cellular and molecular mechanism by which it elicits such broad-based immunity is still unclear. Most reports in the literature have focused on the intrinsic aspects of the humoral immune response with few studies concerning the evaluation of the cellular immune compartment.
Studies of cellular immune response following 17D vaccination demonstrated a significant increase in lymphocytes T CD8+ peaking at day 5 post-vaccination [6], as well as a significant increase in tumour necrosis factor (TNF)-α peaking on days 2 and 7 post-vaccination [7].
Co et al.[8] identified specific cytotoxic T cells against epitopes on non-structural proteins NS1, NS2b, NS3 and structural protein E of the 17D vaccine virus, which are induced early at day 14 post-vaccination and maintained for 18 months.
Despite the relevance of these findings regarding the 17D vaccination, fewer reports have focused attention of the immunological profile following vaccination with the YF substrain 17DD.
In this report we describe a detailed phenotypic investigation of major and minor peripheral blood lymphocyte subpopulations to characterize the kinetics of the adaptive immune response following first-time vaccination with 17DD.
Our findings demonstrate the co-existence of phenotypic features associated with activation events and modulatory pathways. We hypothesize that this controlled microenviroment seems to be the key to prevent the development of serious adverse events, sometimes fatal, reported in the literature as associated with the YF vaccine [9].
Materials and methods
Volunteers
A total of 50 volunteers were screened for the presence of anti-YF antibodies using the plaque reduction neutralization test as described by Stefano et al. (1999) [10]. Tests were performed at the Laboratório de Virologia, Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais by Juliana de Souza Prado under supervision of Dra Erna Geessien Kroon.
Ten healthy individuals, ranging from 21 to 51 years of age, who displayed negative plaque reduction, were selected and considered to be non-vaccinated and not infected previously with the wild-type YF virus.
Each selected volunteer was vaccinated subcutaneously with a single 0·5 ml dose of 17DD YF vaccine (batch no. 007VFA 010Z; Bio-Manguinhos, Fundação Oswaldo Cruz, Brazil). The volunteers were advised to report all clinical symptoms and side affects after vaccination.
Informed written consent was obtained from all participants. This work complied with resolution number 196/1996 from the National Health Council for research involving humans and was approved by the Ethical Committee at Centro de Pesquisas René Rachou, Belo Horizonte, Minas Gerais, Brazil.
Blood samples
Five ml samples of peripheral blood using ethylenediamine tetraacetic acid (EDTA) as the anti-coagulant were collected by trained professional at Laboratório de Doença de Chagas, Centro de Pesquisas René Rachou at four time-points: before vaccination (day 0) and 7, 15 and 30 days after vaccination.
Specific monoclonal antibodies used for immunophenotyping
Mouse anti-human monoclonal antibodies, conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) and tri-colour (TC), specific for cell-surface markers were used simultaneously for two- or three-colour immunocytometric assays. In this study, we used anti-human FITC-conjugated monoclonal antibodies including anti-CD3 (UCHT1), anti-CD4 (RPA-T4), anti-CD5 (L17F12), anti-CD8 (RPA-T8), anti-CD18 (YF118·3), anti-CD28 (15E8), anti-CD32 (FLI8·26), anti-CD62L (DREG-56), anti-CD69 (H1·2F3), anti-CCR3 (61828·111), anti-CXCR3 (49801), anti-CXCR4 (1265) and anti-CCR5 (45531), all purchased from Becton Dickinson (Mountain View, CA, USA). As second-colour reagents, we used anti-human PE-conjugated monoclonal antibodies anti-CD3 (UCHT1), anti-CD4 (RPA-T4), anti-CD23 (M-L233), anti-CD25 (3G10), anti-CD38 (AT13/5), anti-CD54 (15·2), anti-human leucocyte antigen D-related (HLA-DR) (TÜ36) and anti-IL-10R (3F9), all purchased from Becton Dickinson. The third-colour parameters were evaluated using TC-conjugated monoclonal antibodies, including anti-CD4 (RPA-T4), anti-CD8 (RPA-T8) and anti-CD19 (4G7), purchased from Caltag Laboratories (Burlingame, CA, USA). The biotin-labelled monoclonal antibodies anti-CCR2 (48607) and FITC-labelled avidin were purchased from Becton Dickinson.
Flow cytometric analysis of peripheral blood
White blood cell phenotypes were analysed by a modification of the immunofluorescence procedure recommended by Becton Dickinson: 100 µl samples of peripheral blood collected in EDTA-vacutainer tubes (Becton Dickinson) were mixed in 12 × 75 mm tubes with 5 µl of undiluted monoclonal antibodies specific for several cell-surface markers; the tubes were incubated in the dark for 30 min at room temperature. Following incubation, erythrocytes were lysed using 2 ml of fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson Biosciences Pharmingen, San Diego, CA, USA) then washed twice with 2 ml of phosphate-buffered saline containing 0·01% sodium azide. Cell preparations were fixed in 200 µl of FACS fix solution (10 g/l paraformaldehyde, 1% sodium cacodylate, 6·65 g/l sodium chloride and 0·01% sodium azide). Cytofluorimetric data acquisition was performed with a Becton Dickinson FACScalibur instrument using the provided cellquest™ software for data acquisition and analysis.
Statistical analysis
Statistical analysis was performed by paired t-test for multiple comparisons between days 0, 7, 15 and 30, using the GraphPad Prism 3·1 software package (USA). Significant differences are identified in the figures by the letters ‘a’, ‘b’, ‘c’ and ‘d’ in comparison with days 0, 7, 15 and 30, respectively. Pearson's correlation was also computed to investigate associations between phenotypic features. In all cases, the differences were considered significant when the probabilities of equality, P-values, were ≤ 0·05.
Results
Decreased frequency of circulating CD19+ cells at day 7 followed by emerging activation/modulation phenotypic features at day 15 are the hallmarks of the B cell compartment in first-time 17DD YF vaccinees
Analysis of peripheral blood lymphocyte subsets was performed by three-colour flow cytometric assay using FITC, PE or TC-labelled antibodies including anti-CD3, anti-CD4, anti-CD8, anti-CD19 and anti-CD5. Selective analysis of lymphocytes was carried out by first placing an electronic gate on the forward-scatter (FSC) versus side-scatter (SSC) dot plot to select small blood lymphocytes based on their morphometric features. The gated lymphocyte population was characterized further based on its relative fluorescent properties in order to quantify the percentage of fluorescent positive subpopulations as summarized in Table 1. No significant differences were observed in the percentages of CD3+ T cells, CD4+ and CD8+ T cell subsets and the CD4+/CD8+ cell ratio on days 0, 7, 15 and 30.
Table 1.
Phenotypic analysis of peripheral blood lymphocytes following first-time 17DD yellow fever vaccination.*
| Days after 17DD vaccination | ||||
|---|---|---|---|---|
| Cell phenotypes | 0 | 7 | 15 | 30 |
| T cells | ||||
| CD3+ | 64·7 ± 6·6 | 66·8 ± 7·2 | 67·4 ± 7·2 | 66·3 ± 8·3 |
| CD4+ | 39·3 ± 6·2 | 39·6 ± 5·5 | 39·2 ± 6·2 | 38·1 ± 7·3 |
| CD8+ | 26·5 ± 6·0 | 27·9 ± 5·9 | 28·3 ± 5·9 | 27·8 ± 4·5 |
| B cells | ||||
| CD19+ | 13·4 ± 4·8 | 10·5 ± 3·2a.c.d | 13·0 ± 4·7 | 12·9 ± 5·4 |
| CD19+CD5+/CD19+ | 22·0 ± 8·6 | 22·4 ± 6·5 | 23·7 ± 5·5 | 22·1 ± 8·0 |
| CD19+CD5–/CD19+ | 75·6 ± 8·2 | 77·1 ± 6·6 | 75·9 ± 5·7 | 75·8 ± 7·3 |
| Ratios | ||||
| CD3+/CD19+ | 5·3 ± 1·7 | 6·9 ± 2·2a.c.d | 5·7 ± 1·8 | 5·7 ± 1·7 |
| CD4+/CD8+ | 1·6 ± 0·5 | 1·5 ± 0·4 | 1·5 ± 0·4 | 1·4 ± 0·4 |
Data are expressed as mean percentage ± standard deviation (s.d.), except for the ratios.
Significant differences in comparison with days 0, 15 and 30 after vaccination, respectively.
In contrast, the percentage of circulating CD19+ lymphocytes was significantly reduced at day 7 in comparison with days 0, 15 and 30 (P < 0·05), leading to higher T/B cell ratio at day 7 in comparison with all other evaluated time-points (P < 0·05). No significant differences were observed within B cell subpopulations, including conventional (CD19+CD5–) and B1 (CD19+CD5+) cells throughout the evaluation period (Table 1).
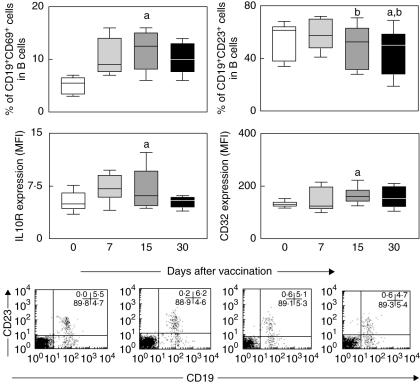
Analysis of B cell activation/modulation phenotypic features was further focused using multi-colour labelling procedures using anti-CD19 monoclonal antibody in parallel with anti-CD69, anti-CD23, anti-interleukin (IL)-10R or anti-CD32. Data analysis was performed using two distinct gating strategies, using bidimensional fluorescence dot plot distribution to quantify the percentages of CD19+CD69+ and CD19+CD23+ within CD19+ lymphocytes and single-colour histograms to evaluate the density of IL-10R and CD32 expression by selected CD19+ cells within gated lymphocytes (Fig. 1).
Fig. 1.
B cell activation/modulation status in peripheral blood of healthy volunteers following first-time 17DD yellow fever (YF) vaccination. Data analysis was performed at days 0 (○), 7 ( ), 15 (
), 15 ( ) and 30 (●) after vaccination. Phenotypic studies were performed using a multi-colour labelling protocol using combinations of anti-CD19 tri-colour (TC), anti-CD23 phycoerythrin (PE), anti-interleukin (IL)-10R PE, anti-CD69 fluorescein isothiocyanate (FITC) or anti-CD32 FITC. The results are expressed in box plot format for CD19+CD69+ (left top panel) and CD19+CD23+ cells (right top panel) and the mean fluorescence intensity (MFI) of IL-10R (left bottom panel) and CD32+ (right bottom panel) expression by CD19+ gated lymphocytes. The box stretches from the lower hinge (defined as the 25th percentile) to the upper hinge (the 75th percentile) and therefore contains the middle half of the score in the distribution. The median is shown as a line across the box. Therefore 25% of the distribution is between this line and the bottom or the top of the box. Significant differences are identified by letters ‘a’ and ‘b’ in comparison to days 0 and 7 after vaccination, respectively. Representative dot plots with quadrant statistics illustrating the increased frequency of CD19+CD23+ cells within gated lymphocytes at day 7 are also provided.
) and 30 (●) after vaccination. Phenotypic studies were performed using a multi-colour labelling protocol using combinations of anti-CD19 tri-colour (TC), anti-CD23 phycoerythrin (PE), anti-interleukin (IL)-10R PE, anti-CD69 fluorescein isothiocyanate (FITC) or anti-CD32 FITC. The results are expressed in box plot format for CD19+CD69+ (left top panel) and CD19+CD23+ cells (right top panel) and the mean fluorescence intensity (MFI) of IL-10R (left bottom panel) and CD32+ (right bottom panel) expression by CD19+ gated lymphocytes. The box stretches from the lower hinge (defined as the 25th percentile) to the upper hinge (the 75th percentile) and therefore contains the middle half of the score in the distribution. The median is shown as a line across the box. Therefore 25% of the distribution is between this line and the bottom or the top of the box. Significant differences are identified by letters ‘a’ and ‘b’ in comparison to days 0 and 7 after vaccination, respectively. Representative dot plots with quadrant statistics illustrating the increased frequency of CD19+CD23+ cells within gated lymphocytes at day 7 are also provided.
Analysis of B cell activation status revealed a significant increase in the percentage of CD19+CD69+ cells, as well as the mean fluorescence intensity of IL-10R expression by CD19+ cells at day 15 in comparison with day 0 (P < 0·05) (Fig. 1, left panels). Correlation analysis between the frequency of CD19+CD69+ and the expression of IL-10R by CD19+ cells at day 15 further confirm a strong relationship between these activation features (Pearson's r = 0·9179, P = 0·0013).
However, analysis of additional B cell phenotypic features also suggested the establishment of modulation phenomena at day 15 as demonstrated by the significant increase in the mean fluorescence intensity of CD32 expression by CD19+ cells in comparison to day 0 (P < 0·05), as well as a significant reduction in the percentage of CD19+CD23+ cells in comparison with day 7 (P < 0·05). A similar drop in the percentage of CD19+CD23+ cells was observed at day 30 in comparison with days 0 and 7 (P < 0·05) (Fig. 1, right panels).
Profile of adhesion molecules expression suggests that the early events of CD8+ T cell activation take place at day 7 after first-time 17DD YF vaccination
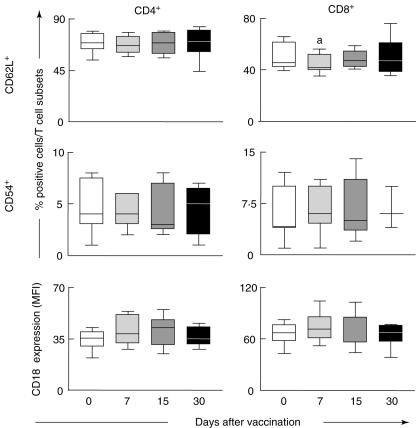
Analysis of the adhesion molecules expression by peripheral blood T cell subsets was performed by multi-colour flow cytometric assay using monoclonal antibodies specific to CD4+ and CD8+ in parallel with anti-CD62L, anti-CD54 or anti-CD18. A selective window was established on the FSC versus SSC dot plot to select the small lymphocyte population followed by two distinct analysis strategies. Analysis of the percentages of fluorescent CD62L+ and CD54+ cells within CD4+ and CD8+ cells was performed using two-colour dot plot distribution. Analysis of CD18 expression by either CD4+ or CD8+ cells was quantified as the mean fluorescence intensity over single-colour histogram representation within the selected T cell subset. No significant differences were found when addressing the adhesion molecules expression on CD4+ cells evaluated at days 0, 7, 15 and 30 (Fig. 2, left panels).
Fig. 2.
Expression of adhesion molecules by T cell subsets in peripheral blood of healthy volunteers following first-time 17DD yellow fever (YF) vaccination. Data analysis was performed at days 0 (○), 7 ( ), 15 (
), 15 ( ) and 30 (●) after vaccination. Phenotypic studies were performed using a triple-labelling protocol with anti-CD4 fluorescein isothiocyanate (FITC) or phycoerythrin (PE), anti-CD8 tri-colour (TC), anti-CD62L FITC, anti-CD54 PE or anti-CD18 FITC. The results are expressed in box plot format for CD62L+, CD54+ cells and the mean fluorescence intensity (MFI) of CD18 expression by CD4+ (left panels) and CD8+ (right panels). The box stretches from the lower hinge (defined as the 25th percentile) to the upper hinge (the 75th percentile) and therefore contains the middle half of the score in the distribution. The median is shown as a line across the box. Therefore 25% of the distribution is between this line and the bottom or the top of the box. Significant differences are identified by letter ‘a’ in comparison to day 0 after vaccination.
) and 30 (●) after vaccination. Phenotypic studies were performed using a triple-labelling protocol with anti-CD4 fluorescein isothiocyanate (FITC) or phycoerythrin (PE), anti-CD8 tri-colour (TC), anti-CD62L FITC, anti-CD54 PE or anti-CD18 FITC. The results are expressed in box plot format for CD62L+, CD54+ cells and the mean fluorescence intensity (MFI) of CD18 expression by CD4+ (left panels) and CD8+ (right panels). The box stretches from the lower hinge (defined as the 25th percentile) to the upper hinge (the 75th percentile) and therefore contains the middle half of the score in the distribution. The median is shown as a line across the box. Therefore 25% of the distribution is between this line and the bottom or the top of the box. Significant differences are identified by letter ‘a’ in comparison to day 0 after vaccination.
Although no changes were observed in the frequency of CD8+CD54+ cells as well as in the expression of CD18 by CD8+ cells, the percentage of CD8+CD62L+ cells was significantly decreased at day 7 in comparison with day 0 (P < 0·05) (Fig. 2, right top graph).
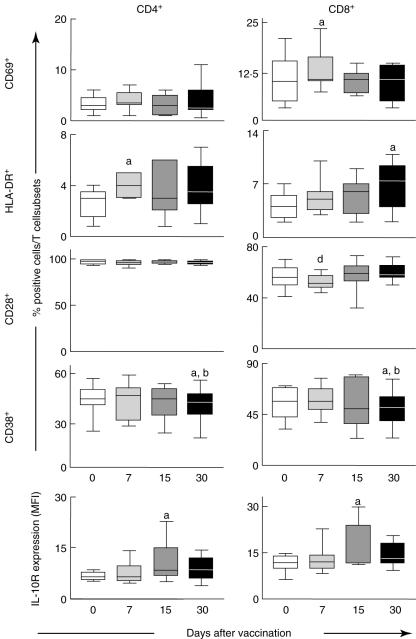
Increased frequency of CD4+HLA-DR+ and CD8+CD69+ cells at day 7 and CD8+HLA-DR+ at day 30 suggests distinct kinetics of cellular activation following first-time 17DD YF vaccination
Analysis of peripheral blood T cell subset activation status was performed using monoclonal antibodies specific to early (CD69), late (HLA-DR) and transmembrane activation-related ecto-enzyme (CD38) as well as the co-stimulatory molecule CD28 (Fig. 3). Data analysis was performed by comparing the frequency of fluorescent-positive cells within the selected T cell subset at the different periods evaluated. Our data demonstrated a cell activation profile suggestive of distinct kinetics of cellular activation within CD4+ and CD8+ T cells, with increased percentages of CD8+ expressing early activation marker (CD69+) and CD4+ bearing late activation marker (HLA-DR+) cells at day 7 in comparison with day 0 (P < 0·05), as well a significant reduction in the percentage of CD8+CD28+ cells at day 7 in comparison with day 30 (P < 0·05) (Fig. 3). Moreover, an increased percentage of CD8+ expressing late activation marker (HLA-DR+) was observed at day 30 in comparison with day 0 (P < 0·05) (Fig. 3).
Fig. 3.
Activation status of T cell subsets in peripheral blood of healthy volunteers following 17DD yellow fever (YF) first-time vaccination. Data analysis was performed at days 0 (○), 7 ( ), 15 (
), 15 ( ) and 30 (●) after vaccination. Phenotypic studies were performed using a double-labelling protocol using anti-CD4 tri-colour (TC), anti-CD8 TC or anti-CD69 fluorescein isothiocyanate (FITC), anti-human leucocyte antigen D-related (HLA-DR) phycoerythrin (PE), anti-CD28 FITC or anti-CD38 PE. The results are expressed in box plot format for CD69+, HLA-DR+, CD28+ and CD38+ and as mean fluorescence intensity (MFI) of IL-10R expression by CD4+ (left panels) and CD8+ (right panels). The box stretches from the lower hinge (defined as the 25th percentile) to the upper hinge (the 75th percentile), and therefore contains the middle half of the score in the distribution. The median is shown as a line across the box. Therefore 25% of the distribution is between this line and the bottom or the top of the box. Significant differences are identified by letters ‘a’, ‘b’ and ‘d’ in comparison to days 0, 7 and 30 after vaccination, respectively.
) and 30 (●) after vaccination. Phenotypic studies were performed using a double-labelling protocol using anti-CD4 tri-colour (TC), anti-CD8 TC or anti-CD69 fluorescein isothiocyanate (FITC), anti-human leucocyte antigen D-related (HLA-DR) phycoerythrin (PE), anti-CD28 FITC or anti-CD38 PE. The results are expressed in box plot format for CD69+, HLA-DR+, CD28+ and CD38+ and as mean fluorescence intensity (MFI) of IL-10R expression by CD4+ (left panels) and CD8+ (right panels). The box stretches from the lower hinge (defined as the 25th percentile) to the upper hinge (the 75th percentile), and therefore contains the middle half of the score in the distribution. The median is shown as a line across the box. Therefore 25% of the distribution is between this line and the bottom or the top of the box. Significant differences are identified by letters ‘a’, ‘b’ and ‘d’ in comparison to days 0, 7 and 30 after vaccination, respectively.
The up-regulation of modulatory phenotypic features on CD4+ and CD8+ cells at day 15 may be the key event leading to lower frequency of CD38+ T cells at day 30 following first-time 17DD YF vaccination.
Single-colour histogram analysis was used to quantify the mean fluorescence intensity of IL-10R expression by either CD4+ or CD8+ cells. Data analysis demonstrated a significant increase in the mean fluorescence intensity of IL-10R expression by CD4+ and CD8+ cells at day 15 in comparison with day 0 (P < 0·05). Consistent with this profile of immune modulation observed at day 15, our findings showed a significant decrease in the percentage of CD4+CD38+ cells as well as CD8+CD38+ cells at day 30 in comparison with days 0 and 7 (P < 0·05) (Fig. 3, bottom panels).
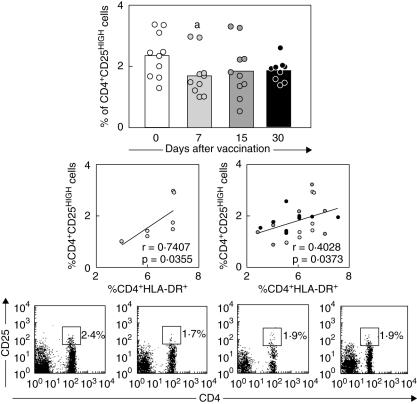
Positive correlation between CD4+HLA-DR+ cells and CD4+CD25high regulatory T cells at day 7 further support that activation/modulation events may take place simultaneously after first-time 17DD YF vaccination.
Quantification of CD4+CD25high regulatory T cells was carried out by first gating on lymphocytes based on their morphometric features on forward- versus side-scatter dot plots, followed by the selection of CD4+ cells presenting high expression of CD25 [11]. Our data demonstrate a significant reduction in the percentage of CD4+CD25high cells at day 7 in comparison with day 0 (P < 0·05) (Fig. 4, top graph). We also found a positive correlation between the percentages of CD4+CD25high cells and CD4+HLA-DR+ cells at 7 day after vaccination (P < 0·05) (Fig. 4, left middle graph). Additionally, we observed a positive correlation between CD4+CD25high cells and CD4+HLA-DR+ throughout the evaluated post-vaccination period (Fig. 4, right middle graph).
Fig. 4.
Frequency of CD4+CD25high regulatory T cells in peripheral blood of healthy volunteers following first-time 17DD yellow fever (YF) vaccination. Data analysis was performed at days 0 (○), 7 ( ), 15 (
), 15 ( ) and 30 (●) after vaccination. A double-labelling panel involving anti-CD4 fluorescein isothiocyanate (FITC) and anti-CD25 phycoerythrin (PE) was used to identify regulatory cells. The results are expressed as scattering of individual values and mean percentage of CD4+CD25high regulatory T cells within gated lymphocytes (top panel). Significant differences are identified by letter ‘a’, in comparison to day 0 after vaccination. Confirmatory correlation analysis validated the positive correlation between CD4+ human leucocyte antigen D-related (HLA-DR+) and CD4+CD25high cells at day 7 after vaccination (left middle panel) as well as throughout the evaluated post-vaccination process (right middle panel). Correlation indexes (r and P-values) are shown in the figures. Representative dot plots with quadrant statistics illustrating the lower frequency of CD4+CD25high regulatory cells within gated lymphocytes at day 7 are also provided.
) and 30 (●) after vaccination. A double-labelling panel involving anti-CD4 fluorescein isothiocyanate (FITC) and anti-CD25 phycoerythrin (PE) was used to identify regulatory cells. The results are expressed as scattering of individual values and mean percentage of CD4+CD25high regulatory T cells within gated lymphocytes (top panel). Significant differences are identified by letter ‘a’, in comparison to day 0 after vaccination. Confirmatory correlation analysis validated the positive correlation between CD4+ human leucocyte antigen D-related (HLA-DR+) and CD4+CD25high cells at day 7 after vaccination (left middle panel) as well as throughout the evaluated post-vaccination process (right middle panel). Correlation indexes (r and P-values) are shown in the figures. Representative dot plots with quadrant statistics illustrating the lower frequency of CD4+CD25high regulatory cells within gated lymphocytes at day 7 are also provided.
In spite of the up-regulation of type 1 chemokine receptor (CXCR3) at day 15 the expression of type 0 chemokine receptor (CCR2) shows a better correlation with the activation status of CD4+ and CD8+ cells observed at day 30 following first-time 17DD YF vaccination.
Recent studies have demonstrated that the standard of expression of chemokine receptors can be used as an indicator of the establishment of a type 1 or type 2 immune response. Thus, we have performed the chemokine receptor analysis in peripheral blood T cell subsets using two-colour flow cytometric platform with antibodies specific to CD4 or CD8 in parallel with type 0 (CCR2, CXCR4), type 1 (CXCR3 and CCR5) and type 2 (CCR3). Selective investigation of lymphocytes was performed by first placing an electronic gate small blood lymphocytes followed by selective analysis of the mean fluorescence intensity of chemokine receptor expression by either CD4+ or CD8+ cells, using single-colour histogram distributions. Our results showed a significant increase in the mean fluorescence intensity of type 1 CXCR3 expression by CD4+ and CD8+ cells at day 15 in comparison to day 7 (P < 0·05). An additional increase in the mean fluorescence intensity of CXCR3 expression by CD4+ was also reported at day 30 in comparison with day 7 (P < 0·05) (Table 2).
Table 2.
Chemokine receptor expression by peripheral blood T cell subsets following first-time 17DD yellow fever vaccination.*
| Days after 17DD vaccination | ||||
|---|---|---|---|---|
| Cell phenotypes | 0 | 7 | 15 | 30 |
| CD4+ | ||||
| CCR2+ | 2·4 ± 0·3 | 2·6 ± 0·4 | 2·8 ± 0·6 | 3·2 ± 0·7a |
| CXCR4+ | 4·0 ± 1·1 | 4·2 ± 0·5 | 4·5 ± 1·0 | 4·7 ± 1·5 |
| CXCR3+ | 22·3 ± 5·5 | 21·8 ± 2·6 | 26·7 ± 4·7b | 26·7 ± 4·1b |
| CCR3+ | 2·6 ± 0·8 | 2·9 ± 0·8 | 2·9 ± 0·8 | 2·7 ± 0·4 |
| CCR5+ | 2·9 ± 0·8 | 3·0 ± 0·6 | 3·3 ± 1·7 | 3·2 ± 0·7 |
| CD8+ | ||||
| CCR2+ | 3·8 ± 0·7 | 4·0 ± 1·0 | 4·7 ± 1·7 | 5·1 ± 1·9a |
| CXCR4+ | 9·9 ± 5·3 | 8·5 ± 2·8 | 8·0 ± 2·0 | 8·1 ± 3·3 |
| CXCR3+ | 27·4 ± 4·9 | 27·3 ± 6·9 | 32·7 ± 6·5b | 30·4 ± 8·6 |
| CCR3+ | 4·1 ± 1·1 | 4·4 ± 1·1 | 3·9 ± 0·7 | 4·0 ± 0·4 |
| CCR5+ | 5·9 ± 2·1 | 6·1 ± 2·2 | 5·4 ± 1·7 | 6·2 ± 1·5 |
Data are expressed as mean fluorescence intensity of chemokine receptor expression within a given T cell subset ± standard deviation (s.d.).
Significant differences in comparison with days 0 and 7 after vaccination, respectively.
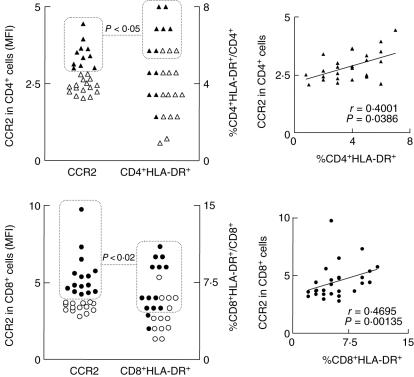
A typical type 0 immunological profile was observed at day 30 in comparison with day 0 characterized by increased mean fluorescence intensity of CCR2 expression by both CD4+ and CD8+ cells (P < 0·05) (Table 2). Interestingly, additional analysis further reinforced the positive correlation between CCR2 expression and the frequency of CD4+ HLA-DR+ and CD8+HLA-DR+ cells throughout the evaluated post-vaccination period (P < 0·05) (Fig. 5).
Fig. 5.
Analysis of CCR2 chemokine receptor expression and the frequency of human leucocyte antigen D-related (HLA-DR+) cells within CD4+ (top panels) and CD8+ lymphocytes (bottom panels) in peripheral blood of healthy volunteers following first-time 17DD yellow fever (YF) vaccination. Data are expressed as scattering of individual values corresponding to the mean fluorescence intensity of CCR2 expression and the frequency of HLA-DR+ cells within CD4+ and CD8+ cells, respectively. Dotted rectangles and lines highlight at individual level the significant association (χ2P-values < 0·05) between the expression of CCR2 and the frequency of HLA-DR+ cells within the CD4+ (▵, ▴) and CD8+ T cells (○, ●). Confirmatory correlation analysis validates the association between the expression of CCR2 by CD4+ and CD8+ T cells with the frequency of activated cells within a given T cell subset. Correlation indexes (r and P-values) are provided in the figure.
Discussion
The increased use of 17DD YF vaccine as well as the changes in the Brazilian policy regarding mass immunization to prevent urbanization of YF have stimulated further investigations to improve our knowledge regarding the immunological events underlying this highly successful prophylactic tool [12]. Moreover, recent data reporting the occurrence of sporadic cases of adverse viscerotropic disease following YF vaccination [13] inspired our efforts to elucidate the cellular and molecular basis of 17DD YF vaccination in order to understand such adverse reactions. Herein, we have developed a detailed phenotypic investigation of the immunological events triggered by first-time 17DD YF vaccination. Our studies, focusing on major and minor peripheral blood lymphocyte subpopulations, allowed us to identify both activation and modulation events within adaptive immunity cells which emerge simultaneously after 17DD YF first-time vaccination.
Although there is a large literature on the humoral immune response featuring neutralizing antibodies titres, few studies have paid attention to the status of the B cell compartment of peripheral blood following 17DD YF vaccination. Our data demonstrate a significant decrease in the frequency of circulating CD19+ cells at day 7 (Table 1) with major phenotypic changes on B lymphocytes taking place at day 15 after vaccination. These findings are consistent with the seroconversion period already described for 17DD YF vaccine by Theiler and Smith [3]. We hypothesize that the lower proportion of circulating B lymphocytes may reflect the compartmentalization of B cell activation, restricted to hyperplasic germinal centres at lymphoid follicles that preceded plasma cell differentiation and antibody production. Upon activation, B lymphocytes are known to undergo a massive change on their surface molecules, which participate as activation markers and co-stimulatory molecules as well as modulatory key elements. Our data demonstrated the most pronounced changes on the B cell compartment at day 15 with phenotypic features related to activation/modulation emerging simultaneously, with up-regulation of IL-10R and increased frequency of CD69+ occurring in parallel with up-regulated levels of CD32 and decreased levels of CD23+ B lymphocytes (Fig. 1). The IL-10/IL-10R complex is a potent pathway for proliferation and maturation of B cells [14], as well a switch factor for IgG1 and IgG3 [15] and also enhances the survival of mature B lymphocytes according to the activation state of these cells [16]. Our results revealed a significant up-regulation of IL-10R expression by CD19+ cells at day 15 in parallel with the higher frequency of activated CD69+ B lymphocytes consistent the increased level of immunoglobulin observed 1–2 weeks following 17DD YF vaccination [3].
On the other hand, we also observed at day 15 the simultaneous presence of phenotypic features suggestive of modulation events, including lowering frequency of CD23+ B cells (Fig. 1). It has been demonstrated that CD23 expression on B cells tends to down-regulate following stimulation with IgG1 immunoglobulins and may also be related to antibody-induced B cell modulation [17]. Moreover, CD32, the major Fc-γR expressed by mature B cells, associated with negative B cell receptor (BCR) signalling [18,19] was found to be up-regulated on B lymphocytes at day 15. Therefore, it is most probable that the increased level of immunoglobulin observed 1–2 weeks following 17DD YF vaccination [3] may interact with CD32 in order to modulate BCR signalling, reflected by the lower frequency of CD23+ B cells at day 30.
We also found distinct kinetics of CD4+ and CD8+ T cell activation following 17DD vaccination. Increased frequency of CD4+ expressing the late activation marker (HLA-DR+) at day 7 suggest that CD4+ T cells are triggered during earlier events following 17DD YF vaccination. On the other hand, increased percentages of CD8+CD69+ cells and a lower frequency of CD8+CD62L+ cells at day 7, besides increased levels of CD8+HLA-DR+ at day 30, suggest that activation of CD8+ cells takes place later after vaccination (Fig. 3).
We noted that CD4+ T cell activation is closely related to the establishment of modulator events mediated by regulatory T cells (Fig. 4). Whereas the entire population of CD4+CD25+ T cells exhibits the regulatory function in the mouse, in humans only the CD4+CD25high population, the natural regulatory T cells [20,21], exhibits strong regulatory function [20,22]. These cells mediate their suppressive effects in vitro in a cell contact-dependent manner controlling disease processes, whereas adaptive regulatory T cells suppress immune responses by producing anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF)-α[23]. Despite the lower frequency of CD4+CD25high cells observed at day 7, suggesting the absence of regulatory mechanism, at this time our data demonstrated an association between the levels of regulatory T cell and the increased levels of activated CD4+HLA-DR+ cells (Fig. 4). Additional analysis further confirmed the positive correlation between CD4+CD25high cells and CD4+HLA-DR+ cells throughout the post-vaccination period indicating that in a given volunteer with higher levels of activated CD4+ T cells there is also a higher frequency of regulatory CD4+CD25high cells following vaccination (Fig. 4). We hypothesize that lower frequency of circulation regulatory T cells may reflect their migration to secondary lymphoid tissues. Recent reports also suggested that the production of interferon (IFN)-γ and perforin as well as NK and CD8+ T cell cytotoxicity are decreased by regulatory T cells [24].
We also observed other changes in the CD4+ T cell compartment, apart from changes related to cell activation and regulatory events mediated by regulatory T cells (CD4+CD25high), which related to immunomodulation demonstrated by increased expression of IL-10R at day 15 (Fig. 3). IL-10 strongly inhibited cytokine production and proliferation of CD4+ T cells and T cell clones via its down-regulatory effect on APC function [25,26]. IL-10 also directly affects the function of T cells and inhibits IL-2, TNF-α and IL-5 production, depending on activation conditions [27–29], as well as expression of CXCR4 and chemotaxis in response to the CXCR4 ligand SDF1 [30].
Our data have indicated a distinct kinetics for the expression of activation-related phenotypic features within the CD8+ T cell compartment. We have also described the existence of simultaneous activation/modulation phenotypic features within these cells. Our findings reveal that significant activation of CD8+ T cells happen after 17DD YF vaccination with an increased percentage of CD8+CD69+ cells and decreased frequency of CD8+CD62L+ cells at day 7 (Fig. 2), with a later increment of CD8+HLA-DR+ cells at day 30 (Fig. 3). However, phenotypic features of CD8+ cells at day 15 revealed enhanced levels of IL-10R expression, showing the putative establishment of additional activation/modulation events with regard to the cytotoxic compartment (Fig. 3). Despite the well-established function of IL-10 down-regulation of CD4+ T cells and monocytes, IL-10 may have inhibitory/stimulatory effects on human CD8+ T cells, inducing their recruitment, modulating or activating their cytotoxic activity and proliferation, depending on the additional cytokine microenvironment [31–34]. Additional investigations focusing on the cytokine milieu triggered by 17DD YF vaccination into the innate and adaptive immunity cells are currently under evaluation in our laboratory, and certainly will contribute to further characterize these immunological events.
Consistent with the establishment of modulatory mechanisms, starting at day 7 post-vaccination (with CD4+CD25high), reinforced by IL-10R up-regulation at day 15, our results showed a reduction in frequency of activated CD38+ T cells at day 30 (Fig. 3). The reduction in CD38+ T cells suggests the clearing of vaccine virus, studies with HIV-positive individuals having shown a positive correlation between viraemia and CD8+CD38+ cells [35]. Moreover, the observation of high levels of CD8+HLA-DR+ and lower levels of CD8+CD38+ re-emphasize the synchronized establishment of activation/modulation events following 17DD YF vaccination.
Moreover, in agreement with the existence of simultaneous activation/modulation mechanisms of the 17DD YF vaccine immune response, our results show that despite the up-regulation of type 1 CXCR3 [36–38] expression by CD4+ and CD8+ cells at day 15, a significant enhancement in the expression of a type 0 CCR2 by CD4+ and CD8+ cells was observed at day 30 (Table 2). The chemokine receptor CCR2 ligand for MCP-1 and CCL2 is expressed predominantly on monocytes/macrophages and on a subset of memory T cells [39,40]. It is well accepted that distinct groups of chemokine receptors play a pivotal role during inflammatory/anti-inflammatory events. As chemokine receptors can be expressed differentially on T cells, depending on their expression pattern a polarization or mixed profile of immune response can take place [41]. Upon activation, T cells acquire new chemokine receptor patterns, particularly, type 1, type 2 or type 0 (generated under the influence of IL-12, IL-4 or mixed cytokine profile, respectively) [42]. Interestingly, our data further reinforce the existence of a positive correlation between CCR2 expression and the frequency of CD4+HLA-DR+ and CD8+HLA-DR+ cells throughout the post-vaccination response (Fig. 5).
Taken together, the phenotypical changes observed on days 7, 15 and 30 after 17DD YF vaccination demonstrated a very rapid simultaneous establishment of activation/modulation mechanisms generated at the levels of cellular and humoral immunity. Considering that none of the volunteers included in this investigation displayed post-vaccine adverse events, the establishment of this mixed immunological profile may be important for the development of an efficient immune response free of adverse events. In this context the activation phenotypic features, including up-regulation of CD69 and HLA-DR on circulating lymphocytes associated with modulatory events mediated by up-regulation of FcγRII receptor on B cells, enhanced levels of regulatory T cells, and increased density of IL-10R and CCR2 expression by T cells seem to be the most reliable hallmarks of this complex immune response following 17DD YF vaccination free of adverse events.
Acknowledgments
We are grateful to Dr Dirk E. Teuwen from the Global Strategic Pharmacovigilance, Sanofi Pasteur, Lyon, France and Dr Rafick-P. Sékaly from the Research, Development and Strategic Planning, L'Université de Montreal, Canada for their critical review of this manuscript.
References
- 1.Vasconcelos PF. Yellow fever: reflections on the disease, prospects for the century and risk of re-urbanization. Braz J Epidemiol. 2002;5:244–58. [Google Scholar]
- 2.Massad E, Coutinho FAB, Burattini MN, Lopez LF. The risk of yellow fever in dengue infested area. Trans R Soc Trop Med Hyg. 2001;95:370–4. doi: 10.1016/s0035-9203(01)90184-1. [DOI] [PubMed] [Google Scholar]
- 3.Theiler M, Smith HH. The use of yellow fever virus modified by in vitro cultivation for human immunization. J Exp Med. 1937;65:787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monath TP, Cetron MS. Prevention of yellow fever in persons traveling to the tropics. Clin Infect Dis. 2002;34:1369–78. doi: 10.1086/340104. [DOI] [PubMed] [Google Scholar]
- 5.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull WHO. 1981;59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhardt B, Jaspeert Niedrig M, Kostner C, L'age-stehr J. Development of viremia and activation after vaccination with Yellow fever virus strain 17D: a model of human flavivirus infection. J Med Vir. 1998;56:159–67. doi: 10.1002/(sici)1096-9071(199810)56:2<159::aid-jmv10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Hacker UT, Jelinek T, Erhardt S, et al. In vivo synthesis of tumor necrosis factor-α in healthy human after yellow fever vaccination. J Infect Dis. 1998;177:774–8. doi: 10.1086/517806. [DOI] [PubMed] [Google Scholar]
- 8.Co MDT, Terajima M, Cruz J, Ennis FA, Rothhman AL. Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: identification of HLA-B335-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology. 2002;293:151–63. doi: 10.1006/viro.2001.1255. [DOI] [PubMed] [Google Scholar]
- 9.Centers for disease Control (CDC). Adverse events associated with 17D-derived yellow fever vaccination. United States, 2000–02. MMWR. 2002;51:989–93. [PubMed] [Google Scholar]
- 10.Stefano I, Sato HK, Pannuti CS, et al. Recent immunization against measles does not interfere with the sero-response to yellow fever vaccine. Vaccine. 1999;17:1042–6. doi: 10.1016/s0264-410x(98)00320-x. [DOI] [PubMed] [Google Scholar]
- 11.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 12. http://portal.saude.gov.br/portal/saude/visualizar_texto.cfm?idtxt=21545.
- 13.Vasconcelos P, Luna E, Galler R, et al. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Brazilian Yellow Fever Vaccine Evaluation Group. Lancet. 2001;358:91–7. doi: 10.1016/s0140-6736(01)05326-0. [DOI] [PubMed] [Google Scholar]
- 14.Rousset F, Garcia E, Defrance T, et al. IL-10 is a potent growth and differentiation factor for human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malisan F, Briere F, Bridon JM, et al. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937–47. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy Y, Broue TJC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–8. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschbaum J, Forschner K, Rasche C, Worm M. Modulation of lymphocyte phenotype and function by immunoglobulins. Br J Dermatol. 2006;154:225–30. doi: 10.1111/j.1365-2133.2005.07005.x. [DOI] [PubMed] [Google Scholar]
- 18.Daeron M, Latour S, Malbec O, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–46. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 19.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JVLA. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;369:340. doi: 10.1038/369340a0. [DOI] [PubMed] [Google Scholar]
- 20.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 21.Cassis L, Aiello S, Noris M. Natural versus adaptive regulatory T cells. Contrib Nephrol. 2005;146:121–31. doi: 10.1159/000082072. [DOI] [PubMed] [Google Scholar]
- 22.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target Cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Dittmer U, He H, Messer RJ, et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 24.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004;112:258–67. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.de Waal MR, Haanen J, Spits H, et al. IL-10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presentig capacity of monocytes via downregulation of class II MHC expression. J Exp Med. 1991;174:15–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorentino DF, Zlontinik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 27.de Waal MR, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cells clones and resting T cells. J Immunol. 1993;150:4754–65. [PubMed] [Google Scholar]
- 28.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–71. [PubMed] [Google Scholar]
- 29.Schandene L, Alonso-Vega C, Willems F, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152:4368–74. [PubMed] [Google Scholar]
- 30.Jinquam T, Quan S, Jacobi HH, et al. CXC chemokine receptor 4 expression and stroomal cell-derived factor-1alpha-induced chemotaxis in CD4+ T lymphocytes are regulated by interleukin-10. Immunology. 2000;99:402–10. doi: 10.1046/j.1365-2567.2000.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory andstimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–93. [PubMed] [Google Scholar]
- 32.Santim AD, Hermonat PL, Ravaggi A, et al. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-spsecific CD8(+) cytotoxic T lymphocytes. J Virol. 2000;74:4729–37. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinquan T, Larsen CG, Gesser B, Matsushima K, Thestrup-Pedersen K. Human interleukin 10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocytes migration. J Immunol. 1993;151:4545–51. [PubMed] [Google Scholar]
- 34.Rowbotton AW, Lepper MW, Garland RJ, et al. IL-10 induced CD8 cell proliferation. Immunology. 1999;98:80–9. doi: 10.1046/j.1365-2567.1999.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollub M, Beran O, Kalanin J, Hnykova J, Spala J, Rozsypal H. CD38 antigen as a marker for immunological follow-up in HIV positive patients. Klin Microbiol Infekc Lek. 2004;10:229–51. [PubMed] [Google Scholar]
- 36.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–97. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leuk Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- 38.Cole KE, Strick CA, Paradis TJ, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–21. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herath KB, Jayasuriya H, Ondeyka JG, et al. Isolation and structures of novel fungal metabolites as chemokine receptor (CCR2) antagonists. J Antibiot. 2005;58:686–94. doi: 10.1038/ja.2005.94. [DOI] [PubMed] [Google Scholar]
- 40.Mahad D, Callahan MK, Williams KA, et al. Modulating CCR2 and CCL2 at the blood–brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–23. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]