Abstract
Tumours evade immune recognition and destruction through loss or down-regulation of expression of antigen processing and antigen-presenting molecules such as the human leucocyte antigen (HLA class I) and transporter for antigen presentation (TAP). This study examined the expression of HLA class I, class II and TAP in human pancreatic carcinoma tissue and 19 immortalized pancreatic cancer lines using a panel of antibodies directed against allele-specific as well as monomorphic determinants of these molecules. In tissue samples, reduction or loss of HLA class I and TAP was observed in 76% of cases, loss or down-regulation of TAP expression in 53%. In pancreatic cell lines down-regulation or loss of class I and TAP expression was also observed frequently. However, reductions in class I and TAP expression were reversible upon exposure to interferon-γin vitro, suggesting a regulatory rather than structural defect in these genes. De novo class II expression was observed in 26% of tumours and 42% of cell lines and may reflect the differentiation status of the cells. The high rate of class I and TAP loss has implications for immunotherapy strategies for pancreatic cancer, as such changes could facilitate a selective growth advantage for malignant cells. However, the reinduction of expression of these molecules with cytokines such as interferon-γ may ultimately allow their cytotoxic T cell-mediated destruction.
Keywords: antigen-presenting, cancer, human, pancreatic
Introduction
Pancreatic adenocarcinoma affects approximately 6000 new patients in the United Kingdom each year. It is a malignancy notorious for its late stage of first presentation, aggressive clinical behaviour and its resistance to conventional anti-cancer therapies [1]. Despite these characteristics, pancreatic tumours express a number of cell surface antigens which contain potential epitopes for recognition by the cellular immune system. One of the mechanisms by which cancers evade the immune system in the early stage of their genesis is by alteration of specific molecules involved in antigen processing and presentation. Human leucocyte antigen (HLA) class I molecules are transmembrane proteins composed of two polypeptide chains: a heavy chain and a light chain (β2-microglobulin). They are expressed by most nucleated cells. Their primary function is to present endogenous antigenic peptides to cytotoxic T lymphocytes (CTLs). The heavy chain is highly polymorphic and is encoded by the closely linked loci HLA-A, -B and -C. β2-microglobulin is non-polymorphic and is encoded by a gene on chromosome 15.
Antigen processing is necessary for the expression of class I molecules on the cell surface. Peptide fragments derived from intracellular proteins are produced by the action of the proteosome and are then transported into the endoplasmic reticulum by peptide transporter proteins such as transporter-associated protein [TAP, the product of TAP-1/TAP-2 genes located within the major histocompatibility complex (MHC) class II region on the short arm of chromosome 6] where synthesis of fully assembled trimolecular HLA class I complex occurs before transport to the cell surface. Experimental deletion of TAP genes results in cells unable to present antigen in the context of class I, suggesting that the TAP gene products are an essential part of the class I complex. In a murine tumour model, loss of TAP was associated with increased tumorigenesis and growth advantage compared to normal TAP-expressing counterparts [2]. However, this loss may be reversed by exogenous interferon treatment, suggesting a regulatory rather than a structural defect in TAP expression [3].
HLA class II molecules act as antigen presenters to CD4+ T lymphocytes. They comprise three molecular subsets: HLA-DR, -DP and -DQ. Their expression is limited to mature cell types such as B cells, macrophages, thymocytes, dendritic cells and activated T cells. Peptides for binding to class II molecules are usually produced from extracellular antigens via the endosomal pathway.
There is extensive evidence of altered MHC or HLA expression in a number of murine and human cancers [4–20]. Some of these studies have correlated the presence of these deficiencies with tumour progression [7,9,12,18,21]. Down-regulation or complete loss of class I expression at the cell surface has been correlated with reduced expression of TAP genes in non-small cell lung, cervical, colorectal, breast and renal cell cancers and pancreatic cell lines [3,7,9,17,22,23]. Because the recognition and killing of tumour cells by CTLs requires the TAP proteins as well as the MHC class I molecules, it has been suggested that the loss of MHC class I and TAP expression may represent a mechanism by which these tumours escape CTL-mediated lysis. MHC class II expression de novo has been described in numerous cancers. De novo class II expression has been associated with more aggressive phenotype in melanoma, whereas in breast and laryngeal carcinoma it has been associated with more benign outcome [24–26].
The aim of this study was to assess the extent to which alterations of antigen processing and presentation occur in pancreatic cancer; in particular, evaluation of HLA class I, class II and TAP expression in samples of human pancreatic cancer and in a panel of human pancreatic cell lines being used commonly for in vitro and in vivo studies of pancreatic cancer. Correlation with clinico-pathological data is made in a subset of patient tumour samples.
Materials and methods
Tissue staining
Representative samples of pancreatic specimens were confirmed by light microscopy. All tissues had been snap-frozen and stored at −70°C. Five µm cryostat sections were cut, fixed in acetone for 10 min at room temperature and left overnight to dry before staining. Histological diagnosis, assessment of differentiation and nodal status (where available) were assessed by light microscopy of routinely processed tissue stained with haematoxylin and eosin.
Antibody staining
The primary antibodies were added to dry sections in a moist chamber for 30 min. Anti-mouse immunoglobulins [Dako, Copenhagen, Denmark; 1/50 dilution in Tris-buffered saline (TBS)] and alkaline phosphatase-anti-alkaline phosphatase (APAAP) complex (Dako) were added and incubated for 30 min. This step was repeated for 10-min incubations to enhance the intensity of the final staining. The alkaline phosphatase substrate was applied afterwards for 20 min and the sections were washed in TBS/water and counterstained with haematoxylin and mounted in an aqueous mounting medium. All antibody incubations were followed by 2-min washings in TBS. For the polyclonal antibody AK1-7 there was an additional step of incubation of mouse-anti-rabbit immunoglobulin.
Analysis of class I, class II and TAP expression were performed on cryostat sections of tumour and staining by appropriate antibodies. Antibodies were obtained from the Imperial Cancer Research Fund (now Cancer Research UK: CRUK) hybridoma unit. HLA class I expression was assessed by two antibodies. W6/32 recognizes an antigenic determinant shared along the HLA-A, -B, -C and β2-microglobulin polypeptide chains; BBM-1 is a monoclonal antibody to β2-microglobulin. TAP-1 protein was detected by the polyclonal antibody AK1-7 raised against the carboxy-terminal peptide of TAP-1 sequence [24]. HLA class II expression was assessed by staining with TALB5 (HLA-DRα subunit [25]), B7/21·2 (HLA-DP monomorphic [26]) and L2 (HLA-DQα chain [27], and were all obtained from Imperial Cancer Research Fund.
Tissue sections were scored by two independent pathologists. For HLA class I antibody, stromal staining acted as a positive control within each section, and the tumour staining intensity relative to this was scored (+) when equivalent (i.e. normal) or (+/–) when of lesser intensity and designated (–) only when absent. For class II expression, staining of macrophages acted as a positive control. Tumours were also scored as well-, moderately or poorly differentiated on sections stained with haematoxylin and eosin. For TAP expression in the cytoplasm the positive and negative controls were the lymphoplasmacytoid cell lines LCL721·174 (negative, deletion of both TAP genes) and wild-type LCL721 (positive).
Cell culture
A number of human pancreatic cell lines were used in this study. The pancreatic cell lines PANC-1 and ASPC1 were obtained from the American Type Culture Collection (Rockville, MD, USA). HPAF was donated by Dr R. Metzgar (Durham NC, USA), Colo 357 by Dr R. T. Morgan (Surgical Division, Denver General Hospital, CO, USA), PT45, 818·1 and 818·4 by Dr H. Kalthoff and Dr W. Schmiegel (Department of Immunology, University Hospital Eppendorf, Hamburg, Germany) and T3M4 was kindly provided by Dr T. Okabe (Tokyo, Japan). The CFPac1 cell line was obtained from Dr R. A. Schoumacher (Gregory Fleming James Cystic Fibrosis Research Center, Birmingham, AL, USA), PaTu-2 by Dr M. van Bulow (University of Mainz, Germany). The cell lines HPAF, PANC-1 and T3M4 were all cultured in RPMI-1640 medium. The RAJI cell line was used as a positive control for expression of all antigen-presenting/processing molecules (cultured in RPMI-1640 medium plus 10% fetal calf serum with 2 mM l-glutamine adjusted to contain 1·5 g/l sodium bicarbonate, 4·5 g/l glucose, 10 mM HEPES, 1·0 mM sodium pyruvate). All other cells were grown in Dulbecco's modified Eagle's medium (DMEM). In both cases medium was supplemented with 10% heat-inactivated (65°C for 30 min) fetal calf serum (FCS) and antibiotics (penicillin and streptomycin 100 U/ml). Media were supplied by the ICRF Media Production (Clare Hall, Middlesex, UK) and the serum obtained from Life Technologies Ltd (Paisley, UK).
Fluorescence activated cell sorter (FACS) analysis of pancreatic tumour cell lines
HLA class I and class II expression were determined on cultured pancreatic cancer cells by direct immunofluorescence. Cells were incubated at 4°C for 30 min in 10 µl of fluorescein isothiocyanate (FITC)-conjugated mouse anti-HLA-ABC class I monoclonal antibodies (Serotec, Oxford, UK) and HLA 10 µl of phycoerythrin (PE)-conjugated mouse anti-human HLA-DR, -DP, -DQ monoclonal antibodies (Serotec). Ten µl of FITC-conjugated anti-human IgG2a and 10 µl mouse PE-labelled anti-human IgG2a antibodies (Serotec) were used as isotype controls, respectively. Cells were washed with FACS buffer [phosphate-buffered saline (PBS), 0·5% bovine serum albumin (BSA), 0·01% NaN3; all from Sigma, Milwaukee, WI, USA] and fixed with 250 µl Cytofix (Becton Dickinson, Oxford, UK). Flow cytometry was performed using a FACScan system (Becton Dickinson). Routinely, 10 000 events were collected using a gate on the forward scatter/side scatter profile to exclude debris. Data were analysed by WinMDI 2·8 and expo 32 and presented as the mean of fluorescence intensity versus cell count.
TAP expression in cell lines
TAP expression in cell lines was assessed by Western blotting using the rabbit polyclonal antibody AB3014 (Chemicon International, Temcula, CA, USA). Where appropriate, cells were exposed to 100 units/ml interferon (IFN)-γ (Boehringer Ingelheim, Ingelheim, Germany) for 48 h. AB3014 was used at a dilution of 1 : 1000. Western blotting was performed using 7% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred onto Hybond nitrocellulose and blocked overnight at 4°C with 3% BSA in TBST [Tris base 0·2 M, NaCl 1·5 M, H20 and Tween 20 (polyoxyethylene sorbitane monolaureate) to 0·05%]. The primary antibody was used at 1 : 1000 (diluted in TBST with 1·5% BSA) at room temperature for 1 h. All washes were performed in TBST (i.e. TBS with 0·1% Tween 20). The secondary antibody (30 min at room temperature) was anti-rabbit IgG-horseradish peroxidase (sc-2004 from Santa Cruz) diluted in wash buffer. Bands were visualized using enhanced chemiluminescence (ECL, Amersham, UK) and Kodak Biomax MR film (Amersham, UK); α-actin was used as a loading control.
Genotyping and phenotyping of cell lines
The HLA class I profile of the pancreatic cell lines found to have low/absent MHC class I expression was determined using lambda monoclonal HLA class I tissue typing trays (One Lambda, Inc., Canoga Park, CA, USA). Each well of the tray contained 1 ml of a specific monoclonal and complement mixture. First, 2 × 103 cells/well were incubated with a mixture of complement binding monoclonal antibody and complement for 1 h at room temperature. Monoclonal antibodies used included HLA A25, HLA A26, HLA B7, HLA B38, HLA Bw4 and Bw6 (all Serotec). After incubation, the cells were fluorostained with 5 ml/well acridine orange (Sigma) and 5 ml/well ethidium bromide (Sigma). The reactions between the HLA cell surface antigen and its matched anti-HLA monoclonal antibody were evaluated using a fluorescence microscope. The tests were scored by estimating the percentage of dead cells, which stain red. Molecular HLA class I typing DNA for class I HLA typing was derived from cell lines using a commercial DNA extraction system (Qiagen Ltd, Crawley, UK). Class I HLA typing was performed using a polymerase chain reaction (PCR) sequence-specific primer (SSP)-based technique [28].
Results
Expression in primary tumours
Expression of HLA class I and TAP molecules was assessed by immunocytochemistry on snap-frozen archival tissue from pancreatectomy or subtotal pancreatectomy for a total of 37 patients with pancreatic cancer and five patients with benign pancreatic disease (Table 1). It was possible to obtain specific clinico-pathological data only from a subset of 20 patients, and paucity of tissue did not allow haplotyping of individuals. There were 20 men and 17 women, with a mean age of 56·5 years. The tumours were all stage II in metastases-free individuals and therefore had a relatively good prognosis. With respect to histological appearance of the tumours, seven were moderately differentiated and 19 poorly differentiated and the remainder showed a mixed pattern. Representative examples of the immunohistochemical studies are shown in Fig. 1. For the cancer patients, W6/32 framework class I antibody staining 10 of 37 (24%) showed normal expression, 21 of 37 (50%) had reduced expression and 11 of 37 (26%) lost class I expression. Of the individuals with loss of W6/32 staining, most (nine of 11) lost β2-microglobulin expression. De novo expression of HLA class II expression was seen in 11 of 37 (26%) cases. Analysis of TAP expression showed loss in 19 of 37 (45%) of tumours examined, which was usually (15 of 19 cases) associated with loss of W/632 and/or β2-m. Of the five non-cancer patients, all five were positive for W6/32 and β2-microglobulin, but one individual had no detectable TAP expression. The specimen from this individual was examined in detail for evidence of focal malignancy (which was not found) and the TAP staining repeated on a number of occasions and found to be negative.
Table 1.
Clinico-pathological data from a cohort of 20 patients with pancreatic carcinoma and benign pancreatic disease
| Class I | ||||||
|---|---|---|---|---|---|---|
| Patient | Age/sex | Histology | W6/32 | β-2 m | TAP | Class II |
| 1 | 62/m | m/p | – | – | – | + |
| 2 | 60/m | p | + | + | + | – |
| 3 | 47/f | m/p | – | – | – | – |
| 4 | 60/m | p | – | – | – | – |
| 5 | 55/f | m/p | +/– | + | + | + |
| 6 | 67/f | m/p | +/– | + | + | – |
| 7 | 75/m | w | – | – | – | – |
| 8 | 62/m | m/p | – | + | + | – |
| 9 | 53/f | p | + | + | – | – |
| 10 | 57/f | p | + | + | – | + |
| 11 | 57/m | m/p | – | + | + | – |
| 12 | 70/f | p | +/– | + | – | + |
| 13 | 56/m | m/p | +/– | + | + | – |
| 14 | 56/m | p | +/– | + | – | – |
| 15 | 43/m | p | +/– | + | + | – |
| 16 | 44/f | m | + | + | – | + |
| 17 | 56/m | m/p | + | + | + | – |
| 18 | 49/f | p | +/– | + | – | |
| 19 | 60/m | p | – | – | – | – |
| 20 | 69/m | m | +/– | + | + | + |
| 21 | 61/f | p | +/– | + | – | – |
| 22 | 50/f | m/p | + | + | – | – |
| 23 | 51/f | m | +/– | + | + | – |
| 24 | 53/m | p | – | – | – | + |
| 25 | 61/f | p | +/– | + | – | – |
| 26 | 49/m | p | – | – | + | – |
| 27 | 62/m | m | +/– | + | – | – |
| 28 | 63/f | p | + | + | + | + |
| 29 | 54/m | p | +/– | + | + | – |
| 30 | 60/m | m/p | – | – | – | + |
| 31 | 54/f | p | + | + | + | – |
| 32 | 44/f | p | + | + | + | – |
| 33 | 48/f | m/p | +/– | + | – | – |
| 34 | 53/m | m | – | – | + | + |
| 35 | 60/m | m | +/– | + | – | – |
| 36 | 6/f | p | + | + | + | – |
| 37 | 49/m | p | +/– | + | + | + |
| 1 | 39/m | Pancreatitis | + | + | + | – |
| 2 | 66/m | Pancreatitis | + | + | – | – |
| 3 | 60/m | Adenoma | + | + | + | – |
| 4 | 31/m | Pancreatitis | + | + | – | + |
| 5 | 43/f | Pancreatitis | + | + | + | – |
m: Moderately differentiated, p: poorly differentiated.
Fig. 1.
(a) Transporter-associated protein (TAP) expression in pancreatic cancer. No expression is seen associated with the tumour cells but the infiltrating immune cells, mainly macrophages, stain positive. (b) De novo major histocompatibility complex (MHC) class II expression in pancreatic cancer. Normal pancreatic architecture is replaced by tumour cells, many of which show de novo class II expression.
Clinico-pathological data from 37 patients with pancreatic carcinoma and five patients with benign pancreatic disease are shown in Table 1. In pancreatic tissue surrounding the tumour, HLA class I expression was observed mainly in the interlobular and intralobular ducts; no expression in acinar cells was seen. Aberrant class II expression was mainly ductal and for both class I and class II antibodies there was positive staining of both tumour tissue and often also of stromal tissue. Statistically there was no correlation between age, sex, lymph node status, tumour size or survival, although the cohort was small. Loss of W6/32 expression was associated statistically with higher (i.e. more undifferentiated) tumour grade (χ2 test, P = 0·02).
Expression in pancreatic tumour cell lines
Nineteen pancreatic tumour cell lines were analysed for HLA class I expression using FACS analysis, plus one positive control for class I and class II expression. The results are summarized in Table 2.
Table 2.
Expression of human leucocyte antigen (HLA) class I, HLA class II subsets and transporter-associated protein (TAP) in pancreatic cell lines and the RAJI (positive control) lymphoma cells line. Class I and class II expression was evaluated by fluorescence activated cell sorter (FACS) analysis, the TAP expression by Western blot
| Cell line | HLA-A, -B,-C | DR | DP | DQ | TAP |
|---|---|---|---|---|---|
| PSN1 | – | + | + | – | – |
| PaTu2 | + | – | – | + | – |
| RWP-1 | + | – | – | – | +/– |
| ASPC-1 | – | – | – | – | – |
| SUIT-2 | + | – | – | – | +/– |
| FA6 | + + | – | – | – | +/– |
| T3M4 | + + | – | – | + | +/– |
| CFPAC1 | + | + | – | +/– | ++ |
| Panc1 | + | – | – | – | + |
| Paca44 | + | + | – | – | – |
| PT45 | – | – | – | + | + |
| HPAF | – | – | – | – | + |
| Paca3 | – | – | – | – | + |
| GER | + | – | – | – | +/– |
| MDA Panc3 | + | – | – | – | +/– |
| PancTu1 | + | – | – | – | – |
| BXPC3 | – | – | – | – | +/– |
| 818·1 | – | – | – | + | +/– |
| 818·4 | + | + | – | + | +/– |
| RAJI | + | + | + | + | + |
HLA class I
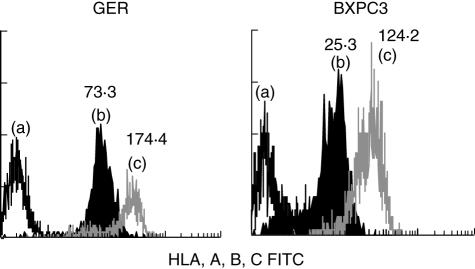
Levels of HLA class I and class II expression were designated as abnormally low (–) (i.e. ‘loss’) if the mean fluorescence index was less than 50, and weakly positive (+/–) if the mean channel fluorescence (MCF) index was 51–100 (after Scupoli et al. [29]). Class I expression was not always distributed uniformly within the cell population (in the cases of PSN1, PT45 and 818·1). Seven of 19 (36·8%) cell lines showed ‘loss’ of class I expression, which was inducible in each line by the addition of 100 units/ml IFN-γ to the culture medium for 48 h. Representative scans are shown in Fig. 2.
Fig. 2.
Expression of major histocompatibility complex (MHC) class is inducible by interferon (IFN)-γ. The class I ‘negative’ cell lines GER and BXPC3 were exposed to 100 µ/ml IFN-γ for 48 h. Class I expression was up-regulated as shown by the histograms (i.e. b–c) confirmed by the increases in mean channel fluorescence (shown above the peaks). In both figures peak (a) is the isotype control.
HLA class II
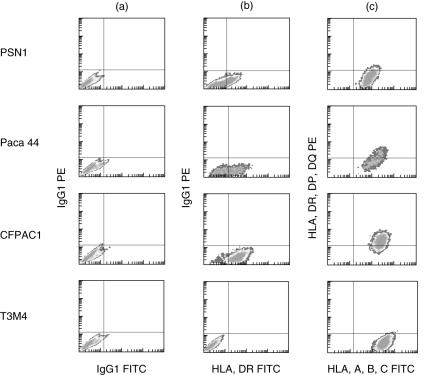
Eight of the 19 cell lines (42%) showed de novo class II expression. The cell line PSN1 (which also showed loss of class I expression) in particular showed expression of both HLA-DR and DP, and the cell line 818·4 expression of HLA-DR and -DQ. Six other cell lines showed expression of one or more of the three class II molecules, but there was no correlation with loss of class I expression. HLA-DP expression was observed in only one cell line compared to -DR and -DQ in four and six cell lines, respectively. Representative scans are shown in Fig. 3.
Fig. 3.
De novo major histocompatibility complex (MHC) class II expression is shown for four cell lines. Column (a) is the isotype control, column (b) shows human leucocyte antigen D-related (HLA-DR) expression (absent in T3M4, high in the others) and column (c) defines cells positive for major histocompatibility complex (MHC) class I (forward scatter) and class II (side scatter, using an antibody which detects all 3 class II subtypes).
TAP expression
TAP expression in cell lines was assessed by Western blot and results summarized in Fig. 4. Normal TAP expression was observed in five of the 19 (26%) pancreatic cell lines. Reduced expression occurred in nine of 19 (47%) and there was loss of TAP expression in five of 19 (26%). Concordance with loss of class I (assessed by W6/32 expression) was seen in four cell lines (PSN1 and ASPC-1, GER and BXPC3). All but one cell line (PT45) expressing class II de novo had either reduced or absent TAP expression. In keeping with previous reports in the literature, TAP expression was inducible upon exposure to IFN-γ for 48 h for all three TAP ‘negative’ cell lines in Fig. 4.
Fig. 4.
Transporter-associated protein (TAP) expression in cell lines is inducible with interferon (IFN)-γ. Three representative cell lines are shown. Up-regulation of TAP expression is seen clearly after in vitro exposure of cells to 100 µ/ml IFN-γ for 48 h; α-actin was used as a loading control.
Characterization of HLA profile of cell lines with loss of class I expression
We undertook more detailed phenotypic and genotypic analysis of cell lines where class I was absent on initial FACS analysis. FA6 cell line was known to express class I normally on FACS was included − however, phenotypic analysis showed detectable A2 and A24 antigens but no B locus antigens. Loss of A locus antigen was confirmed for BXPC3 and ASPC-1 cell lines, whereas for the lines GER, PSN-1 and PT45 A2 or A24 antigens were detected; no B locus antigens were detectable for any of these apart from PSN-1 (Table 3).
Table 3.
Genotypic and phenotypic human leucocyte antigen (HLA) class I profile of cell lines found to be negative for class I on fluorescence activated cell sorter (FACS) analysis using W6/32 antibody. FA6 was included as a ‘positive control’, but was found to have loss of B locus antigens
| Phenotype | |||
|---|---|---|---|
| Cell line | Genotype | A locus antigen | B locus antigen |
| BXPC3 | A1; B37; Cw6 | Not detected | Not detected |
| GER | A2; B62, B44; Cw9, Cw7 | A2 antigen detected | B52 antigen detected |
| PSN-1 | A24; B52; Cw12 | A24 antigen detected | Not detected |
| PT45 | A2; B41, B61; Cw2, Cw17 | Not detected | |
| FA6 | A2, A24; B56, B59; Cw1, Cw4 | A2 and A24 antigens detected | Not detected |
| ASPC-1 | A1, A2, A26; B52, B61, B62; Cw10, Cw12 | Not detected | Not detected |
Discussion
A reduction in expression of HLA class I molecules has been reported in numerous human cancers and may be considered a characteristic of tumour progression. Such down-regulation could facilitate a selective growth advantage for malignant cells by allowing escape from CTL-mediated destruction. Loss of HLA class I can be observed at different levels, i.e. total loss of class I, loss of expression of one locus or one haplotype, or even one specific allele. In this study, immunohistochemical analysis of primary pancreatic cancers has shown a complete loss of HLA class I expression in 24% of cases and reduced expression in 50% of cases. The former figure is in keeping with other studies of total loss of class I expression in breast, lung, cervical and prostatic carcinomas. In addition to the positive immunoreactivity with W6/32 for monomorphic determinants (the ‘framework’ antibody) we were able to assess further the extent of abnormalities of class I expression by looking for deficiencies of β2-microglobulin expression. Due to the paucity of the archival frozen tissue examined it was not possible to perform detailed HLA typing of each patient by microdissection or collection of blood lymphocytes. We found two cases which were negative for W6/32 but positive for the antibody BBM.1. We found that pancreatic carcinoma may be associated with complete loss of HLA class I or selective loss of the A2 allele. Natali et al. [12] reported loss of individual alleles in prostate cancer. In cases where expression of the HLA-A2 allele was lost, five of 10 prostate cancers were positive for W6/32 [29], and in non-small cell lung carcinomas which were also all positive for W6/32, 13 of 43 had lost expression of the HLA-A2 allele [12]. One explanation for the high rate of abnormalities may be because pancreatic cancer presents clinically at an advanced stage. Five cases of benign pancreatic disease retained class I expression: this may be supported by the fact that pancreatitis is associated with infiltration of monocytes and T cells which secrete a large array of lymphokines including IFN-γ, which is a potent inducer of MHC class I and II expression.
A wide variation in the level of class I expression was also observed in pancreatic carcinoma cell lines and loss of expression detected in 43%, which is in keeping with a previous study of pancreatic cancer, but higher than similar studies with melanoma cell lines (16%) [30,31]. Known HLA class I molecular typing of some of the cell lines did not show preferential association between presence of certain alleles and loss of class I expression. Because only W6/32 was used for screening, it was important to establish whether reduced HLA expression was attributable to reduction of locus-specific or allele-specific products. We found that in ASPC-1 and BXPC3 lines serological testing of phenotype confirmed the original findings. However, several ‘negative’ cell lines did have detectable A2 or A24 antigens but had loss of B locus antigen which may explain the FACS findings.
Correlation of class I loss with advanced stage and grade of disease has been made in colorectal carcinoma and melanoma [32,33]. In this study, there was also a statistically significant association between loss of HLA class I expression and de-differentiation of pancreatic tumour. Class I expression has been tested as a prognostic factor in the context of breast and laryngeal cancers. Loss of class I expression has been associated with a significantly poorer clinical outcome [34,35]. In our study, there were insufficient follow-up data to comment on class I or TAP expression and correlation with survival.
It may be that a higher rate of class I down-regulation or loss would be expected in advanced tumours associated with metastatic deposits. In several studies, lymph node metastases from breast [36], colon, bladder, kidney [37] and cervix cancers [7] were virtually always deficient in class I expression, reflecting late stages of a tumour. In our pancreatic cohort there was no correlation with lymph node status, although lymph node tissue from any patients were not available for comparative analysis of HLA class I expression. Reduction in TAP expression in certain malignancies has influenced subsequent biological behaviour. In primary malignant melanoma, loss of TAP expression was associated with the development of metastases and was independent of tumour thickness [38]. In patient tissue samples, loss of TAP expression occurred in almost 50% of cases, all of which were moderately or poorly differentiated. This figure compares to recent studies of breast and lung cancers. This loss was associated commonly with loss of HLA class I expression (W6/32 and/or β2-microglobulin), and implies a common factor causing coordinate dysregulation of genes for class I and TAP which are located on different chromosomes. In pancreatic cell lines, reduction or loss of TAP expression was observed in 14 of 19 cell lines but was inducible with exogenous IFN-γ in all cases, suggesting a regulatory rather than structural defect in the TAP genes. The frequent association between loss of TAP and class I expression implies that in the absence of the transporter protein the antigenic peptide is not able to join the HLA class I molecule, rendering the assembly of the heavy chains and β2-microglobulin impossible. TAP expression was lost in one patient specimen; the cause was uncertain, but there are reports of asymptomatic loss of TAP expression in humans [39].
HLA class II expression has been correlated with well- and poorly differentiated cancers. The functional significance of class II expression is debatable. In our study, there was evidence of de novo HLA class II expression in eight of the pancreatic carcinomas but none in the benign group. Eight cell lines expressed one or more class II molecules; three of eight of these were associated with loss of class I expression. HLA-DR and -DP molecules were expressed most frequently. The induction of HLA class II expression may be a result of local secretion of cytokines such as IFN-γ and TNF-α by tumour infiltrating lymphocytes, reflecting the differentiation status of the cell or as a result of oncogenic transformation. In head and neck cancers, class II expression was associated with CD8+ CD3+ T cell infiltration compared with HLA-DR negative patients expressing CD4+ CD3+ cells [40]. The non-coordinate expression of HLA class II subsets has been observed in other tumour cell lines: gastric, colonic, hepatocellular and cervical in origin. In addition a differential expression of class II molecules was observed in melanoma lesions, particularly between primary and metastatic tumours [41].
The ability of tumour cells to evade T cell responses has important implications for both our understanding of tumorigenesis and the potential efficacy of immunotherapy for cancer. Future therapeutic strategies will need to address the potential loss or down-regulation of expression of antigen-processing and -presenting molecules such as HLA class I and TAP. Further investigation into the mechanisms of TAP down-regulation could provide a tool to restore antigen presentation and render cells sensitive to CD8+ cytotoxic T cell-mediated therapy.
Acknowledgments
We are very grateful to George Elia, Ruth Peat and Suzie Keating from Cancer Research UK for their expert technical assistance. HP, NR and AR were funded by Cancer Research UK (previously as the Imperial Cancer Research Fund).
References
- 1.Hruban RH. Pancreatic cancer: from genes to patient care. J Gastro Intest Surg. 2001;5:583–7. doi: 10.1016/s1091-255x(01)80099-8. [DOI] [PubMed] [Google Scholar]
- 2.Johnsen AK, Templeton DJ, Sy M, Harding CV. Deficiency of transporter for antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J Immunol. 1999;163:4224–31. [PubMed] [Google Scholar]
- 3.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpan RS, Zhang M, Pardee AB. Cell cycle-dependent expression of TAP1, TAP2, and HLA-B27 messenger RNAs in a human breast cancer cell line. Cancer Res. 1996;56:4358–61. [PubMed] [Google Scholar]
- 5.Chen HL, Gabrilovich D, Tampe R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13:210–3. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 6.Cromme FV, Airey J, Heemels MT, et al. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med. 1994;179:335–40. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cromme FV, van Bommel PF, Walboomers JM, et al. Differences in MHC and TAP-1 expression in cervical cancer lymph node metastases as compared with the primary tumours. Br J Cancer. 1994;69:1176–81. doi: 10.1038/bjc.1994.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnsen A, France J, Sy MS, Harding CV. Down-regulation of the transporter for antigen presentation, proteasome subunits, and class I major histocompatibility complex in tumor cell lines. Cancer Res. 1998;58:3660–7. [PubMed] [Google Scholar]
- 9.Kaklamanis L, Leek R, Koukourakis M, Gatter KC, Harris AL. Loss of transporter in antigen processing 1 transport protein and major histocompatibility complex class I molecules in metastatic versus primary breast cancer. Cancer Res. 1995;55:5191–4. [PubMed] [Google Scholar]
- 10.Kaklamanis L, Townsend A, Doussis-Anagnostopoulou IA, Mortensen N, Harris AL, Gatter KC. Loss of major histocompatibility complex-encoded transporter associated with antigen presentation (TAP) in colorectal cancer. Am J Pathol. 1994;145:505–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Keating PJ, Cromme FV, Duggan-Keen M, et al. Frequency of down-regulation of individual HLA-A and -B alleles in cervical carcinomas in relation to TAP-1 expression. Br J Cancer. 1995;72:405–11. doi: 10.1038/bjc.1995.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC. Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer. 1996;73:148–53. doi: 10.1038/bjc.1996.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurokohchi K, Carrington M, Mann DL, et al. Expression of HLA class I molecules and the transporter associated with antigen processing in hepatocellular carcinoma. Hepatology. 1996;23:1181–8. doi: 10.1002/hep.510230537. [DOI] [PubMed] [Google Scholar]
- 14.Maeurer MJ, Gollin SM, Martin D, et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 1996;98:1633–41. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe M, Khanna R, Jacob CA, et al. Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein–Barr virus latent membrane protein-1: coordinate up-regulation of peptide transporters and HLA-class I antigen expression. Eur J Immunol. 1995;25:1374–84. doi: 10.1002/eji.1830250536. [DOI] [PubMed] [Google Scholar]
- 16.Sanda MG, Restifo NP, Walsh JC, et al. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–5. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seliger B, Hohne A, Knuth A, et al. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin Cancer Res. 1996;2:1427–33. [PubMed] [Google Scholar]
- 18.Seliger B, Hohne A, Knuth A, et al. Analysis of the major histocompatibility complex class I antigen presentation machinery in normal and malignant renal cells: evidence for deficiencies associated with transformation and progression. Cancer Res. 1996b;56:1756–60. [PubMed] [Google Scholar]
- 19.Singal DP, Ye M, Ni J, Snider DP. Markedly decreased expression of TAP1 and LMP2 genes in HLA class I-deficient human tumor cell lines. Immunol Lett. 1996;50:149–54. doi: 10.1016/0165-2478(96)02531-x. [DOI] [PubMed] [Google Scholar]
- 20.Vitale M, Rezzani R, Rodella L, et al. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res. 1998;58:737–42. [PubMed] [Google Scholar]
- 21.Dotto GP, Parada LF, Weinberg RA. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985;318:472–5. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- 22.Kaklamanis L, Gatter KC, Hill AB, et al. Loss of HLA class-I alleles, heavy chains and beta 2-microglobulin in colorectal cancer. Int J Cancer. 1992;51:379–85. doi: 10.1002/ijc.2910510308. [DOI] [PubMed] [Google Scholar]
- 23.Torres MJ, Ruiz-Cabello F, Skoudy A, et al. Loss of an HLA haplotype in pancreas cancer tissue and its corresponding tumour derived cell line. Tissue Antigens. 1996;47:372–81. doi: 10.1111/j.1399-0039.1996.tb02572.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee JE, Abdalla J, Porter GA, et al. Presence of the human leukocyte antigen class II gene DRB1*1101 predicts interferon gamma levels and disease recurrence in melanoma patients. Ann Surg Oncol. 2002;9:587–93. doi: 10.1007/BF02573896. [DOI] [PubMed] [Google Scholar]
- 25.Adams TE, Bodmer JG, Bodmer WF. Production and characterization of monoclonal antibodies recognizing the alpha-chain subunits of human ia alloantigens. Immunology. 1983;50:613–24. [PMC free article] [PubMed] [Google Scholar]
- 26.Watson AJ, DeMars R, Trowbridge IS, Bach FH. Detection of a novel human class II HLA antigen. Nature. 1983;304:358–61. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- 27.Loiseau P, Lehn P, Dautry F, et al. Correlation between an HLA-DQ alpha length polymorphism of messenger RNA and serologically defined specificities (DQw1, DRw53, DR3+5) Immunogenetics. 1986;23:111–14. doi: 10.1007/BF00377970. [DOI] [PubMed] [Google Scholar]
- 28.Welsh K, Bunce M. Molecular typing for the MHC with PCR-SSP. Rev Immunogen. 1999;1:157–76. [PubMed] [Google Scholar]
- 29.Scupoli MT, Sartoris S, Tosi G, et al. Expression of MHC class I and class II antigens in pancreatic adenocarcinomas. Tissue Antigens. 1996;48:301–11. doi: 10.1111/j.1399-0039.1996.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 30.Natali PG, Nicotra MR, Bigotti A, et al. Selective changes in expression of HLA class I polymorphic determinants in human solid tumors. Proc Natl Acad Sci USA. 1989;86:6719–23. doi: 10.1073/pnas.86.17.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–94. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 32.Momburg F, Degener T, Bacchus E, Moldenhauer G, Hammerling GJ, Moller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986;37:179–84. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Nevot MA, Garcia E, Romero C, Oliva MR, Serrano S, Garrido F. Phenotypic and genetic analysis of HLA class I and HLA-DR antigen expression on human melanomas. Exp Clin Immunogenet. 1988;5:203–12. [PubMed] [Google Scholar]
- 34.Concha A, Esteban F, Cabrera T, Ruiz-Cabello F, Garrido F. Tumor aggressiveness and MHC class I and II antigens in laryngeal and breast cancer. Semin Cancer Biol. 1991;2:47–54. [PubMed] [Google Scholar]
- 35.Esteban F, Concha A, Huelin C, et al. Histocompatibility antigens in primary and metastatic squamous cell carcinoma of the larynx. Int J Cancer. 1989;43:436–42. doi: 10.1002/ijc.2910430316. [DOI] [PubMed] [Google Scholar]
- 36.Gudmundsdottir I, Gunnlaugur Jonasson J, Sigurdsson H, Olafsdottir K, Tryggvadottir L, Ogmundsdottir HM. Altered expression of HLA class I antigens in breast cancer association with prognosis. Int J Cancer. 2000;89:500–5. doi: 10.1002/1097-0215(20001120)89:6<500::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Cordon-Cardo C, Fuks Z, Drobnjak M, Moreno C, Eisenbach L, Feldman M. Expression of HLA-A, B, C antigens on primary and metastatic tumour cell populations of human carcinomas. Cancer Res. 1991;51:6372–80. [PubMed] [Google Scholar]
- 38.Kamarashev J, Ferrone S, Seifert B, et al. TAP1 down-regulation in primary melanoma lesions: an independent marker of poor prognosis. Int J Cancer. 2001;95:23–8. doi: 10.1002/1097-0215(20010120)95:1<23::aid-ijc1004>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Zimmer J, Andres E, Donato L, Hanau D, Hentges F, de la Salle H. Clinical and immunological aspects of HLA class I deficiency. Q J Med. 2005;98:719–27. doi: 10.1093/qjmed/hci112. [DOI] [PubMed] [Google Scholar]
- 40.Samukawa T. Expression of HLA-DR antigen on head and neck carcinomas − immunohistological study. pp. 88–97. [DOI] [PubMed]
- 41.van Vreeswijk H, Ruiter DJ, Brocker EB, Welvaart K, Ferrone S. Differential expreesion of HLA-DR, DQ and DP antigens in primary and metastatic melanoma. J Invest Dermatol. 1988;90:755–60. doi: 10.1111/1523-1747.ep12560951. [DOI] [PubMed] [Google Scholar]