Abstract
Clinical evidence implicates polymorphonuclear leucocytes in the pathogenesis of vasculitis in Kawasaki disease. We examined modulation of expression of adhesion molecules (CD11b and CD62L) on polymorphonuclear leucocytes and how this expression is related to serum cytokine concentrations. In 18 patients with Kawasaki disease and 15 control subjects, adhesion molecule expression was determined by two-colour immunofluorescence staining of blood leucocytes and flow cytometry. Eight cytokines and chemokines were also measured. In patients with Kawasaki disease, mean fluorescence intensity for CD11b before giving intravenous immunoglobulin was significantly higher than in normal subjects (P < 0·005). After intravenous immunoglobulin, mean fluorescence intensity for CD11b decreased significantly. With coronary artery lesions present, mean CD11b fluorescence intensity was significantly higher than without coronary artery lesions (P= 0·005 before intravenous immunoglobulin; P = 0·024 after intravenous immunoglobulin). No differences were seen in CD62L expression on polymorphonuclear leucocytes between patients with Kawasaki disease and normal subjects. CD11b expression on polymorphonuclear leucocytes correlated positively with serum interleukin (IL)-6, IL-10, granulocyte colony-stimulating factor, percentage of neutrophils among white cells and C-reactive protein. Polymorphonuclear leucocytes from patients with Kawasaki disease showed increased CD11b expression, which was associated with increased serum cytokines and appeared to be related to coronary artery lesions.
Keywords: CD11b, coronary artery lesions, cytokine, intravenous immunoglobulin, Kawasaki disease
Introduction
Kawasaki disease (KD), an acute multi-system vasculitis of unknown aetiology that affects primarily infants and children, is a major cause of acquired heart disease [1]. The most widely used therapy is intravenous immunoglobulin (IVIG) given with oral aspirin [2], although approximately 10–20% of patients have persistent fever after completion of IVIG treatment and 5% of patients still may develop coronary artery lesions (CAL) despite this therapy [3]. Clinical evidence suggests that polymorphonuclear leucocytes (PMN) may participate importantly in pathogenesis of vasculitis in KD. During the acute phase, PMN are seen in the peripheral blood, together with an overall leucocytosis including a left shift [4]. Children with KD have high serum granulocyte colony-stimulating factor (G-CSF) concentrations in the acute phase, which decrease rapidly to normal after resolution of the acute phase [5,6]. Furthermore, G-CSF has shown association with coronary artery dilation [7]. Histologically, vascular lesions in the acute phase of KD are associated with evidence of PMN activation and damage to endothelial cells caused by PMN extravasation [8,9]. PMN in the acute phase of KD are likely to damage the endothelium through secretion of oxygen radicals [10], matrix metalloproteinase and elastase [11]. Thus, excessively activated PMN and their inflammatory responses may be associated with coronary artery damage in KD.
One of the major influences on PMN migration from the blood to an inflammatory site involves modulation of adhesion molecules on both PMN and endothelial cells. In particular, proinflammatory mediators induce shedding of l-selectin (CD62L) [12] and increase expression of aMb2 (CD11b/CD18) on the PMN surface as major events in transendothelial migration [13]. Such altered adhesion molecule expression can influence migration of PMN to inflammatory sites, where they release excessive reactive oxygen species that initiate tissue damage. Johnson et al. suggested that up-regulation of aMb2 by antibodies directed against neutrophil cytoplasmic antigens may represent part of the mechanism mediating neutrophil–endothelial cell interactions in systemic vasculitis [14]. However, the specific surface molecules on PMN that participate in the acute phase of KD remain unclear.
In this study, we evaluated modulation of expression of the adhesion molecules CD11b and CD62L on PMN in patients with KD. We also investigated how various circulating cytokines might be related to adhesion molecule expression on surfaces of PMN.
Materials and methods
Study populations
The study was reviewed and approved by the Gunma University Ethics Committee in May 2000. A total of 18 patients with KD were studied after written informed consent had been obtained from their parents. All patients were admitted to our hospital between September 2000 and August 2004 and fulfilled at least five of six criteria for diagnosis of KD. Patients were excluded when clinical or laboratory evidence of any other disease known to mimic KD was present or when KD was atypical. The patients received IVIG (1 g/kg/day for 2 consecutive days), aspirin (30 mg/kg/day) and dipyridamole (2 mg/kg/day). The IVIG used was S-sulphonated human immunoglobulin (Kenketu Venilon I; Teijin Pharma, Tokyo, Japan). CAL were diagnosed by two-dimensional echocardiography performed in all patients at the time of enrolment, at 2 weeks of illness and at 4 weeks of illness by the same paediatric cardiologist. CAL were defined as present if the internal luminal diameter was 3 mm or more in patients < 5 years old or 4 mm or more in patients 5+ years old; if the internal diameter of a segment was at least 1·5 times as large as that of an adjacent segment or if luminal contour was clearly irregular. Control values for cytokines and PMN expression of CD11b and CD62L were obtained from 15 healthy children who had no past history of KD.
Immunostaining and flow cytometric analysis
Blood samples were obtained before IVIG treatment, shortly after IVIG treatment (within 24 h) and in the convalescent phase (14–21 days after IVIG treatment). Samples were stored at room temperature and processed for flow cytometry within 1 h. Expression of adhesion molecules was determined by two-colour immunofluoroscent staining of blood leucocytes and flow cytometry. Saturating concentrations of fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD11b (Beckman Coulter, Tokyo, Japan) and phycoerythrin (PE)-conjugated mouse anti-human CD62L (Beckman Coulter) were added to the blood sample. After cells were exposed to antibodies for 30 min at room temperature in darkness, 0·5 ml of red cell lysing solution (Optilyse C; Beckman Coulter) was added for 10 min of incubation at room temperature in darkness. Cells were then suspended in phosphate-buffered saline and stored in darkness at room temperature for 15 min. After cells were centrifuged at 400 g for 5 min and the supernatant discarded, they were resuspended in 0·5 ml of phosphate-buffered saline and analysed with an EPICS XL cytometer (Beckman Coulter) within 5 min. Isotype-matched control antibodies, PE-conjugated IgG2 and FITC-conjugated IgG2 (Beckman Coulter) were used to define the cut-off for fluorescence positively as the 99th percentile of the distribution of the cells labelled with control antibody. PMN were gated based on forward- and side-scatter on the display. Ten thousand events were acquired from each sample. Antigen expression was determined as mean fluorescence intensity.
Cytokine and chemokine measurements
We measured eight cytokine and chemokine concentrations in a small volume of serum (50 µl) using a highly sensitive fluorescent microsphere system (Japan Bio-Rad Laboratories, Tokyo, Japan). Blood samples were centrifuged at 1500 g for 10 min, and the sera were stored at −80°C until use. Serum tumour necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-2, IL-4, IL-6, IL-8, IL-10 and G-CSF were measured in duplicate using the Bioplex Protein Array system (Japan Bio-Rad Laboratories). Capture antibodies and detection antibodies for microspheres coupled with TNF-α, IFN-γ, IL-2, IL-4, IL-6, IL-8, IL-10 and G-CSF were obtained from Japan Bio-Rad Laboratories. The appropriate standard and samples (50 µl) diluted in assay buffer (phosphate-buffered saline, 1% bovine serum albumin-fraction V and 0·02% azide, pH 7·4) were added to each well of a filter plate pre-equilibrated with assay buffer. Samples were incubated with 50 µl of the antibody-coupled microspheres at room temperature for 30 min on a plate shaker oscillating at 300 r.p.m. Freshly diluted detection antibody (25 µl) was added to wells for incubation at room temperature for 30 min on the shaker. Streptavidin–PE (50 µl) was added to wells, which were incubated further at room temperature for 10 min on the shaker. Samples were analysed by the Bioplex suspension array system according to the manufacturer's instructions. The lower limit of detection for each cytokine and chemokine measurement was 3–6 pg/ml.
Statistical analysis
All analyses were carried out by means of an spss statistical software package version 13·0 J (SPSS Japan Inc., Tokyo, Japan). Data are expressed as the mean ± standard deviation (SD) or median with range. Continuous variables were compared with the Mann–Whitney U-test or Wilcoxon's rank sum test. Using data from pre-IVIG, post-IVIG and convalescent phase, correlations between cell adhesion molecule expression on PMN and other laboratory values were assessed using Pearson's correlation coefficient. Differences with two-tailed P-values below 0·05 were considered significant.
Results
Baseline characteristics and clinical outcomes of patients
Table 1 shows baseline characteristics and clinical outcomes of patients with KD and characteristics of normal subjects. Patients with KD (11 boys and seven girls with an age range of 3–62 months; median 17 months) and normal subjects (11 boys and four girls with an age range of 3–136 months) were studied. CAL were found in three patients with KD (17%).
Table 1.
Baseline characteristics and clinical outcomes of patients with Kawasaki disease
| Kawasaki patients | Normal controls | |
|---|---|---|
| Number | 18 | 13 |
| Male/female | 11/7 | 9/4 |
| Age (months) | 24·2 ± 21·1 (range 3–12) | 40·5 ± 33·7 (range 3–136) |
| Days of illness at initial IVIG | 4·7 ± 1·2 (range 3–8) | |
| Clinical symptoms | ||
| 6/6 | 10/18 | |
| 5/6 | 8/18 | |
| 4/6 | 0/18 | |
| Length of fever (days) | 6·4 ± 2·9 (range 3–15) | |
| Coronary artery lesions (+) | 3/18 | |
Data are presented as mean ± standard deviation. IVIG: intravenous immunoglobulin.
Serial changes in CD11b and CD62L expression on PMN
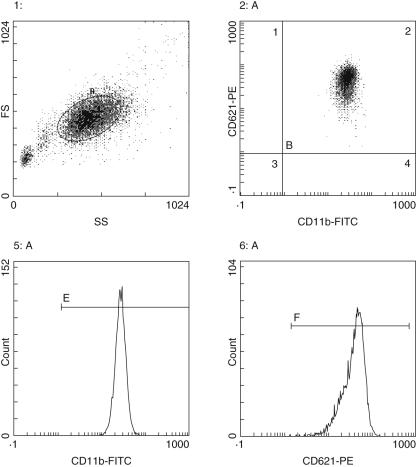
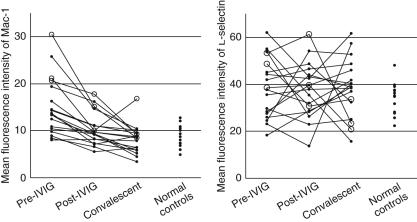
Figure 1 shows fluorescence activated cell sorting plots displaying the gates and gating sequences used. Figure 2 depicts CD11b and CD62L expression on PMN in pre-IVIG, post-IVIG and convalescent phases of KD, compared with expression in normal subjects. In patients with KD, mean fluorescence intensity of CD11b before IVIG treatment was significantly higher than that in normal subjects (P < 0·005). IVIG treatment caused a significant reduction in mean fluorescence intensity of CD11b from that before treatment (P < 0·001). More importantly, the mean fluorescence intensity of CD11b in KD patients with CAL was significantly higher than in KD patients without CAL (P= 0·005 pre-IVIG, P = 0·024 post-IVIG). In contrast, no significant differences in CD62L expression on PMN were seen between patients with KD and normal subjects or between KD patients with CAL and without CAL.
Fig. 1.
Flow-cytometer measurements of CD11b and CD62L in peripheral blood of patients with Kawasaki disease.
Fig. 2.
Serial changes of mean fluorescence intensity of CD11b and CD62L on polymorphonuclear leucocytes. Open circles, Kawasaki disease patients with coronary artery lesions; closed circles, Kawasaki disease patients without coronary artery lesions. IVIG, intravenous immunoglobulin.
Serum cytokines and chemokines: correlation with expression of CD11b on PMN
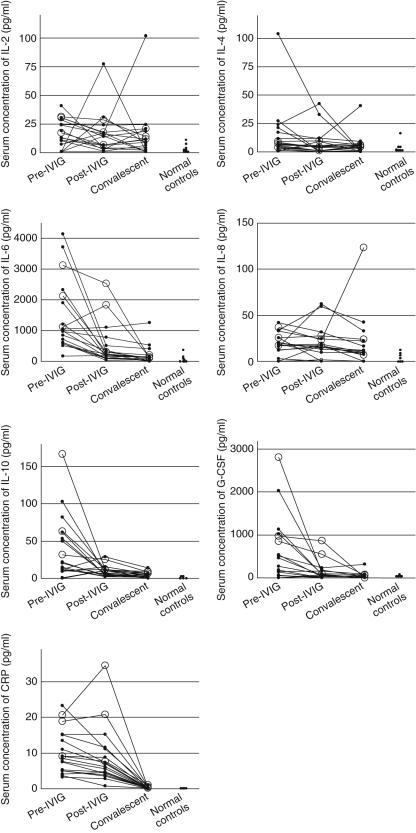
To investigate how cytokines and chemokines are related to CD11b expression on PMN, we measured serum concentrations of eight cytokines and chemokines in KD patients. Serum IL-2, IL-4, IL-6, IL-8, IL-10 and G-CSF in patients with KD before IVIG treatment were significantly higher than in control subjects and IL-6, IL-10, G-CSF and CRP were significantly lower after IVIG treatment than before treatment (Fig. 3). Before IVIG treatment, serum IL-6, IL-10 and G-CSF concentrations tended to be higher in KD patients with CAL than in patients without CAL, while falling short of significance (IL-6, 2118·5 ± 1006·1 versus 1356·5 ± 1182·0 pg/ml, P = 0·164; IL-10, 87·2 ± 70·7 versus 30·9 ± 31·5 pg/ml, P = 0·074; G-CSF, 639·6 ± 321·7 versus 248·7 ± 363·6 pg/ml, P = 0·076). In the post-IVIG phase, serum IL-6 and G-CSF were significantly higher in KD patients with than without CAL (IL-6, 1556·0 ± 1131·3 versus 304·9 ± 291·9 pg/ml, P = 0·027; G-CSF, 265·6 ± 171·7 versus 32·3 ± 32·0 pg/ml, P = 0·011), while serum IL-10 tended to be higher in KD patients with CAL (16·1 ± 8·0 versus 8·5 ± 6·9 pg/ml, P = 0·066). Table 2 shows correlations between CD11b expression on PMN and serum cytokines and chemokines. CD11b expression on PMN showed a positive correlation with serum concentrations of IL-6, IL-10, G-CSF, CRP and percentage of neutrophils.
Fig. 3.
Serial changes of serum cytokine levels in Kawasaki disease patients and normal controls. Open circles, Kawasaki disease patients with coronary artery lesions; closed circles, Kawasaki disease patients without coronary artery lesions. IVIG, intravenous immunoglobulin; IL, interleukin; G-CSF, granulocyte colony stimulating factor; CRP, C-reactive protein.
Table 2.
Correlations with CD11b expression on polymorphonuclear leucocytes and other laboratory data
| IL-2 | IL-4 | IL-6 | IL-8 | IL-10 | |
|---|---|---|---|---|---|
| R | 0·065 | 0·067 | 0·410 | 0·060 | 0·574 |
| P value | 0·612 | 0·601 | 0·001 | 0·638 | < 0·001 |
| TNF-α | IFN-γ | G-CSG | % PMN | CRP | |
| R | 0·014 | −0·029 | 0·473 | 0·569 | 0·479 |
| P value | 10·910 | 0·819 | < 0·001 | < 0·001 | < 0·001 |
IL: interleukin; TNF: tumour necrosis factor; IFN: interferon; G-CSF: granulocyte colony-stimulating factor; PMN: polymorphonuclear leucocytes; CRP: C-reactive protein.
Discussion
The main findings in this study were, first, that CD11b expression on PMN from patients with KD was increased significantly in the acute phase and decreased rapidly after therapy; secondly, CD11b expression on PMN was higher in KD patients with CAL; and thirdly, CD11b expression on PMN correlated with serum concentrations of several pro- and anti-inflammatory cytokines and chemokines, the percentage of PMN among white cells and CRP. To the best of our knowledge, this is the first report to demonstrate increased CD11b expression on PMN in the acute phase of KD.
Although we could not confirm the pathogenetic consequences of increased expression of CD11b on PMN and the high concentrations of various cytokines in acute KD in vitro, we postulated as follows. In general, CD11b promotes firm attachment of the PMN to the endothelium, which allows transendothelial migration into inflamed tissues. CD11b expression is up-regulated mainly by proinflammatory cytokines such as IL-6 and G-CSF. In addition, PMN generate large amounts of reactive oxygen species (superoxide radicals, hydrogen peroxide and hypochlolite) and release toxic granules containing myeloperoxidase. These toxic compounds may be associated with cardiovascular system injuries in KD patients. Therefore, enhanced expression of CD11b induced by circulating inflammatory cytokines is likely to promote adhesion and transendothelial migration of leucocytes in KD. In turn, these migrated leucocytes are likely to be an important source of inflammatory cytokines that further induced endothelial cell surface expression of intercellular adhesion molecules 1–3, resulting in progression of vasculitis in KD. Indeed, histopathological findings in KD show PMN adherent to endothelial cells and infiltration of neutrophils, monocytes and lymphocytes into the walls of small and medium-sized blood vessels [9,15–17]. However, our study lacked sufficient data to demonstrate that increased CD11b expression on polymorphonuclear cells actually promotes adhesion and transmigration of leucocytes in acute KD patients. Further examination using autopsy specimens or animal models of KD vasculitis is needed to prove our hypotheses.
Several clinical studies have reported that activation of PMN may contribute to the severity of KD. Suzuki et al. reported that increased peripheral blood white blood cell counts, particularly neutrophil counts with a morphological left shift, could be helpful in predicting CAL [18]. Niwa et al. suggested that oxygen radical generation and proteolytic enzymes produced by neutrophils induce endothelial cell damage in KD [10]. Senzaki et al. demonstrated a positive association between plasma concentrations of neutrophil proteinases such as elastase and matrix metalloproteinases with CAL formation in acute-phase KD patients [11]. Several studies have suggested that concentrations of proinflammatory cytokines known to stimulate neutrophils could predict CAL [18,19]. Taken together, these results suggest that PMN from the CAL group may have strong adhesive capacities as well as enhanced ability to release cytotoxic substances. However, further examinations will be needed to use CD11b expression on PMN as a predictor of CAL, because only three patients with CAL were included in this study.
We confirmed evaluation of serum concentrations of six of the eight cytokines and chemokines that we measured during the acute phase of KD. Evaluation of the anti-inflammatory cytokine IL-10 as well as proinflammatory cytokines correlated positively with CD11b expression on PMN. The latter findings would seem to be a matter of debate, because IL-10 has been reported to reduce the inflammatory response of monocytes and macrophages and to inhibit cytokine production by T helper 1 cells and inhibit CD11b expression on PMN in vitro[20]. However, in acute inflammation, IL-10 production is considered increasingly to be a modulatory response to proinflammatory cytokine production, tending to counteract excesses of proinflammatory mediators. In this study, serum concentrations of IL-10 showed strong correlations with serum concentrations of IL-6 (R= 0·614, P < 0·0001), G-CSF (R= 0·705, P < 0·0001), IL-8 (R= 0·214, P = 0·036) and CRP (R= 0·381, P = 0·0001). Thus, CD11b expression on PMN may stimulate synthesis indirectly, via proinflammatory cytokines.
No significant difference in CD62L expression on PMN was observed in the various phases of KD treatment in this study. The results are consistent with a report finding that during acute KD changes in soluble l-selectin in serum were not significant, despite dramatic changes in soluble E-selectin and P-selectin [21,22]. However, diminished l-selectin expression on cell surfaces as the result of a shedding process, associated with increased soluble l-selectin in blood, has been reported during PMN activation. Decreased CD62L expression, associated with increased CD11b surface expression, has been described in patients with inflammatory diseases other than KD [23]. Unfortunately, no such disease controls were included in the present study. Further studies will be necessary to determine whether the finding is specific to KD.
In conclusion, PMN from KD patients showed increased CD11b expression. This abnormality is associated with increased serum cytokines and chemokines, and appears to predict CAL. Understanding of the molecular mechanisms underlying ligand binding by CD11b, as well as the roles of CD62L in PMN functions, should guide us in regulating neutrophil function in KD patients.
Acknowledgments
This study was supported in part by Grants-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and Initiatives for Attractive Education in Graduate Schools from MEXT.
References
- 1.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–44. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Beiser AS, et al. Single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–9. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 3.Yanagawa H, Nakamura Y, Yashiro M, et al. Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics. 2001;107:E33. doi: 10.1542/peds.107.3.e33. [DOI] [PubMed] [Google Scholar]
- 4.Koyanagi H, Yanagawa H, Nakamura Y, et al. Leukocyte counts in patients with Kawasaki disease: from the results of nationwide surveys of Kawasaki disease in Japan. Acta Paediatr. 1997;86:1328–32. doi: 10.1111/j.1651-2227.1997.tb14907.x. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi H, Hatake K, Tomizuka H, et al. High serum levels of M-CSF and G-CSF in Kawasaki disease. Br J Haematol. 1999;105:613–5. doi: 10.1046/j.1365-2141.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Kato M, Kobayashi T, et al. Increased circulating granulocyte colony-stimulating factor in acute Kawasaki disease. Pediatr Int. 1999;41:330–3. doi: 10.1046/j.1442-200x.1999.01074.x. [DOI] [PubMed] [Google Scholar]
- 7.Samada K, Igarashi H, Shiraishi H, et al. Increased serum granulocyte colony-stimulating factor correlates with coronary artery dilatation in Kawasaki disease. Eur J Pediatr. 2002;161:538–41. doi: 10.1007/s00431-002-1018-5. [DOI] [PubMed] [Google Scholar]
- 8.Naoe S, Takahashi K, Masuda H, et al. Kawasaki disease. With particular emphasis on arterial lesions. Acta Pathol Jpn. 1991;41:785–97. doi: 10.1111/j.1440-1827.1991.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Oharaseki T, Naoe S, et al. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr Int. 2005;47:305–10. doi: 10.1111/j.1442-200x.2005.02049.x. [DOI] [PubMed] [Google Scholar]
- 10.Niwa Y, Sohmiya K. Enhanced neutrophilic functions in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J Pediatr. 1984;104:56–60. doi: 10.1016/s0022-3476(84)80589-2. [DOI] [PubMed] [Google Scholar]
- 11.Senzaki H, Masutani S, Kobayashi J, et al. Circulating matrix metalloproteinases and their inhibitors in patients with Kawasaki disease. Circulation. 2001;104:860–3. doi: 10.1161/hc3301.095286. [DOI] [PubMed] [Google Scholar]
- 12.Palecanda A, Walcheck B, Bishop DK, et al. Rapid activation-independent shedding of leukocyte l-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992;22:1279–86. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- 13.Luscinskas FW, Kiely JM, Ding H, et al. In vitro inhibitory effect of IL-8 and other chemoattractants on neutrophil–endothelial adhesive interactions. J Immunol. 1992;149:2163–71. [PubMed] [Google Scholar]
- 14.Johnson PA, Alexander HD, McMillan SA, et al. Up-regulation of the granulocyte adhesion molecule Mac-1 by autoantibodies in autoimmune vasculitis. Clin Exp Immunol. 1997;107:513–9. doi: 10.1046/j.1365-2249.1997.d01-956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley AH, Eckerley CA, Jack HM, et al. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997;159:5946–55. [PubMed] [Google Scholar]
- 16.Rowley AH, Shulman ST, Mask CA, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis. 2000;182:1183–91. doi: 10.1086/315832. [DOI] [PubMed] [Google Scholar]
- 17.Brown TJ, Crawford SE, Cornwall ML, et al. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–3. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Noda E, Miyawaki M, et al. Serum levels of neutrophil activation cytokines in Kawasaki disease. Pediatr Int. 2001;43:115–9. doi: 10.1046/j.1442-200x.2001.01362.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin CY, Lin CC, Hwang B, et al. Cytokines predict coronary aneurysm formation in Kawasaki disease patients. Eur J Pediatr. 1993;152:309–12. doi: 10.1007/BF01956740. [DOI] [PubMed] [Google Scholar]
- 20.Laichalk LL, Danforth JM, Standiford TJ. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol Med Microbiol. 1996;15:181–7. doi: 10.1111/j.1574-695X.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 21.Furui J, Ishii M, Ikeda H, et al. Soluble forms of the selectin family in children with Kawasaki disease: prediction for coronary artery lesions. Acta Paediatr. 2002;91:1183–8. doi: 10.1080/080352502320777414. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita S, Dobashi H, Nakatani K, et al. Circulating soluble selectins in Kawasaki disease. Clin Exp Immunol. 1997;108:446–50. doi: 10.1046/j.1365-2249.1997.3852128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Amaro R, Sanchez-Madrid F. Cell adhesion molecules: selectins and integrins. Crit Rev Immunol. 1999;19:389–429. [PubMed] [Google Scholar]