Abstract
Tumour-derived chaperone-rich cell lysate (CRCL), which is made up of numerous heat shock proteins, has been used successfully to generate tumour-specific T cell responses and protective immunity against a wide range of murine tumours. In this study, we have investigated the potency of human ovarian cancer-derived CRCL to activate dendritic cells (DC) and to generate tumour-specific T cells in vitro. CRCL was generated from primary ovarian cancers and SKOV3-A2, a HER2/neu, Wilm's tumour gene 1 (WT1) and human leucocyte antigen (HLA)-A2 positive human ovarian tumour cell line. Peripheral blood mononuclear cells from both HLA-A2+ healthy donors and HLA-A2+ ovarian cancer patients were stimulated weekly with autologous DC loaded with ovarian tumour-derived CRCL. After four to six stimulations in vitro, specific cytokine secretion and cytotoxicity were measured. CRCL promoted interleukin (IL)-12 secretion and enhanced the immunostimulatory capacity of DC. T cells from healthy controls and from ovarian cancer patients secreted higher amounts of interferon-γ following in vitro restimulation with ovarian cancer-derived CRCL than with HER2/neu or WT1 peptide-pulsed DC. We were also able to generate cytotoxic T lymphocyte activity against cancer-specific antigens such as HER2/neu and WT1 from all healthy donors, but from only one of the four ovarian cancer patients with bulky disease. These preliminary results substantiate further the concept that CRCL may prove to be a potent adjuvant for women suffering from ovarian cancer and that this personalized vaccine may be a promising approach for active immunotherapy.
Keywords: cancer vaccine, chaperone-rich cell lysate (CRCL), dendritic cell (DC), heat shock protein (HSP), ovarian cancer
Introduction
Although ovarian cancer accounts for only 3% of all new cancer cases, it is the fifth leading cause of cancer-related death in women and the leading cause of mortality from gynaecological malignancies, with approximately 15 000 deaths annually [1]. Despite dosage escalation of cancer chemotherapy and increasingly radical surgery, the overall survival has not changed significantly. Thus, if successful adjuvant treatment could be developed for ovarian cancer that would prevent the extremely high rate of recurrence, the survival from ovarian cancer could be improved. Cancer vaccines have minimal toxicity, can target the patient's immune response to tumours and may circumvent the intrinsic drug resistance that underlies present chemotherapeutic measures.
Ovarian cancer has been described as multiple diseases [2] and, in fact, more than 40 histological types contribute to the World Health Organization's classification of epithelial tumour categories [3]. Thus, treating all ovarian cancers as if they were the same disease without tailoring treatment to each individual tumour has a high likelihood of failure. To develop effective immunotherapeutic strategies, there would be a need to identify different immunogenic tumour antigens. Tumour-derived heat shock protein (HSP, also referred as chaperone protein) vaccine is an individualized approach that circumvents the intricate hurdle of identifying immunogenic antigens of individual ovarian cancers. The observation that HSPs chaperone antigenic peptides of the cells from which they are derived makes them an attractive and novel personalized approach against cancer, which allows for vaccination of the host against a large repertoire of individual tumour antigens [4–7]. In fact, tumour-derived HSPs (e.g. glucose regulated protein (GRP)94/gp96, HSP90, HSP70, calreticulin HSP110 and GRP170) have been shown to be effective immunogens in both animal models and in clinical trials [4–6,8–17].
Instead of utilizing individual HSP, our laboratory has focused its efforts on studying the potential benefits of tumour-derived HSP vaccines containing multiple HSPs. We have employed a free solution-isoelectric focusing method (FS-IEF) that enriches for immunogenic chaperones from clarified tumour lysates to obtain chaperone-rich cell lysates (CRCL) [11,18]. Given the limitations of preparing tumour vaccines from autologous tumour, CRCL offers an important advantage, in that with the same quantity of starting material we are able to obtain up to 10–20 times as much CRCL-derived proteins as with conventionally purified individual HSP [18,19]. This can be achieved in less time and with less labour [20]. Moreover, we have demonstrated that CRCL is a more effective adjuvant than purified HSPs [21,22], and we were able to generate tumour-specific responses in multiple murine models [20,21]. Thus, its rapid preparation, high-yield and potent anti-tumour activity makes CRCL a desirable immunogenic material for clinical use.
We have not studied previously the efficacy of CRCL in the human system. Preclinical studies, particularly using human peripheral blood mononuclear cells (PBMC), are needed to explore the feasibility of CRCL for immunotherapy in human cancers. This is necessary for advancing CRCL-based vaccines in phase I clinical trials. Therefore, the aim of this study was to investigate whether ovarian cancer-derived CRCL can be used to activate human T cells and elicit a cell-mediated immune response. Specific cytolytic T lymphocytes (CTL) were generated from all human leucocyte antigen (HLA)-A2+ healthy individuals, but only from one ovarian cancer patient following stimulation with autologous CRCL-pulsed dendritic cells (DC). These HLA-A2-specific CTL were able to lyse original ovarian tumour cells but not irrelevant tumour cells in a major histocompatibility complex (MHC) class I-restricted manner. Additionally, we were able to generate tumour-specific CTL against HER2/neu and Wilm's tumour gene 1 (WT1) antigen, indicating that CRCL can chaperone multiple peptides. This correlated with the higher immunostimulatory capacity of CRCL-pulsed DC and secretion of interferon (IFN)-γ by the tumour-specific CTL.
Materials and methods
Cell lines
Ovarian tumour cells SKOV3 (HLA-A2–) and SKOV3-A2 (HLA-A2+) [23] were kindly provided by Dr Mary L. Disis (University of Washington). SKOV3 cells were cultured in complete RPMI-1640 medium (Cellgro, Herdon, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Omega Scientific, Tarzana, CA, USA), 1000 U/ml penicillin–streptomycin and 2 mM of l-glutamine. SKOV3-A2 was cultured in complete RPMI-1640 in the presence of G-418 selection medium (500 μg/ml G-418 (Invitrogen, Rockville, MD, USA). T2 cells (CRL-1992; American Type Culture Collection, Manassas, VA, USA) were used as an antigen-presenting cell line for 51Cr release assays. T2 cells, K562 [natural killer (NK)-sensitive chronic myelogenous leukaemia cell line], K562-A2 (HLA-A2+) cells kindly provided by Dr Lonnnie P. Lybarger (University of Arizona) were cultured in complete RPMI-1640 medium.
Generation of ovarian tumour-derived CRCL
Human ovarian tumour tissues were collected from chemotherapy naive patients undergoing cytoreductive surgery for metastatic ovarian cancer. All patients signed an Institutional Review Board-approved consent form prior to surgery for procurement of tumour samples for research purposes. SKOV3-A2 cells were also harvested as another source of CRCL. FS-IEF enrichment of CRCL was performed as described previously [11,20]. Briefly, tumours were homogenized in lysis buffer and a 100 000 g supernatant was obtained and quantified by bicinchoninic acid (BCA) protein assay (Pierce Endogen, Rockford, IL, USA). The high-speed supernatant was subjected to FS-IEF in a Bio-Rad Rotofor cell (Hercules, CA, USA) for 5 h at 15 W constant power. Twenty fractions were harvested, and each fraction was analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot probing with specific antibodies for the chaperone proteins HSP70, HSP90, GRP94/gp96 and calreticulin. Fractions selected to be pooled for vaccines were those that contained all four of the above HSPs. Pooled fractions were then concentrated using Centricon devices (Millipore, Bedford, MA, USA). Detergents were removed by passage over an Extractigel matrix (Pierce Endogen). The fractions were reconstituted in phosphate-buffered saline (PBS), quantified and stored at − 70°C until use. Endotoxin level of the CRCL is lower than 0·01 endotoxic units (EU)/µg of CRCL as examined by Limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, MD, USA).
Generation of DC
Human leukopheresis was obtained from HLA-A2+ healthy donors or from ovarian patients after undergoing cytoreductive surgery and prior to receiving chemotherapy, according to the guidelines set forth by the Human Subjects Committee at the University of Arizona. All patients and healthy donors signed an Institutional Review Board-approved consent prior to leukopheresis. PBMC were purified using standard white blood cell separation by density centrifugation with Ficoll-Hypaque Plus (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Aliquots of PBMC were cryopreserved in liquid nitrogen until use. DC were generated from PBMC by a modification of the methods described by Romani et al. [24]. In brief, PBMC were cultured in T75 flasks in 10 ml of medium (AIM-V; Gibco brl, Carlsbad, CA, USA) and were allowed to adhere for 2 h at 37°C in 5% CO2. To remove the non-adherent PBMC fraction, flasks were washed several times with PBS. X-Vivo 15 (BioWhittaker, Walkersville, MD, USA) supplemented with 1000 IU/ml of granulocyte–macrophage colony-stimulating factor (GM–CSF; Berlex, Wayne, NJ, USA) and 1000 IU/ml of IL-4 (Peprotech, Rocky Hill, NJ, USA) was added to adherent PBMC and the culture was continued for 5–6 days.
Generation of SKOV3-A2-specific CTL
HLA-A2+ CTL were generated by repeated stimulation of autologous T cells with CRCL or peptide-pulsed mature DC. Briefly, immature DC were incubated for 24 h with SKOV3-A2 CRCL or HER2/neu peptide at a final concentration of 25 µg/ml or 10 µg/ml at 37°C in 5% CO2 followed by the addition of 100 ng/ml tumour necrosis factor [(TNF)-α; R&D Systems, Minneapolis, MN, USA] and 10 µm prostaglandin E2 (PGE2; R&D) for 48 h to induce DC maturation. On day 0, non-adherent PBMC (1 × 106/ml) were plated in 2 ml of Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Rockville, MD, USA) with 25 mM HEPES, supplemented with 10% heat-inactivated human AB serum (Gemini Bioproducts, Calabasas, CA, USA), 1000 U/ml penicillin-streptomycin, 2 mM l-glutamine and with pulsed mature DC (1 × 105/ml) in 24-well plates at 37°C in 5% CO2 (10 : 1 ratio of effector cells to stimulator DC). IL-7 (R&D Systems) was added at a concentration of 10 ng/ml. Starting on day 1, 300 IU/ml of IL-2 (R&D Systems) was added every 2–3 days. The T cells were restimulated weekly with pulsed autologous mature DC from 7 days after the first stimulation. At the end of the third or fourth stimulation, IFN-γ enzyme-linked immunospot (ELISPOT) assays were performed. After four to six cycles of in vitro stimulation and selection, cytotoxicity assays (based on 51Cr release) were performed. The CTL cultures were separated further into CD4 and CD8 populations after sorting using a fluorescence activated cell sorter (FACScan; Becton Dickinson, Franklin Lakes, NJ, USA).
ELISPOT assays
ELISPOT assays were performed to measure the IL-12 secretion by DC and IFN-γ production by PBMC. For IL-12 detection, 1 × 106 day 5 DC were cultured with 50 µg/ml lipopolysaccharide (LPS), ovarian tumour-derived lysate or CRCL in the presence of 1000 IU/ml GM–CSF and IL-4 for 48 h on Millipore MultiScreen-HA 96-well plates (Millititer; Millipore, Bedford, MA, USA). The plates were coated previously overnight with anti-IL-12 p70 capture antibody (10 µg/ml, BD PharMingen, San Diego, CA, USA). DC were then washed out with copious amounts of PBS + 0·05% Tween 20 (PBST). Biotinylated anti-IL-12 antibody (2 µg/ml, BD PharMingen) was added for 2 h. Free antibody was washed out, and the plates were incubated with horseradish peroxidase (HRP)-linked avidin (ABC Elite reagent, 1 drop each of Reagent A and Reagent B per 10 ml PBS; Vector Laboratories, Burlingame, CA, USA) for 1 h, following extensive washing with PBST, and then washing with PBS. Spots were visualized by the addition of the HRP substrate 3-amino-9-ethylcarbazole (AEC; Sigma Chemical, St Louis, MO, USA) prepared in acetate buffer (pH 5·0) with 0·015% hydrogen peroxide. Spots were counted using a dissecting microscope. Wells of interest were photographed with a microscope-mounted Cool SNAP CCD camera (RS Photometrics, Tucson, AZ, USA), and images were captured with RS Image, version 1·07 (Roper Scientific, Tucson, AZ, USA). The image of each well was optimized electronically to visualize the maximum number of spots. ELISPOT assays were also performed to measure IFN-γ secretion from stimulated PBMC. DC (2·5 × 104 cells/well) were loaded with the indicated peptide or CRCL (25 μg/ml) and incubated with effector cells (5 × 104 cells/well) in a total of 200 μl of X-Vivo 15 for 36 h at 37°C in 5% CO2. A mouse anti-human IFN-γ monoclonal capture antibody (10 μg/ml/well; Pharmingen) and a biotinylated anti-human IFN-γ monoclonal capture antibody (2·5 μg/ml/well; Pharmingen) were applied to visualize spots.
Mixed leucocyte reaction (MLR)
In order to assay for allogeneic or autologous lymphocyte proliferation, varying numbers of day 5 DC were plated in a 96-well round-bottomed plate (Falcon, San Jose, CA, USA) and pulsed with 25 μg/ml of lysate, CRCL or 100 ng/ml TNF-α and 10 µm PGE2, or were left untreated for 48 h. DC were plated in triplicate in X-Vivo medium containing 1000 IU/ml of GM–CSF and 1000 IU/ml of IL-4. DC were treated with 100 µg/ml mitomycin-C (Sigma Chemical Co.) for 20 min, and then washed three times with PBS. Allogeneic PBMC (2 × 105 cells) were added to the DC in a total volume of 200 µl of medium. DC were serially diluted and incubated with the ratio of PBMC to DC ranging from 5 : 1 to 40 : 1. On day 3 of culture, cells were pulsed with 1 μCi/well [3H]-thymidine (MP Biomaterial, Costa Mesa, CA, USA) for 18 h and were then harvested using a 96-well Packard cell harvester and the radioactivity measured on a Packard beta counter (Packard Biosciences, Meriden, CT, USA) [21].
Cytolytic activity of stimulated PBMC
51Chromium release cytotoxicity assays were performed to evaluate the ability of stimulated PBMC to lyse the following target cells: SKOV3-A2, SKOV3, K562, K562-A2 or T2 cells pulsed with 10 μg/ml of HLA-A2 restricted peptides, HER2/neu (KIFGSLAFL), WT1 (RMFPNAPYL) or hepatitis B virus (HBV) peptide (FLPSDYFPSV). Targets (5 × 103 cells/well) were labelled with 100 μCi of 51Cr (Amersham Pharmacia Biotech) in complete IMDM for 1 h. Percentage of specific lysis was calculated as [(experimental 51Cr release-spontaneous release)/(maximum release – spontaneous release)] × 100.
Flow cytometry
Fluorochromes fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-conjugated monoclonal antibodies (BD PharMingen) were added to cell pellets, incubated for 30 min on ice and washed before analysed by flow cytometry (FACScan; Becton Dickinson).
Results
Effect of ovarian tumour-derived CRCL on human DC maturation
To generate CRCL-specific T cells from HLA-A2+ PBMC, autologous DC were used as antigen-presenting cells (APC). Previous data have demonstrated that murine DC incubated with tumour-derived CRCL had higher expression of CD40 and MHC class II on their cell surface, produced more IL-12 and had superior immunostimulatory capacity in MLR [21]. In this study, we have evaluated whether these findings would translate into the human system using CRCL from ovarian cancers. CRCL was derived from ovarian tumours or from the SKOV3-A2 cell line. DC were generated from either healthy volunteers or from ovarian cancer patient PBMC, as described in Materials and methods.
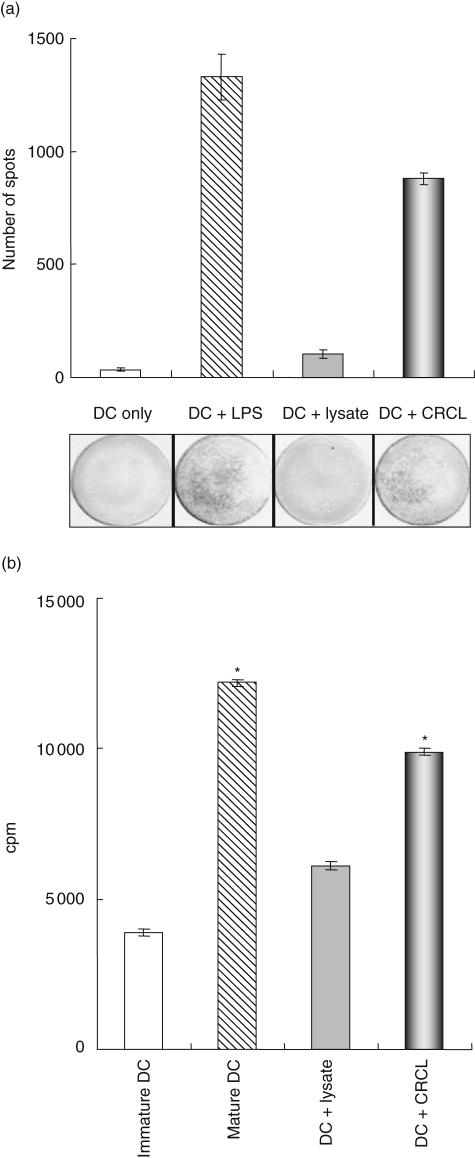
DC IL-12 secretion following stimulation with 50 μg/ml CRCL was evaluated and compared to LPS as the positive control. Compared with lysate and medium alone, CRCL-stimulated DC had increased IL-12 production as assessed by ELISPOT (Fig. 1a). We also examined whether DC pulsed with ovarian cancer CRCL would be more effective stimulators in a MLR assay when compared to DC exposed to tumour lysate or to TNF-α and PGE2. Our results indicate that ovarian cancer-derived CRCL has superior activating effects on DC compared to tumour lysate (Fig. 1b). However, in terms of surface maturation markers, unlike our previous work in murine models, the expressions of HLA-DR, CD40, CD80, CD86 and CD83 were not significantly up-regulated by CRCL alone (data not shown).
Fig. 1.
Dendritic cell (DC) maturation induced by chaperone-rich cell lysates (CRCL) (a). DC pulsed with CRCL show increased interleukin (IL)-12 production. Enzyme-linked immunospot (ELISPOT) assays were performed to measure IL-12 production by normal human DC. At day 5 of culture, 1 × 106 DC were cultured with medium, 50 µg/ml tumour-derived lysate, CRCL or maturation lipopolysaccharide (LPS) each for 48 h. All the cultures were kept in the presence of granulocyte–macrophage colony-stimulating factor (GM–CSF) and IL-4. (*P < 0·05 versus DC or DC + lysate; representative data from three experiments are shown). (b). Ovarian tumour-derived CRCL increases DC capacity to stimulate peripheral blood mononuclear cell (PBMC) proliferation. DC were cultured as described above, harvested, treated with mitomycin-C and washed. Allogeneic PBMC were added (1 × 105 per well) and cultured with the pretreated DC for 3 days. [3H]-thymidine was added and the cells were cultured for an additional 18 h before the incorporated radioactivity was counted (*P < 0·05 versus DC or DC + lysate; representative data from three experiments are shown); cpm: counts per minute. These data are representative of DC derived from both healthy donors and ovarian cancer patients and incubated with either patient ovarian tumour tissue or SKOV3-A2-derived CRCL.
Generation of tumour-specific T cells
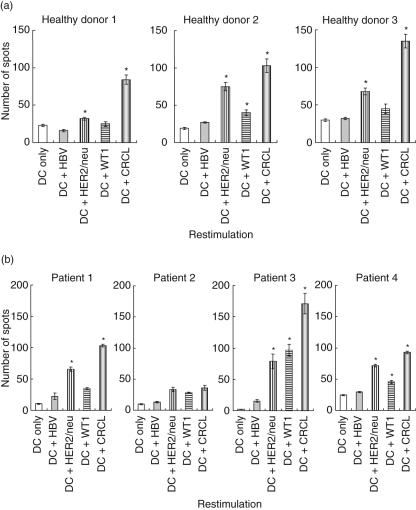
Initially we tested the immunogenicity of the ovarian cancer-derived cell line, SKOV3-A2, in DC/PBMC combinations from healthy HLA-A2+ individuals. To maximize the stimulatory capacity of DC, we generated mature DC by two rounds of stimulation. First, immature DC were stimulated with CRCL for 24 h and this was followed by TNF-α and PGE2 exposure for another 48 h. PBMC were monitored for cytokine production by IFN-γ ELISPOT assays after four rounds of CRCL-pulsed DC stimulation. Additional experimental groups included DC that had been pulsed with irrelevant HBV peptide or relevant HER2/neu or WT1 peptide. SKOV3-A2 CRCL-pulsed DC elicited the greatest antigen-specific IFN-γ responses from responding PBMC derived from healthy individuals (Fig. 2a). This suggests that improved DC stimulation and/or a larger antigenic repertoire was provided by CRCL. The stimulated healthy donor PBMC demonstrated IFN-γ secretion following encounters with HER2/neu peptide in all cases, albeit less than CRCL, and WT1 peptide elicited only modest responses in two of three healthy donors. Similarly, stimulation of patient PBMC with CRCL resulted in specific responses in three of the four ovarian patients demonstrated by increased IFN-γ secretion (Fig. 2b). The IFN-γ responses from individual peptides were clearly not as strong as those from broader antigen exposure driven by SKOV3-A2 CRCL restimulation (Fig. 2b). These results suggest that T cells generated in the presence of DC/CRCL stimulation from both healthy donors and ovarian cancer patients could recognize either individual tumour antigen or a broader antigen repertoire encompassed in the tumour CRCL.
Fig. 2.
Tumour-specific responses detected from human leucocyte antigen (HLA)-A2+ healthy donors (a) and ovarian cancer patients (b) by interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assays. Peripheral blood mononuclear cells (PBMC) were stimulated for 4 weeks by autologous dendritic cells (DC) loaded with SKOV3-A2 chaperone-rich cell lysates (CRCL). PBMC were from both healthy donors and ovarian cancer patients. DC (2·5 × 104 cells/well) pulsed with SKOV3-A2 CRCL and indicated peptides (25 μg/ml) were used to stimulate bulk PBMC cultures (5 × 104 cells/well). DC pulsed with hepatitis B virus (HBV) peptide and unpulsed DC were used as control stimulation. (Results from healthy donors 1–3 and patients 1–4 are shown). (*P < 0·05 versus DC or DC + HBV).
Cytolytic activity of tumour-specific T cells
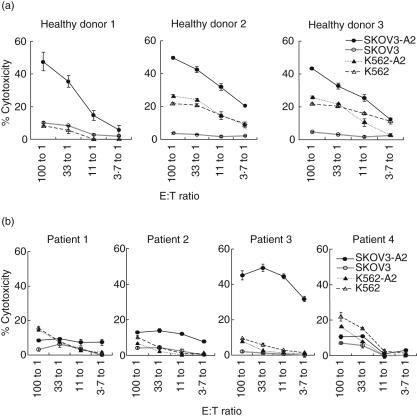
After six weekly stimulations with CRCL-pulsed mature DC, cytotoxicity assays were performed to address whether antigen-specific CTL could lyse the SKOV3-A2 targets. Stimulated PBMC from all three healthy individuals (Fig. 3a) lysed the SKOV3-A2 targets. To determine MHC restriction of these T cell lines, we examined the cytotoxicity against cells not expressing HLA-A2 (SKOV3) and no lysis was observed. Some NK-mediated cytotoxicity was observed as indicated by lysis of the NK sensitive target K562. K562 cells transfected to express A2 had comparable lysis to A2 negative parental K562. These data demonstrated that tumour-derived CRCL is capable of generating CTL specific to the tumour of origin of CRCL. In contrast, CRCL loaded DC-stimulated PBMC from ovarian cancer patients demonstrated specific cytotoxicity in only one of the four patients studied (Fig. 3b). Although recognition of tumour antigen was observed as documented by IFN-γ secretion (Fig. 2b), lysis of target tumour cells was not detected in patients 1 and 4 (Fig. 3b). Patient 3, whose T cells demonstrated substantial lysis of SKOV3-A2 targets, also demonstrated the highest IFN-γ secretion by ELISPOT (Fig. 2b).
Fig. 3.
Cytotoxicity of target cells by stimulated peripheral blood mononuclear cells (PBMC) generated by weekly stimulation with SKOV3-A2 tumour-derived chaperone-rich cell lysates (CRCL)-pulsed dendritic cells (DC). Specific T cells were generated by 6-week stimulation of DC loaded with SKOV3-A2 CRCL from the PBMC of healthy donor (a) and ovarian cancer patients (b). Effectors (at the indicated E : T ratios) were incubated with 51Cr-labelled target cells (5 × 103 cells/well), and 51Cr release from target cells was measured after 6–8 h. Lysis of the natural killer (NK)-sensitive cell line, K562, and K562 expressing human leucocyte antigen (HLA)-A2 served as background controls. Lysis of the HLA-A2+ or HLA-A2– ovarian cancer cells (SKOV3-A2 and SKOV3) was also evaluated. (Results from healthy donors 1–3 and patients 1–4 are shown). (Error bars represent the mean ± s.e.m. of triplicate samples).
Cytolytic activity of CRCL-stimulated versus peptide-stimulated T cells
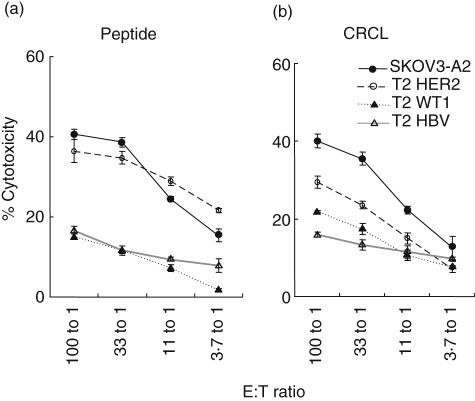
HLA-A2 restricted peptides have been shown to be potent CTL stimulators and antigens. We compared our CRCL stimulation with that by HER2/neu peptide. As shown in Fig. 4a, HER2/neu-stimulated T cells lysed T2 cells pulsed with HER2/neu peptide and SKOV3-A2 cells, which express and present this peptide. Importantly, CRCL-stimulated T cells lysed T2 cells pulsed with HER2/neu in addition to lysing the original SKOV3-A2 cells (Fig. 4b), correlating the results of our IFN-γ ELISPOT assays. This implies the presence of the HER2/neu antigen in the SKOV3-A2-derived CRCL preparations.
Fig. 4.
Cytotoxicity of target cells by stimulated peripheral blood mononuclear cells (PBMC) generated by SKOV3-A2 tumour-derived chaperone-rich cell lysates (CRCL) and by HER2/neu peptide. Specific T cells were generated by 6-week stimulation of PBMC by dendritic cells (DC) loaded with HER2/neu peptide (a) or SKOV3-A2 CRCL (b). Effectors (at the indicated E : T ratios) were incubated with 51Cr-labelled target cells (5 × 103 cells/well), and 51Cr release from target cells was measured after 6–8 h. Lysis of T2 cells pulsed with hepatitis B virus (HBV) peptide was background control. Lysis of the ovarian cancer cells SKOV3-A2 or T2 cells pulsed with HER2/neu, Wilm's tumour gene 1 (WT1) peptide was evaluated. (Representative data from two experiments were shown. Error bars represent the mean ± s.e.m. of triplicate samples). These data are representative of both healthy donors and ovarian cancer patient 3.
Cytotoxicity of the stimulated PBMC is mediated mainly by CD8+ T cells
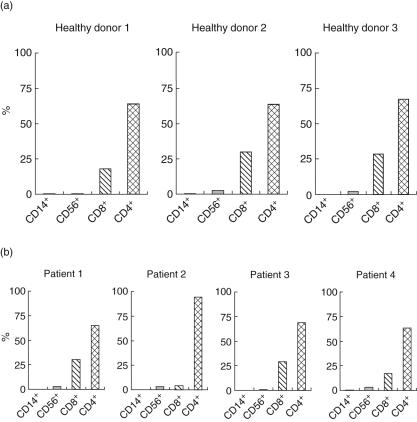
Flow cytometric analysis demonstrated that CRCL-stimulated PBMC were comprised mainly of CD4+ and CD8+ T cells. In all healthy donors and patients 1, 3 and 4, CD4+ and CD8+ T cells attributed to 60–70% and 20–30%, respectively, of bulk cell culture after six weekly stimulations (Fig. 5a,b). In contrast, the CD8+ T cell population of patient 2 was dramatically lower at only 6% compared to 25% before stimulation (data not shown), suggesting that there were defects in the CD8+ T cell growth in this patient. This lower number of CD8+ T cells correlated with lower IFN-γ (Fig. 2b) production and cytotoxicity (Fig. 3b) in this patient. The percentages of CD56+ (NK cells) and CD14+ (monocytes) cells were low (1% and 1–3%, respectively) in both healthy donors and ovarian patients.
Fig. 5.
Phenotype of stimulated peripheral blood mononuclear cells (PBMC). Stimulated PBMC were generated from healthy donors (a) or ovarian cancer patients (b) by weekly stimulation with dendritic cells (DC) loaded with SKOV3-A2 chaperone-rich cell lysates (CRCL). Stimulated cells were double-stained for CD14+ (monocyte), CD56+[natural killer (NK) cell], CD8+, and CD4+ T cells. Percentage of positive cells was analysed using flow cytometry.
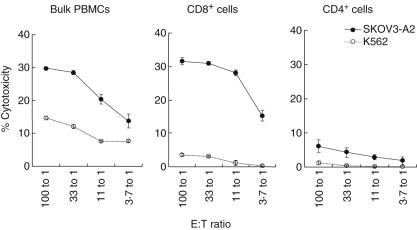
To confirm that the cytotoxicity of the stimulated PBMC was mediated by CTL, we isolated CD4+ and CD8+ T cells from bulk cultures and performed 51Cr release assays. As demonstrated in Fig. 6, SKOV3-A2 targets were lysed primarily by CD8+ T cells. There was some K562 cell lysis induced by bulk cell culture compared to CD4+ or CD8+ T cells, suggesting that the overall cytotoxicity of bulk-stimulated PBMC was also comprised of NK cell-mediated lysis, but at much lower levels than that of CD8+ T cells. These results, together with the absence of cytotoxicity against SKOV3 (HLA-A2-negative) target cells, indicate that the cytotoxicity against the tumour, from which CRCL originated (SKOV3-A2), can be attributed mainly to MHC class I (HLA-A2)-restricted CD8+ T cells.
Fig. 6.
Cytotoxicity against target cells by bulk peripheral blood mononuclear cells (PBMC) or by separated CD8+ or CD4+ T cells derived from stimulated PBMC. Stimulated PBMC were generated as mentioned previously. Target SKOV3-A2 and K562 cells were labelled with 51Cr. The bulk PBMC or sorted CD8+ and CD4+ T cells were co-incubated (at the indicated E : T ratios) with labelled target cells (5 × 103 cells/well) for 6–8 h. The percentage of target cell lysis was then determined. Representative data from patient 3 are shown. Data from healthy controls were similar. Error bars represent the mean ± s.e.m. of triplicate samples.
Discussion
Tumour cells are able to escape immunological destruction induced by certain tumour antigens due to the antigenic variability that arises from genetic instability. A single tumour antigen or a single epitope vaccine may lead to tumour escape variants and tolerance to therapy due to the immune pressure [25,26]. Tumour-derived HSPs are personalized vaccines that carry multiple antigens of the original tumour [27,28], circumventing the need to identify each tumour's antigens. Compared to individual HSP, CRCL is a multiple HSP complex which can chaperone a wide variety of tumour-specific antigens. In this pilot human study, we have demonstrated for the first time that CRCL can maintain the advantages of individual HSPs as vaccines (i.e. no need to identify antigens, multivalency of the antigen ensemble and specificity of the class I restricted response) in a human in vitro system. We have focused on whether ovarian cancer-derived CRCL was able to generate HLA-A2 specific responses. Stimulation of autologous human DC with ovarian cancer-derived CRCL was able to generate specific CTL that were able to recognize and lyse ovarian tumour targets. Additionally we were able to generate CTL against two different tumour antigens such as HER2/neu and WT1 in a class I-restricted manner. Therefore, as we have reported previously in mice, human CRCL is capable of generating a larger repertoire of tumour-specific CTL which are polyclonal in nature. Such T cells may respond to subdominant epitopes, which would be an important consideration as high-affinity T cells derived from self-antigens can be deleted in the thymus. Additionally, the chaperone proteins themselves have potent natural adjuvant- or cytokine-like activities on APC such as DC, thus enhancing the DC ability to process/present antigen and stimulate T cell responses, as demonstrated by IL-12 production and higher immunostimulatory capacity of CRCL-stimulated DC.
We have demonstrated that CRCL can be generated from patient tumour tissue. Compared to 1–2 mg of CRCL from 1 g of murine tumour, 0·5–1 mg CRCL could be generated from human ovarian tumour tissue. However, the overall yield of CRCL is still much higher than that of individual HSPs. Given the limitations of preparing tumour vaccines from autologous tumour, CRCL offers an important advantage in that with the same quantity of starting material we are able to obtain up to 10 times as much CRCL-derived proteins as with conventionally purified individual HSPs. This makes the method desirable from a clinical standpoint in terms of high yield from a potentially limited tumour source, and with a rapid turn-around time from tumour harvest to treatment of the patient. CRCL was also been isolated successfully from an ovarian cancer cell line culture SKOV3-A2. Instead of using solid tumour tissue, cell pellets of cell cultures after centrifugation were homogenized and applied to FS-IEF. The vaccine generated in this way was as efficient in activating DC as that from solid tumour, suggesting that cells in culture may provide another feasible source of CRCL if inadequate tumour is available.
To measure the potential efficacy of a tumour vaccine, CTL responses against tumour antigens have been regarded as an important indicator of activity. CTL usually recognizes cytoplasmic antigens of target cells that are processed and presented as peptide complexes with MHC class I molecules [29]. Exogenous antigens can be cross-presented on MHC class I molecules of professional APCs to CD8+ T cells in certain circumstances, with the help of HSPs [28,30–33]. This mechanism had been utilized in our study to generate CTL in vitro following stimulation with mature DC pulsed with tumour-derived CRCL. Because immature DC are highly effective in taking up and processing antigens compared with mature DC, we pulsed immature DC with CRCL and induced DC to maturation after antigen pulsing. Indeed, CRCL alone could activate immature human DC as indicated by augmented IL-12 secretion and immunostimulatory capacity in MLR. Because cancer patients are immunosuppressed, their DC may have a functional defect which has been suggested to be one of the causes for T cell dysfunction in cancer patients [34,35]. Following ovarian cancer CRCL stimulation, DC from both healthy donors and ovarian cancer patients revealed an increased cytokine secretion and T cell stimulatory capacity.
We have demonstrated that following CRCL stimulation of human DC, we were able to promote the expansion of both CD4+ and CD8+ T cells. Examining the ratio of CD4+versus CD8+ T cells, there was preferential expansion of CD4+ T cells at the end of 6 weeks of restimulation. This is in sharp contrast to single purified HSP such as gp96, where an activated CD8+ T cell bias was observed [36]. This result suggests that there are qualitative differences in CRCL stimulation compared to single HSPs. However, the cytotoxicity was mainly attributed primarily to MHC class I-restricted CD8+ T cells and therefore the exact contribution of CD4+ T cells in CRCL-stimulated culture needs further investigation. It is important to note that in spite of expansion of CD4+ T cells in the patient PBMC cultures, the CD8+ T cells were generally not able to kill tumour-specific targets, indicating that these patients may have ongoing immunosuppression. The causes of this immunosuppression need to be defined further.
Unlike other preclinical studies which have isolated PBMC from vaccinated patients [4–6,17,37], we have not yet acquired approval to immunize patients with CRCL. Therefore, we examined the function of CRCL-stimulated DC in stimulating anti-tumour T cell responses in an in vitro cell culture system. With the help of autologous DC loaded with ovarian cancer-derived CRCL, we stimulated PBMC, obtained by leukopheresis, in vitro from both healthy donors and ovarian carcinoma patients. These experiments are tedious, requiring extended culture of PBMC and weekly generation of new DC for pulsing and restimulation.
Specific IFN-γ secretion was observed in the stimulated PBMC from healthy donors and from three of four ovarian cancer patients. However, cytolytic function of stimulated T cells from ovarian cancer patients with untreated bulky disease was generally suppressed. Published preclinical studies using HSP vaccines have generally evaluated the IFN-γ secretion, without cytolytic assays, with the cytokine data correlating with the clinical responses to HSP vaccination [4–6,17]. It should be kept in mind that the absence of positive data in 51Cr release assays does not predict lack of in vivo responses. Moreover, the experimental in vitro conditions used to generate CTL are artificial, given that other cells and cytokines that are normally present in vivo may be absent in culture. However, the difference in the generated cytotoxicity between healthy volunteers and patients was striking and cannot be ignored, and suggests that chemo-naive patients with widely metastatic ovarian cancer may be so immunosuppressed that their T cells have a defective response to CRCL. The nature of the defects in generating CTL in these ovarian cancer patients are not clear and need to be defined. The host immunosuppressive environment may play a vital role in this defect, which requires further study. We have observed diminished IL-2 output from Jurkat cells incubated with albumin purified from ovarian cancer patient ascites fluid, suggesting a possible systemic immunosuppression in these patients [38]. It is possible that patients may respond better after their tumour burden has been reduced with surgery and chemotherapy. These results indicate the importance of a pilot study to immunize patients with CRCL and to measure their immunological response. It also indicates that piloting the time of administration will be critical.
This study is the first to test the immunostimulatory effects of CRCL in the human system. The uniqueness of this system is that we were able to activate multiple antigenic determinants, including shared tumour antigens such as HER2/neu and WT1. Therefore it is conceivable that CRCL could deliver a broader repertoire of tumour-specific antigens that makes CRCL vaccination as individualized therapy desirable. Individual HSP such as HSP70 and gp96 have already been applied to phase II clinical trials [5,37]. The objective of this study was not to compare CRCL with individual HSPs in vitro, as our data from the murine in vivo models indicate that CRCL has significantly more activity than individual HSPs. Our goal in undertaking this study was to examine the efficacy of obtaining CRCL from human tumours and generating immunological responses in vitro. Our pilot data demonstrate that this approach may constitute a promising immunotherapeutic strategy to treat patients with ovarian cancer and therefore warrants further investigation.
Acknowledgments
We are grateful to Dr Douglas F. Lake and Dr Sara O. Dionne for providing access to their laboratory resources, Roger Fiederlein for the valuable assistance during the study and Lorry Velasco and Kathy Schmidt for their excellent nursing work. This study was supported by National Institutes of Health, grant no. R21 CA102410, by the Arizona Biomedical Research Commission, Tee up for Tots and Raise a Racquet for Kids.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Auersperg N, Edelson MI, Mok SC, Johnson SW, Hamilton TC. The biology of ovarian cancer. Semin Oncol. 1998;25:281–304. [PubMed] [Google Scholar]
- 3.Rabban JT, Bell DA. Current issues in the pathology of ovarian cancer. J Reprod Med. 2005;50:467–74. [PubMed] [Google Scholar]
- 4.Janetzki S, Palla D, Rosenhauer V, et al. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232–8. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Pilla L, Patuzzo R, Rivoltini L, et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM–CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol Immunother. 2006;55:958–68. doi: 10.1007/s00262-005-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Coppa J, Carrabba MG, et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235–45. [PubMed] [Google Scholar]
- 7.Oki Y, Younes A. Heat shock protein-based cancer vaccines. Exp Rev Vaccines. 2004;3:403–11. doi: 10.1586/14760584.3.4.403. [DOI] [PubMed] [Google Scholar]
- 8.Li Z. Priming of T cells by heat shock protein–peptide complexes as the basis of tumor vaccines. Semin Immunol. 1997;9:315–22. doi: 10.1006/smim.1997.0087. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava PK, Amato RJ. Heat shock proteins: the ‘Swiss Army knife’ vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–7. doi: 10.1016/s0264-410x(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 10.Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797–802. doi: 10.1084/jem.189.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graner M, Raymond A, Romney D, et al. Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res. 2000;6:909–15. [PubMed] [Google Scholar]
- 12.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–20. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 13.Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–403. [PubMed] [Google Scholar]
- 14.Park JE, Facciponte J, Chen X, et al. Chaperoning function of stress protein grp170, a member of the hsp70 superfamily, is responsible for its immunoadjuvant activity. Cancer Res. 2006;66:1161–8. doi: 10.1158/0008-5472.CAN-05-2609. [DOI] [PubMed] [Google Scholar]
- 15.Manjili MH, Wang XY, Chen X, et al. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171:4054–61. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 16.Belli F, Testori A, Rivoltini L, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 2002;20:4169–80. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 17.Rivoltini L, Castelli C, Carrabba M, et al. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma-specific T cells. J Immunol. 2003;171:3467–74. doi: 10.4049/jimmunol.171.7.3467. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother. 2006;55:329–38. doi: 10.1007/s00262-005-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graner M, Raymond A, Akporiaye E, Katsanis E. Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother. 2000;49:476–84. doi: 10.1007/s002620000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother. 2003;52:226–34. doi: 10.1007/s00262-002-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood. 2003;101:4485–91. doi: 10.1182/blood-2002-10-3108. [DOI] [PubMed] [Google Scholar]
- 22.Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood. 2003;101:245–52. doi: 10.1182/blood-2002-05-1580. [DOI] [PubMed] [Google Scholar]
- 23.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–17. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 26.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–8. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 29.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–92. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004;5:678–84. doi: 10.1038/ni1082. [DOI] [PubMed] [Google Scholar]
- 31.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 32.Inaba K, Turley S, Yamaide F, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–73. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 34.Lenahan C, Avigan D. Dendritic cell defects in patients with cancer: mechanisms and significance. Breast Cancer Res. 2006;8:101. doi: 10.1186/bcr1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satthaporn S, Robins A, Vassanasiri W, et al. Dendritic cells are dysfunctional in patients with operable breast cancer. Cancer Immunol Immunother. 2004;53:510–8. doi: 10.1007/s00262-003-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez SR, Singh-Jasuja H, Warger T, et al. Glycoprotein 96-activated dendritic cells induce a CD8-biased T cell response. Cell Stress Chaperones. 2005;10:221–9. doi: 10.1379/CSC-117R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Qiao Y, Liu B, et al. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin Cancer Res. 2005;11:4460–8. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 38.Graner MW, Likhacheva A, Davis J, et al. Cargo from tumor-expressed albumin inhibits T-cell activation and responses. Cancer Res. 2004;64:8085–92. doi: 10.1158/0008-5472.CAN-04-1871. [DOI] [PubMed] [Google Scholar]