Abstract
Thyroid-associated ophthalmopathy (TAO) is a common yet poorly understood component of Graves' disease involving inflammation, congestion and soft tissue remodelling of the orbit. Unlike most autoimmune disorders, TAO has variable severity but follows a predictable course and is usually self-limited. The objective of this study was to investigate the phenotypic profile of peripheral blood mononuclear cells in euthyroid patients with TAO. The study was a prospective, consecutive analysis of the peripheral blood mononuclear cell phenotype in patients with TAO and normal controls. We demonstrate that the fraction of T cells expressing CD69, CD25 or CXCR4 is significantly greater in patients with TAO compared to control donors. In addition, the fraction of CD19+ CD25+ B cells is significantly greater. We did not find differences between the two groups of subjects in monocytes expressing these markers. There is a phenotypic shift in peripheral blood lymphocytes associated with TAO that appears durable and persists beyond the hyperthyroid phase of Graves' disease. These changes may support the immune reaction provoking orbital disease development.
Keywords: autoimmunity, Graves' disease, lymphocyte phenotype, thyroid associated ophthalmopathy
Introduction
Graves' disease (GD) represents a common and poorly understood autoimmune syndrome involving the thyroid, orbital connective tissue and skin. The pathogenesis of this disease has eluded our understanding, despite intense interest. It is an organ-specific process, and its clinical presentation is almost invariably dominated by the presence of circulating antibodies against the TSH receptor (TSHR) [1]. At the core of both thyroid and connective tissue manifestations of GD is the infiltration of T and B lymphocytes, macrophages and mast cells. It is currently thought that these recruited bone marrow-derived cells drive the extensive tissue remodelling found in affected tissues [2,3]. Thyroid-associated ophthalmopathy (TAO) is characterized by lymphocytic infiltration accompanied by exaggerated accumulation of hyaluronan with extensive inflammatory changes [4,5]. The orbital disease involves the biosynthesis of proinflammatory molecules by fibroblasts. The unique consequences of a complex interplay between these fibroblasts and immunocompetent cells appears to represent the basis for the site-selective involvement of the orbit in TAO [6–8].
Previous studies have surveyed peripheral mononuclear cells (PBMCs) from patients with GD [9]. A number of phenotypic markers have been examined. These earlier studies have focused on patients with hyperthyroidism whether or not they manifested TAO. They demonstrated distinct differences in phenotypic profiles of lymphocytes during the hyperthyroid phase of GD compared to control subjects [9]. However, limited conclusions can be drawn from these earlier studies with regard to the systemic aspects of the immune disease that might underlie development and progression of TAO, because any contributions from hyperthyroxinaemia per se were difficult to dissect out from those related specifically to the orbital process.
CD69 expression by lymphocytes is associated with cellular activation [10]. Moreover, cross-linking this protein promotes T cell proliferation [11,12]. Peripheral blood lymphocytes have little basal expression of CD69, but stimulation of the TCR/CD3 complex by phorbol esters through a protein kinase C (PKC)-mediated mechanism causes rapid (2–3 h) induction of this marker on T, B and natural killer (NK) cells [11,12]. Recent data indicate that the function of CD69 may be more complicated than previously recognized. CD69 is expressed by lymphocytes at sites of chronic inflammation, including affected joints of patients with rheumatoid arthritis (RA) [13]. This molecule may regulate immune responses negatively through production of transforming growth factor (TGF)-β[14].
In addition to CD69, several other molecules are expressed on the T cell surface following activation in vitro. The CD25 antigen, considered a hallmark of cellular activation, is induced by direct stimulation of the TCR/CD3 complex or by treatment with phorbol ester [15]. CD25 is also displayed constitutively by a subset of lymphocytes having unique regulatory properties [16]. Human leucocyte antigen D-related (HLA-DR), a human class II major histocompatibility complex (MHC) antigen, is expressed on B lymphocytes, monocytes, macrophages and particularly during the later stages of activation on T and NK lymphocytes [17]. While CXCR4 is not considered an activation-associated molecule, its expression is elevated in patients with RA and may be functionally related to cell trafficking to sites of inflammation [18,19].
In this study, we examine for the first time the phenotype of PBMCs in euthyroid patients with clinically significant TAO. These individuals were treated previously for hyperthyroidism and were uniformly euthyroid at the time of their participation in the study. Our findings suggest that these peripheral immune cells display a distinctly activated phenotype different from those derived from control donors. The sustained activation of bone marrow-derived cells following successful treatment of hyperthyroidism may promote orbital inflammation and the subsequent tissue activation and remodelling in late-stage TAO.
Materials and methods
Materials
Ficoll Hypaque was purchased from Sigma Aldrich (St Louis, MO, USA). FacLyse buffer, Cytofix, anti-CD3 CyChrome, anti-CD14 fluorescein isothiocyanate (FITC), anti-CD4-FITC, anti-CD19 CyChrome, anti CD25 allophycocyanin (APC), isotype mouse IgG1 FITC, phycoerythrin (PE) and CyChrome were purchased from BD Biosciences (San Jose, CA, USA). Anti-forkhead box P3 (Foxp3) CyChrome and proprietary permeabilization buffers were obtained from eBioscience (San Diego, CA, USA). Staining buffer (SB) was prepared from phosphate-buffered saline, 4% fetal bovine serum with 0·1% sodium azide (Sigma Aldrich). Fetal bovine serum was purchased from Life Technologies (Grand Island, NY, USA).
Patients
Subjects, aged 20–65 years, were recruited from the patient population of Jules Stein Eye Institute. Informed consent was obtained as specified by the Institutional Review Boards of the Center for Health Sciences at University of California Los Angeles (UCLA) and Harbor–UCLA Medical Center. The study population comprised patients evaluated for TAO with either active orbital inflammation or disease without evidence of inflammation. These patients were euthyroid as determined by standard laboratory testing. Control subjects were healthy volunteers without known GD or other autoimmune disease who presented for aesthetic or functional eyelid surgery. Individuals excluded from the study included those with non-thyroid autoimmune disease, asthma, granulomatous disease, sinusitis or HIV infection.
Clinical data including age, sex, medications, smoking history, orbital physical examination and laboratory values are summarized in Table 1. Complete ophthalmic and orbital examination, including measurement of proptosis, eyelid motility, assessment of orbital and periorbital inflammation, was performed. In addition, digital photos documented orbital and periorbital disease involvement. Ophthalmic examination was quantified according to the Clinical Activity Score (CAS). Careful examination of the skin failed to detect evidence of thyroid-related dermopathy in any of the participants.
Table 1.
Clinical profile of patients with thyroid-associated ophthalmopathy (TAO) and those from the control population
| Patient number | |
|---|---|
| Graves' | 22 |
| Control | 12 |
| Sex M/F | |
| Graves' | 3/19 |
| Control | 2/10 |
| Age (mean) | |
| Graves' | 46 |
| Control | 42 |
| Interval since diagnosis | |
| Mean (months) | 27 |
| Range (months) | 3–112 |
| Smoking history | |
| Graves' | 0/22 |
| Control | 0/12 |
| Graves' CAS > 3 | 5/22 |
Flow cytometry
Peripheral blood (approx. 5 ml) was obtained and stored in tubes containing heparin. Staining for flow cytometry was performed within 24 h of collection, according to the manufacturer's instructions (BD Biosciences). Briefly, 100 µl whole blood was placed in 12 × 75 mm polypropylene tubes and fluorochrome-conjugated monoclonal antibodies were added (1 µg/106 cells). These were then incubated in the dark for 20 min at room temperature. Fluorescence activated cell sorter (FACS) lyse solution (2 ml) was added for 10 min at room temperature to disrupt red blood cells (RBCs). Cells were washed twice with SB, resuspended in Cytofix (BD Biosciences) and kept in the dark at 4°C until cytometric analysis (within 72 h). Analysis was performed using a live cell gate on a FACSCalibur flow cytometer (BD Biosciences). Staining of regulatory T cells was performed according to the manufacturer's guidelines (eBioscience). Briefly, human PBMCs were isolated by density gradient centrifugation and washed three times. Surface staining of CD4-FITC, CD3-PE and CD25-APC was performed as described above. After 30-min incubations, cell pellets were washed and resuspended in 1 ml fixation/permeabilization solution (eBiosciences) and incubated for another 30 min. Cells were washed once with the addition of permeabilization buffer and anti-Foxp3 Cychrome was added and incubated for 30 min. After washings, cells were analysed as described above. In all experiments, non-specific isotype-matched control antibodies were used to define the gating strategy for each individual experiment. Fluorescence intensity greater than isotype control was considered positive for specific expression.
Statistics
Statistical analysis was performed using a two-sample Student's t-test with a confidence level of > 95%.
Results
The fraction of T cells expressing CD69 is increased in patients with TAO
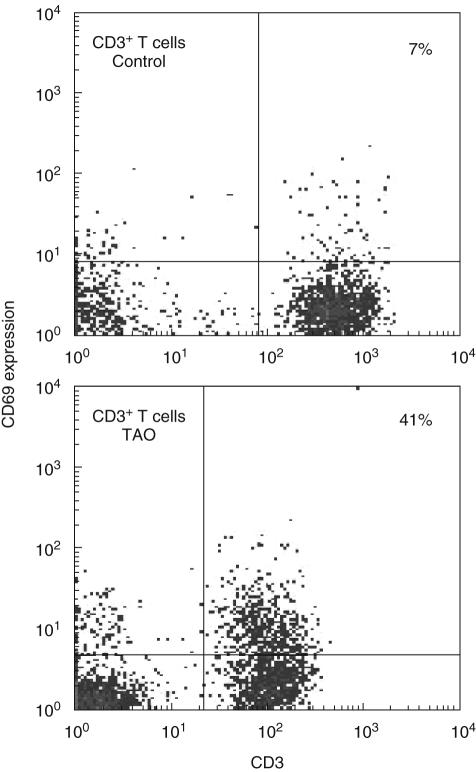
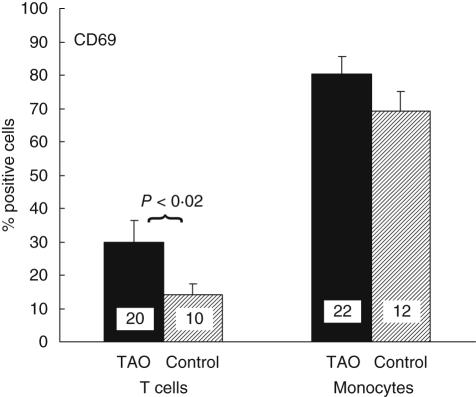
Using multi-parameter flow cytometry, we examined the surface expression of CD69 by peripheral blood T cells and monocytes. Because CD69 is expressed soon after antigen activation and in chronic inflammatory states, we quantified levels of this protein in TAO patients [11,13,20]. T cells, B cells and monocytes were identified by the expression of CD3, CD19 and CD14, respectively. An example of CD69 expression on T cells is shown in Fig. 1. As the data indicate, 41% of T cells from patients with TAO display CD69. In contrast, the protein is detectable in 7% of T cells from the control donor. Figure 2 demonstrates that the fraction of CD3 T cells from patients with TAO expressing CD69 was 30 ± 7% (mean ± SE, n = 20) and was significantly greater than that of control CD3 T cells (14 ± 3%, n = 10, P < 0·02). The percentage of T cells expressing CD69 varied among donors. The range for TAO patients was 9–98% while that for controls was 3–35%. There was no correlation between disease activity and expression of CD69 on T cells. The percentage of cells expressing CD69 from patients with CAS > 3 was 30 ± 15% (n = 5) compared to 29 ± 7% from patients with CAS < 3 (n = 15). In addition, the relative mean fluorescence intensity of CD69 expressing cells [mean fluorescence intensity (MFI) positive expression/isotype] was similar in control and TAO lymphocytes, indicating that no differences in CD69 antigen-density were detected on positive cells. In contrast to T cells, the fraction of CD14 monocytes expressing CD69 was similar in control and TAO samples (TAO, 81 ± 5%, range 48–100%, n = 22 versus control, 69 ± 5%, n = 12, range 30–100%).
Fig. 1.
A larger fraction of T cells from patients with thyroid-associated ophthalmopathy (TAO) display CD69 than do those from control donors. Representative dot plots demonstrate expression of CD69 using multi-parameter flow cytometry. Peripheral blood mononuclear cells (PBMCs) were isolated and stained with fluorochrome-labelled antibodies against CD3, CD19, CD14 and CD69. Analysis of CD3 positive cells is demonstrated using a live cell gate. Isotype matched control antibodies were used to establish gating parameters such that positive cells constituted less than 0·5% of the quadrant gate for the isotype. The fraction of T cells expressing CD69 is smaller in control donors (7%) compared to TAO (41%).
Fig. 2.
Increased fraction of T cells from patients with thyroid-associated ophthalmopathy (TAO) express CD69. Display of CD69 by CD3+ T cells and CD14+ monocytes was examined by multi-parameter flow cytometry. 30 ± 7% CD3+ T cells from patients with TAO (n = 20, dark bar) express CD69 compared to 14 ± 3% from control donors (n = 10, shaded bar: P < 0·02). A similar fraction of monocytes from TAO patients (81 ± 5%, n = 22) and from control donors (69 ± 5%, n = 12) express CD69.
The fraction of T cells and B cells expressing CD25 is increased in patients with TAO
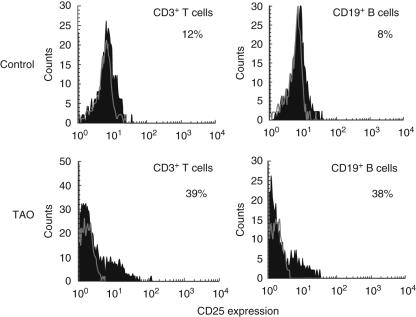
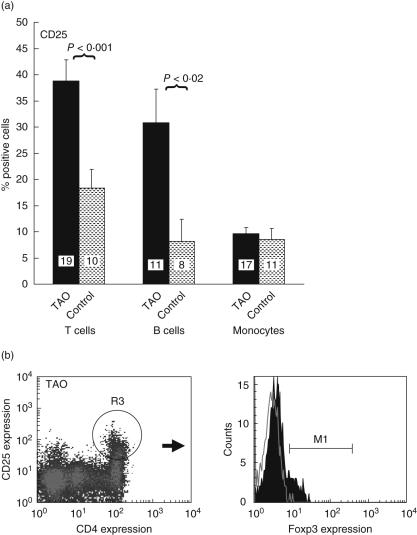
Surface expression of CD25 is up-regulated following T lymphocyte activation and confers responsiveness to interleukin (IL)-2 [10]. It is also a phenotypic marker for regulatory T cells [16]. We therefore investigated the expression of this antigen in patients with TAO. A representativehistogram analysing CD3 T cells and CD19 B cells demonstrates a larger fraction of disease-derived lymphocytes from both lineages constitutively express CD25 (Fig. 3). We then examined PBMCs from several different patients and their control donors (Fig. 4a). With regard to TAO T cells, 38 ± 4% (n = 19) express CD25 compared to control (19 ± 4%, n = 10, P < 0·001). The increased fraction of T cells expressing CD25 was not accounted for by increased regulatory T cells. Figure 4b demonstrates that a relatively large fraction of TAO CD3+ CD4+ T cells express CD25, but relatively few (< 5%) of these T cells express Foxp3 and thus are not regulatory T cells; 31 ± 6% (n = 11) B cells from patients express CD25 compared to controls (11 ± 4%, n = 8, P < 0·02) (Fig. 4a). Expression of CD25 was greater on T cells than on B cells from both control donors and patients. As was the case for CD69, T and B cell expression of CD25 was heterogeneous among patients with TAO (T cells, range 13–95%; B cells, range 10–70%). No correlation could be established between antigen levels in active versus stable orbital disease. The percentage of T cells expressing CD25 from patients with CAS > 3 was 40 ± 12% (n = 5) compared to 38 ± 3% from patients with CAS < 3 (n = 14). Similar expression of CD25 on monocytes was found in TAO and control populations (Fig. 4a, TAO, 10 ± 1% versus control, 9 ± 2%, n.s.).
Fig. 3.
A larger fraction of T cells from patients with thyroid-associated ophthalmopathy (TAO) display CD25 expression. Representative histograms demonstrate expression of CD25 using multiparameter flow cytometry. Peripheral blood mononuclear cells (PBMCs) were isolated and stained with fluorochrome-labelled anti-CD3, CD19, CD14 and CD69 antibodies. A live cell gate was performed and analysis of CD3+ cells is demonstrated. Isotype matched labelled control is shown as an open tracing while CD25 expression is represented by the solid histogram. The fraction of T cells expressing CD25 in TAO T cells (39%) is greater than that from control donors (12%). The fraction of control B cells expressing CD25 is smaller (8%), compared to those from patients with TAO (38%).
Fig. 4.
(a) Increased fraction of T cells from patients with thyroid-associated ophthalmopathy (TAO) express CD25. Expression of CD25 by CD3+ T cells, CD19+ B cells and CD14+ monocytes from control donors and those with TAO was examined by multi-parameter flow cytometry. With regard to T cells, 38 ± 4% from donors with TAO (dark bar, n = 19) express CD25 compared to 19 ± 4% of those from controls (shaded bar, n = 10: P < 0·001). In the case of B cells, 31 ± 6% from TAO (dark bar, n = 11) express CD25 compared to 11 ± 4% of those from controls (shaded bar, n = 8: P < 0·02). Similar fractions of monocytes from patients with TAO (10 ± 1%) and from controls (9 ± 2%) express CD25. (b) An increased fraction of CD3+ CD4+ T cells from patients with TAO express the antigen, but this population does not express forkhead box P3 (Foxp3). CD3+ CD4+ and CD25+ cells are gated as shown (density plot) and minimally express Foxp3 (solid histogram, < 5%).
The fraction of T cells expressing CXCR4 is greater in patients with TAO
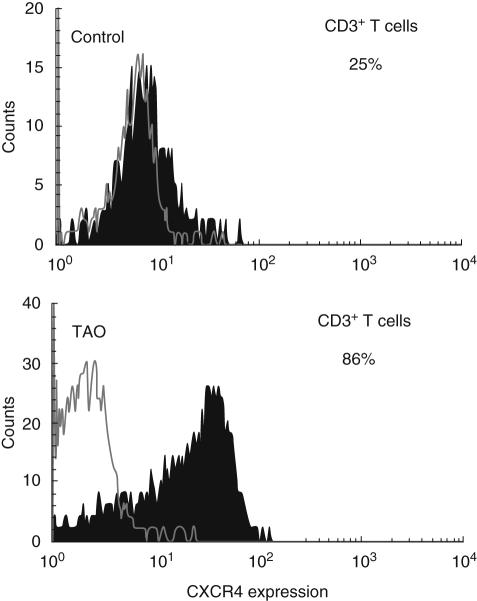
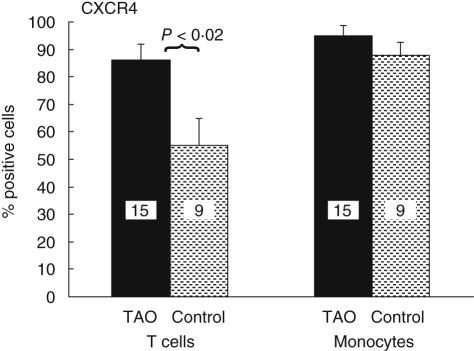
CXCR4 and its ligand CXCL12 (sdf-1) are important factors in B cell development and have been implicated in autoimmune diseases including RA and thyroid autoimmunity [21,22]. CXCL12 is over-expressed in thyroid autoimmune disease. Moreover, CXCR4 overexpression in RA may contribute to local T cell recruitment [23,24]. The fraction of TAO-derived T cells expressing CXCR4 was significantly greater than that from controls, as shown in Figs 5 and 6 (TAO, 86 ± 6% (n = 15) versus control, 55 ± 10% (n = 9, p < 0·02). The fraction of monocytes expressing CXCR4 was similar by TAO and control samples (TAO, 97 ± 3% versus control 96 ± 3%).
Fig. 5.
A greater fraction of T cells from patients with thyroid-associated ophthalmopathy (TAO) display CXCR4 expression. A live cell gate was performed and analysis of CD3+ cells is demonstrated. Isotype-matched labelled control is shown as an open histogram while CXCR4 expression is represented by the solid histogram. CXCR4 is greater in TAO (86%) versus controls (25%).
Fig. 6.
Increased fraction of T cells from patients with TAO express CXCR4. Expression of CXCR4 by CD3+ T cells was determined by multi-parameter flow cytometry. TAO, 86 ± 6% (dark bar, n = 15) versus control, 55 ± 10% (shaded bar, n = 9: P < 0·02).
HLA-DR expression is similar in T cells from donors with TAO and controls
HLA-DR is expressed relatively late in T cell activation and may be critical to sustained antigen presentation [10]. This molecule is displayed abundantly by B cells and monocytes from both TAO and control donors (data not shown). We determined the fraction of T cells expressing HLA-DR to be heterogeneous among donors with TAO. However, the fraction of T cells expressing HLA-DR is similar in control versus those from patients with TAO (TAO, 29 ± 6%, n = 13 versus control, 21 ± 6%, n = 9).
Discussion
Our studies focusing on euthyroid patients with TAO include individuals with active inflammation and others with relatively stable disease. We demonstrate an increased fraction of T cells expressing CD69 in patients with TAO compared to control subjects, regardless of CAS or duration of disease (Fig. 2). CD69 is among the earliest proteins up-regulated following mononuclear cell activation. Its mRNA is detectable within 30 min and protein can be demonstrated on the membrane within 1 h [11]. CD69 is also rapidly down-regulated, and thus is an ideal marker for very early lymphocyte activation in vitro[10]. Contradictory results have emerged from studies conducted in vitro and in vivo regarding the role of CD69 in cell activation. CD69 is not expressed typically by lymphocytes until they become activated through the CD3/TCR complex in vitro. However, absence of CD69 in vivo does not reduce T cell responsiveness [14]. Data from animals with chronic autoimmune conditions including collagen-induced arthritis (CIA) have revealed CD69 mediated inhibitory activity associated with the regulated production of TGF-β in inflammatory lesions [14]. CD69-deficient mice develop more severe CIA than do wild-type mice and the production of TGF- β in the joints of these mutant mice is lower than mice with wild-type CD69 expression [14]. Consistent with functions described elsewhere, TGF-β appears to inhibit inflammation and promote scar formation [25]. CD69 expression and the production of TGF-β appear causally related, as cross-linking CD69 on T cells, NK and macrophages leads to production of that cytokine [26].
The role of CD69 in vivo is as yet undefined, but it may be a marker for T cell subsets which inhibit inflammation through the expression of TGF-β[14]. CD69 is expressed at sites of chronic inflammation, including on T cells isolated from the joints of patients with RA [13]. Previous studies have demonstrated increased CD69 display on T cells in patients with GD but without clinical TAO [20,27]. Those studies included individuals soon after the diagnosis of GD and during treatment with methimazole [27]. The results were compared with age- and sex-matched controls and another group of patients with GD in long-term remission. In those reports, CD69 expression in T cells was increased in hyperthyroid patients and was found to be reduced by methimazole. The level of CD69 expression in patients with long-term remission from hyperthyroidism was similar to that in controls [27]. In a subsequent study, CD69 and HLA-DR display were examined in CD45RA+ (naive) and CD45RA– (memory) CD4+ T cells as a well as in CD8+ T cells from untreated, hyperthyroid patients [20]. Methimazole therapy was found to decrease CD69 display while enhancing HLA-DR expression in CD4+ and CD8+ T cells [20]. In other studies, immunophenotype failed to predict clinical course [28]. None of our patients received either methimazole or propylthiouracil at the time of their participation in the current study. Moreover, their hyperthyroidism had already been treated definitively with either radioactive iodine or surgical thyroidectomy. They had been euthyroid for many months prior to entry into the study. Thus continued CD69 expression in chronic disease states may define a regulatory cell subset, inhibit chronic inflammation, and promote orbital tissue remodelling. These findings may be especially relevant in TAO that is marked by immune infiltration and subsequent scar formation.
Another molecule associated with cell activation is CD25. Following CD69 expression, the low affinity IL-2 receptor becomes displayed and levels remain elevated for many hours [10]. We also report that the fraction of CD25 B cells and T cells from TAO patients is increased relative to control donors. IL-2 is a potent co-stimulator of T cell activation, proliferation and cytokine production. Previous studies have suggested that IL-2 and IL-6 may play significant roles in the development of GD and TAO [29,30]. Hyperthyroid patients have significantly greater numbers of T and B cells expressing CD25 compared to euthyroid patients with GD [31]. In addition, all GD patients displayed greater numbers of B cells bound to IL-2 [29]. These studies did not examine patients with TAO, but suggest a central role for IL-2-mediated functions in GD. In addition to the growth promoting effects of IL-2 receptor expression, a unique subset of CD25+ T cells have been identified which actively regulate immune mediated events and display TCR skewed toward autoreactivity [16,32]. Our finding that a greater fraction of CD25+ T cells are present in TAO compared to control donors may reflect an expansion of this regulatory cell population. Future studies will address their functional role in TAO.
CXCR4 is a chemokine receptor expressed by naive T cells [33]. Antigenic stimulation or T cell differentiation to a memory phenotype might result in the down-regulation of its expression [34,35]. CXCR4 up-regulation has been demonstrated in synovial lymphocytes from joints affected with RA, implying its functional role in lymphocyte recruitment [18,19]. Patients with autoimmune thyroid disease express elevated levels of CXCL12, the ligand for CXCR4 [22]. Ligation of this cognate pair results in the generation of robust chemoattractant activity and CXCL12 has been implicated in B cell and germinal centre development [36]. We report ubiquitous expression of CXCR4 by T cells from patients with TAO, raising the possibility that interplay between the receptor and its ligand may sustain an activated immune state in late stage disease.
Our current results demonstrate a unique profile of peripheral blood T and B cell phenotypes in euthyroid patients with TAO. We postulate that these lymphocytes may reflect site-specific immune interactions in the orbit. GD orbital fibroblasts display characteristic responses to proinflammatory cytokines and biosynthetic attributes including increased expression of lymphocyte co-stimulatory molecules such as CD40 [37–39]. The interaction of the T and B cells with GD orbital fibroblasts may form the basis for site-specific disease. Thus, characterizing lymphocyte populations in TAO should represent an important step toward identifying molecular interactions driving orbital disease.
Acknowledgments
The authors are grateful to Ms Debbie Hanaya for her expert assistance in the preparation of this manuscript. This work was funded in part by grants EY016339 (to R. S. D), RR017304 (to A. G. G.), EY008976, EY011708, DK063121 (to T. J. S) and RR00425 from the National Institutes of Health. The authors gratefully acknowledge the continued support of Steve and Kathleen Flynn and the Bell Charitable Foundation.
References
- 1.Weetman AP. Graves' disease. New Engl J Med. 2000;343:1236–48. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 2.de Carli M, D'Elios MM, Mariotti S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves' ophthalmopathy. J Clin Endocrinol Metab. 1994;77:1120–4. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]
- 3.Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves' ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93:2738–43. doi: 10.1172/JCI117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TJ, Hoa N. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–80. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 5.Smith TJ. Unique properties of orbital connective tissue underlie its involvement in Graves' disease. Minerva Endocrinol. 2003;28:213–22. [PubMed] [Google Scholar]
- 6.Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12:197–203. doi: 10.1089/105072502753600133. [DOI] [PubMed] [Google Scholar]
- 7.Smith TJ, Parikh SJ. HMC-1 mast cells activate human orbital fibroblasts in coculture: evidence for up-regulation of prostaglandin E2 and hyaluronan synthesis. Endocrinology. 1999;140:3518–25. doi: 10.1210/endo.140.8.6881. [DOI] [PubMed] [Google Scholar]
- 8.Young DA, Evans CH, Smith TJ. Leukoregulin induction of protein expression in human orbital fibroblasts: evidence for anatomical site-restricted cytokine–target cell interactions. Proc Natl Acad Sci USA. 1998;95:8904–9. doi: 10.1073/pnas.95.15.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aust G, Lehmann I, Heberling HJ. Different immunophenotype and autoantibody production by peripheral blood and thyroid-derived lymphocytes in patients with Graves' disease. Exp Clin Endocrinol Diab. 1996;104:50–8. doi: 10.1055/s-0029-1211422. [DOI] [PubMed] [Google Scholar]
- 10.Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Meth. 2004;293:127–42. doi: 10.1016/j.jim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Testi R, Phillips JH, Lanier LL. T cell activation via Leu-23 (CD69) J Immunol. 1989;143:1123–8. [PubMed] [Google Scholar]
- 12.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–60. [PubMed] [Google Scholar]
- 13.Laffon A, Garcia-Vicuna R, Humbria A, et al. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991;88:546–52. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sancho D, Gomez M, Viedma F, et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J Clin Invest. 2003;112:872–82. doi: 10.1172/JCI19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson AL, Matsumoto H, Janszen M, Maino V, Blidy A, Shye S. Restricted expression of p55 interleukin 2 receptor (CD25) on normal T cells. Clin Immunol Immunopathol. 1990;54:126–33. doi: 10.1016/0090-1229(90)90012-f. [DOI] [PubMed] [Google Scholar]
- 16.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–42. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Tomkinson BE, Wagner DK, Nelson DL, Sullivan JL. Activated lymphocytes during acute Epstein–Barr virus infection. J Immunol. 1987;139:3802–7. [PubMed] [Google Scholar]
- 18.Haas CS, Martinez RJ, Attia N, Haines GK, III, Campbell PL, Koch AE. Chemokine receptor expression in rat adjuvant-induced arthritis. Arthritis Rheum. 2005;52:3718–30. doi: 10.1002/art.21476. [DOI] [PubMed] [Google Scholar]
- 19.Haringman JJ, Smeets TJ, Reinders-Blankert P, Tak PP. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis and reactive arthritis. Ann Rheum Dis. 65:294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gessl A, Waldhausl W. Elevated CD69 expression on naive peripheral blood T-cells in hyperthyroid Graves' disease and autoimmune thyroiditis. discordant effect of methimazole on HLA-DR and CD69. Clin Immunol Immunopathol. 1998;87:168–75. doi: 10.1006/clin.1998.4524. [DOI] [PubMed] [Google Scholar]
- 21.De Klerck B, Geboes L, Hatse S, et al. Pro-inflammatory properties of stromal cell-derived factor-1 (CXCL12) in collagen-induced arthritis. Arthritis Res Ther. 2005;7:R1208–20. doi: 10.1186/ar1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradfield PF, Amft N, Vernon-Wilson E, et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–82. doi: 10.1002/art.11219. [DOI] [PubMed] [Google Scholar]
- 23.Aust G, Sittig D, Steinert M, Lamesch P, Lohmann T. Graves' disease is associated with an altered CXCR3 and CCR5 expression in thyroid-derived compared to peripheral blood lymphocytes. Clin Exp Immunol. 2002;127:479–85. doi: 10.1046/j.1365-2249.2002.01778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aust G, Steinert M, Kiessling S, Kamprad M, Simchen C. Reduced expression of stromal-derived factor 1 in autonomous thyroid adenomas and its regulation in thyroid-derived cells. J Clin Endocrinol Metab. 2001;86:3368–76. doi: 10.1210/jcem.86.7.7654. [DOI] [PubMed] [Google Scholar]
- 25.Fontana A, Constam DB, Frei K, Malipiero U, Pfister HW. Modulation of the immune response by transforming growth factor beta. Int Arch Allergy Immunol. 1992;99:1–7. doi: 10.1159/000236328. [DOI] [PubMed] [Google Scholar]
- 26.Esplugues E, Sancho D, Vega-Ramos J, et al. Enhanced antitumor immunity in mice deficient in CD69. J Exp Med. 2003;197:1093–106. doi: 10.1084/jem.20021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corrales JJ, Lopez A, Ciudad J, Mories MT, Miralles JM, Orfao A. Methimazole therapy in Graves' disease influences the abnormal expression of CD69 (early activation antigen) on T cells. J Endocrinol. 1997;155:491–500. doi: 10.1677/joe.0.1550491. [DOI] [PubMed] [Google Scholar]
- 28.Corrales JJ, Lopez A, Ciudad J, Orfao A. The distribution of the major peripheral blood T, B and NK cell subsets does not predict the clinical outcome of Graves' disease patients after methimazole therapy. J Biol Regul Homeost Agents. 2000;14:193–9. [PubMed] [Google Scholar]
- 29.Corrales JJ, Orfao A, Lopez A, Mories MT, Miralles JM, Ciudad J. Analysis of IL-2 and IL-6 binding to peripheral blood lymphocytes in Graves disease: relationship with disease activity. Cytometry. 1997;30:118–23. doi: 10.1002/(sici)1097-0320(19970615)30:3<118::aid-cyto2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Chen B, Tsui S, Smith T. IL-1β induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotype attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005;175:1310–19. doi: 10.4049/jimmunol.175.2.1310. [DOI] [PubMed] [Google Scholar]
- 31.Gessl A, Wilfing A, Agis H, et al. Activated naive CD4+ peripheral blood T cells in autoimmune thyroid disease. Thyroid. 1995;5:117–25. doi: 10.1089/thy.1995.5.117. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Sebastiani S, Allavena P, Albanesi C, et al. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- 34.Abbal C, Jourdan P, Hori T, Bousquet J, Yssel H, Pene J. TCR-mediated activation of allergen-specific CD45RO(+) memory T lymphocytes results in down-regulation of cell-surface CXCR4 expression and a strongly reduced capacity to migrate in response to stromal cell-derived factor-1. Int Immunol. 1999;11:1451–62. doi: 10.1093/intimm/11.9.1451. [DOI] [PubMed] [Google Scholar]
- 35.Bermejo M, Martin-Serrano J, Oberlin E, et al. Activation of blood T lymphocytes down-regulates CXCR4 expression and interferes with propagation of X4 HIV strains. Eur J Immunol. 1998;28:3192–204. doi: 10.1002/(SICI)1521-4141(199810)28:10<3192::AID-IMMU3192>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Honczarenko M, Le Y, Glodek AM, et al. CCR5-binding chemokines modulate CXCL12 (SDF-1)-induced responses of progenitor B cells in human bone marrow through heterologous desensitization of the CXCR4 chemokine receptor. Blood. 2002;100:2321–9. doi: 10.1182/blood-2002-01-0248. [DOI] [PubMed] [Google Scholar]
- 37.Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273:29615–25. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 38.Smith TJ, Sciaky D, Phipps RP, Jennings TA. CD40 expression in human thyroid tissue: evidence for involvement of multiple cell types in autoimmune and neoplastic diseases. Thyroid. 1999;9:749–55. doi: 10.1089/thy.1999.9.749. [DOI] [PubMed] [Google Scholar]
- 39.Smith TJ, Sempowski GD, Berenson CS, Cao HJ, Wang HS, Phipps RP. Human thyroid fibroblasts exhibit a distinctive phenotype in culture: characteristic ganglioside profile and functional CD40 expression. Endocrinology. 1997;138:5576–88. doi: 10.1210/endo.138.12.5563. [DOI] [PubMed] [Google Scholar]