Abstract
Interleukin (IL)-6 is an inflammatory mediator involved in bone resorption. G/C polymorphism at position −174 of the IL-6 gene has been reported to influence IL-6 expression, with the G allele associated with higher expression levels. The aims of this study were to investigate the expression of IL-6 as well as the incidence of IL-6 (−174) gene polymorphism and their correlation to the severity of periodontitis in Brazilians. Peripheral blood mononuclear cells were collected from 12 non-smoker individuals with periodontitis for evaluation of IL-6 expression using flow cytometry. We observed a positive correlation between the mean clinical attachment loss and intensity of expression of IL-6, in which the greater the attachment loss, the higher the expression of IL-6 (P= 0·007, R2 = 0·52). Also, patients with severe periodontitis displayed a higher intensity of IL-6 expression compared to moderate periodontitis (P= 0·04). To determine the occurrence of IL-6 gene polymorphism, DNA was obtained from oral swabs of 209 Brazilian individuals with and without periodontitis. Polymerase chain reaction, restriction endonuclease digestion and electrophoresis were performed, allowing for detection of the IL-6 (−174) polymorphism. We observed that non-smokers with moderate periodontitis (P= 0·05) and control (P= 0·04) groups displayed a higher incidence of the G– genotype when compared to severe periodontitis. This suggests that the G– genotype may represent a protective role in severity of periodontitis. Thus, the increased expression of IL-6 and IL-6 (−174) polymorphism are associated with periodontal disease severity in Brazilian individuals.
Keywords: cytokine, IL-6, periodontitis, polymorphism, severity
Introduction
Interleukin (IL)-6 is a pleiotropic cytokine produced by many cell types such as stimulated monocytes, fibroblasts, endothelial cells and T and B lymphocytes [1]. It is not expressed constitutively, but is highly inducible and is produced in response to a number of inflammatory stimuli such as IL-1, tumour necrosis factor (TNF)-α, bacterial products and viral infection [2]. This cytokine has diverse functions, including differentiation and/or activation of macrophages and T cells, growth and differentiation of B cells, stimulation of haematopoiesis and neural differentiation [2]. IL-6 is also a potent stimulator of osteoclast differentiation and bone resorption [3] and an inhibitor of bone formation [4].
IL-6 is implicated in the pathology of several diseases, such as Sjögren's syndrome [5], Alzheimer's disease [6], systemic onset juvenile chronic arthritis [7] and psoriasis [8]. With regard to periodontitis, IL-6 is expressed by a variety of cells in the periodontal lesion and, in common with IL-1 and TNF-α, enhances bone resorption [9,10]. Elevated levels of IL-1β and IL-6 have been shown to be induced by periodontal pathogens and are correlated with the continuous tissue destruction observed in periodontitis [11].
A number of parameters may influence cytokine secretion levels, including the producing cell type, the nature of the stimulus and the genetic background of the source cells [12]. Polymorphisms in the promoter region of the IL-6 gene may result in interindividual variation in transcription and expression of this cytokine [2]. A single nucleotide polymorphism, G/C (−174), located within the IL-6 promoter, has been reported to influence IL-6 expression, with the G allele being associated with higher expression levels [7]. GG and GC individuals have been shown to have higher IL-6 levels in plasma, higher IL-6 gene transcriptional activity and higher inducible IL-6 responses when compared to CC individuals [5,7]. This polymorphism has been associated with several diseases including systemic onset juvenile chronic arthritis [7], aggressive breast cancer [13] and Alzheimer's disease [14]. Few studies have evaluated IL-6 (−174) polymorphism in individuals with periodontitis. Trevilatto et al. [15] have demonstrated that the genotype GG may be associated with chronic periodontitis (CP) in a Caucasian Brazilian population and D'Aiuto et al. [16] observed that, in patients from London with severe periodontal infections, increased soluble IL-6 was associated with the presence of the C allele.
Because IL-6 is a crucial cytokine for the regulation of bone metabolism [17] and the frequency of many genetic alleles varies between ethnic groups [18], the aims of this study were to investigate the association of phenotypic expression of this cytokine and of the IL-6 (−174) gene polymorphism with the severity of periodontitis in Brazilians. This study demonstrated the existence of a correlation between both IL-6 phenotypic expression and IL-6 gene polymorphism with severity of periodontitis.
Materials and methods
Patients
The study employed a cross-sectional design involving individuals from the State of Minas Gerais from the South-eastern region of Brazil. We performed our initial sample size calculation using the genotype frequency published by Trevilatto et al. [15], using an equal number of case and control individuals. Thus, using a control frequency of 0·33 (for the G/G genotype) and severe periodontitis group frequency of 0·71 (for the G/G genotype), an alpha of 0·05 and power of 0·98, we obtained a suggested sample size of a total of 120 individuals (combining severe periodontitis and control groups). A total of 209 patients receiving treatment at the Dentistry School, Federal University of Minas Gerais, were included in this study. Diagnosis of disease was made considering the patient's medical and dental histories, radiographic findings and observation of clinical signs and parameters including probing depth, assessment of clinical attachment loss (CAL), observation of tooth mobility, bleeding on probing and presence of plaque/calculus. Measurements of probing depth and CAL were assessed at six locations around each tooth. The severity of disease was characterized on the basis of the mean of CAL. Assessment of CAL was performed by insertion of a periodontal probe in the gingival sulcus, and the measurement corresponding to the distance from the cemento–enamel junction to the location of a periodontal probe tip was defined as CAL. Results were expressed as mean CAL; that is, the average of CAL in all six sites of the affected teeth. Patients exhibiting mean CAL ≥ 6 mm were considered to have severe periodontitis and those exhibiting 3 mm ≤ CAL > 6 mm were considered to have moderate periodontitis, following the criteria used previously by Holla et al. [19]. At the time of sample collection, healthy control individuals included in this study did not have periodontal disease, as determined by the absence of CAL and no sites with probing depth > 3mm. Moreover, upon questionnaire and clinical evaluation, no control individuals had a history of periodontal disease.
After clinical diagnosis of periodontitis and evaluation of severity of disease, a sample of 12 non-smokers individuals was selected for phenotypic analysis of IL-6 expression by peripheral blood mononuclear cells (PBMC). Considering the severity of disease, these patients were stratified into two groups: subjects with moderate periodontitis (MP, n = 6) and subjects with severe periodontitis (SP, n = 6). In order to perform genotypic analysis, a sample of 209 patients was selected and an oral swab was collected from each patient. The patients were stratified into three groups: subjects with moderate periodontitis (MP, n = 88), subjects with severe periodontitis (SP, n = 67) and healthy volunteers as the control group (C, n = 54).
A questionnaire was completed by all individuals enrolled in this study in order to obtain information regarding dental history, family history of periodontal disease, smoking habit and general health concerns. Use of orthodontic appliances, chronic use of anti-inflammatory drugs, history of diabetes, hepatitis or HIV infection, immunosuppressive chemotherapy, bleeding disorders, severely compromised immune function, pregnancy or lactation were regarded as exclusion criteria. Except for the presence of periodontitis, the patients included in this study were systemically healthy. Because tobacco smoking is an important risk factor for periodontitis, smoking was also included in our data analysis. ‘Smokers’ were defined as current smokers/former smokers (more than 10 cigarettes/day) and ‘non-smokers’ included individuals who had never smoked. Table 1 summarizes the patient data, as well as their classification into different groups.
Table 1.
Characteristics of the study groups
| Clinical forms | Severe periodontitis | Moderate periodontitis | Healthy controls |
|---|---|---|---|
| Number of individuals (n) | 67 | 88 | 54 |
| Gender | |||
| Male (%) | 27 (40·3) | 22 (25) | 23 (42·6) |
| Female (%) | 40 (59·7) | 66 (75) | 31 (57·4) |
| Age range (years) | 15–59 | 15–67 | 19–70 |
| Mean ± s.d. (years) | 36·9 ± 12·4 | 40·2 ± 11·6 | 29·4 ± 9·6 |
| Smoking status | |||
| Non-smokers (%) | 47 (70·2) | 59 (67) | 52 (96·3) |
| Smokers (%) | 20 (29·8) | 29 (33) | 2 (3·7) |
This study was approved by Universidade Federal de Minas Gerais's Ethics Committee (numbers 003/03 and 132/00) and signed informed consent was obtained from all participants.
Preparation of PBMC for flow cytometric analysis
Blood samples (20 ml) were collected from 12 non-smoker patients with periodontitis by puncture of the peripheral vein into vaccuntainer tubes containing heparin. PBMC were obtained by separating blood cells over a Ficoll gradient, as performed previously by us [20]. Briefly, blood was diluted 1 : 2 with phosphate-buffered saline (PBS), applied over a Ficoll gradient, centrifuged, and the mononuclear ring was collected. Cells were then washed and counted. A concentration of 107 cells/ml diluted in RPMI-1640 was used in all flow cytometry analyses to evaluate the expression of IL-6.
Analysis of expression of IL-6 by flow cytometry
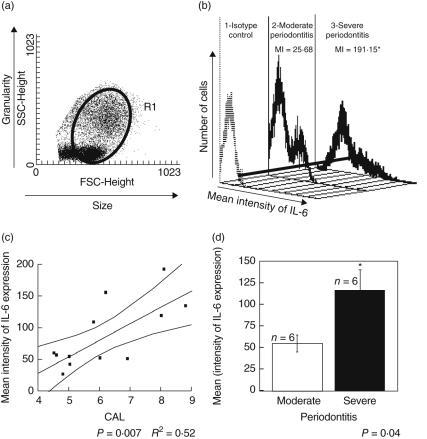
We analysed the expression of cytokine IL-6 in PBMC isolated from patients using flow cytometry, as performed previously by us [20]. Briefly, 2 × 105 cells were incubated with α-CD3/CD28 polyclonal stimulus in RPMI-1640 for approximately 18 h. The use of α-CD3/CD28 leads to amplification of cytokine secretion by activating the T cells, allowing for cytometric detection. During the last 4 h of activation, brefeldin A (1 µg/ml) was added to the cultures in order to avoid protein secretion by the cells. Cells were harvested and, after fixation in a 2% formaldehyde solution for 20 min at room temperature, washed in PBS pH 7·2 and permeabilized by incubation for 10 min with a 0·5% saponin solution. Permeabilized cells were incubated with phycoerythrin (PE)-labelled anti-IL-6 monoclonal antibody for 20 min. Samples were washed, suspended in PBS and analysed using a fluorescence activated cell sorter (FACS) (Vantage; Becton-Dickinson, San Jose, CA, USA). For all samples we collected 20 000 cells and analysed a minimum of 5000 events/staining, selecting the gate that included lymphocytes and monocytes (Fig. 1a). The PE-labelled anti-IL-6 monoclonal antibody was purchased from Caltag (Carlsbad, CA, USA). PE-labelled isotype control, also from Caltag, was added to the staining protocol for each patient.
Fig. 1.
Analysis of interleukin (IL)-6 expression by flow cytometry, considering the severity of periodontal disease. (a) Representative dot-plot of peripheral blood mononuclear cells (PBMC) distribution considering the size (forward scatter, x-axis) and granularity (side scatter, y-axis). R1 = representative gate of the region containing lymphocytes and monocytes used for flow cytometric analysis. (b) Representative histogram of individuals with moderate and severe periodontitis considering the mean intensity of IL-6 and number of cells. Histogram 1 shows an isotype control, histogram 2 shows the mean intensity (MI) of IL-6 in an individual with moderate periodontitis (MI = 25·68) and histogram 3 shows the mean intensity of IL-6 in an individual with severe periodontitis (MI = 191·15). (c) Positive correlation between the intensity of IL-6 expression and clinical attachment loss (CAL) (P= 0·007; R2 = 0·52). (d) Comparison between individuals with moderate periodontitis and severe periodontitis in regards to expression of IL-6 (P= 0·04). White bars are representative of individuals with moderate periodontitis and black bars are representative of individuals with severe periodontitis. *Statistical difference with P = 0·04.
Collection of oral swab and DNA extraction
Cells from 209 individuals were obtained through an oral swab performed with a sterile plastic spatula. After gentle scraping of oral mucosa, the tip of the spatula was immersed in 2 ml sterile microtubes containing 1500 µl of Krebs buffer (NaCl 20%, KCl 2%, CaCl2,2%, H2O 2%, MgSO4, KH2PO4, C6H12O6). DNA extraction was performed as described by Boom et al. [21] and modified as follows. A pellet of epithelial cells was obtained by centrifugation at 200 g for 5 min. The supernatant was removed and 20 µl of silica (SiO2; Sigma, St. Louis, MO, USA) and 450 µl of lysis buffer (6·0 M GuSCN, 65 mM Tris-HCl pH = 6·4, 25 mM ethylenediamine tetraacetic acid (EDTA) and 1·5% Triton X-100) were added. Samples were homogeneized by using a vortex and incubated for 30 min at 56°C. After this incubation, samples were submitted to another centrifugation and the supernatant was discharged. The pellet obtained (with DNA adsorbed on the silica) was washed twice with 450 µl washing buffer (6·0 M GuSCN, 65 mM Tris-HCl pH = 6·4), twice with 450 µl of 70% ethanol, once with 450 µl acetone and dried at 56°C for 20 min. Finally, 100 µl of TE buffer (10 mM Tris-HCl pH = 8·0 and 1 mM EDTA) was added and incubated at 56°C for 12 h. After incubation, the solution was homogenized, centrifuged and the supernatant containing DNA was obtained.
Polymerase chain reaction (PCR) and restriction endonuclease digestion
IL-6 (−174) polymorphism was assessed by PCR amplification and digestion. The primers used were 5′-CAGAAGAACTCAGATGACTG-3′ and 5′-GTGGGGCTGATTGGAAACC-3′ with product size of 431 base pairs (bp), as described previously [1]. PCR was carried out in a total volume of 50 µl, containing 10 µl of solution DNA, pre-mixed buffer (50 mM KCl, 10 mM Tris-HCl pH = 8·4, 0·1% Triton X-100, 1·5 mM MgCl2, deoxynucleotide triphosphates, Taq DNA polymerase) and primers (20 pmol/reaction). The amplification conditions consisted of 94°C for 3 min followed by 35 cycles of 94°C for 30 s, 56°C for 35 s and 72°C for 30 s. The run was terminated by final elongation at 72°C for 5 min. The products were digested with 6 units of Hsp92II at 37°C for 4 h and digestion products of 229 + 122 + 51 + 29 bp and 229 + 173 + 29 bp were obtained for the C and G alleles, respectively. The visualization was performed in a 2% high-resolution agarose gel electrophoresis, stained with ethidium bromide.
Statistical analysis
FACS data were analysed using the CellQuest software. Non-parametric analysis of variance and the Tukey–Kramer test from SAS (jmp software) was applied to ascertain statistically significant differences between the groups under comparison. Comparisons that had a P-value < 0·05 were considered statistically significant.
Genotypic data were analysed using the jmp statistical software (SAS). The χ2 likelihood ratio test was used to compare the genotypes distributions between the C and MP groups, the C and SP groups and the MP and SP groups [3 × 2 contingency table, degrees of freedom (d.f.) = 2]. The G/C allele and G+/G– genotype distribution between the C and MP groups, the C and SP groups and the MP and SP groups were assessed in 2 × 2 contingency table (d.f. = 1). A one-tailed Fisher's exact test was performed when a value of less than 5 occurred. The study groups were tested for the Hardy–Weinberg equilibrium comparing the expected with the observed genotypes frequencies. To exclude the possible confounding effect of smoking, in a second analysis we excluded smokers from all the different clinical groups. Although smoker individuals were excluded from the analysis, the remaining number of controls and severe periodontitis subjects (99 individuals) allows for a statistical power of 0·96. A P-value < 0·05 was considered significant.
Results
Intensity of expression of IL-6 by PBMC is correlated with CAL
To determine if an association existed between the intensity of IL-6 production and periodontal disease severity, IL-6 expression was determined by flow cytometry from 12 individuals with moderate to severe CAL. The results showed a positive correlation between the mean CAL and intensity of IL-6 expression by PBMC (P= 0·007, R2 = 0·52; Fig. 1c). No correlation was observed when we compared the percentage of cells expressing IL-6 and the mean CAL (data not shown). We also observed that the severe periodontitis group displayed a higher mean intensity of IL-6 expression, as measured by mean intensity of fluorescence in the FACS analysis, when compared with the moderate periodontitis group (P= 0·04; Fig. 1d). This higher intensity of IL-6 expression is observed clearly in the representative histogram of the mean intensity (MI, Fig. 1b) from an individual with moderate periodontitis (MI = 25·68) and from an individual with severe periodontitis (MI = 191·15).
Non-smoker individuals with moderate periodontitis display higher incidence of G– genotype
Once the expression of IL-6 was correlated to severity of periodontal disease, we sought to investigate if the severity of disease is associated to the occurrence of a functional genetic polymorphism. To determine if an association existed between the IL-6 (−174) functional polymorphism and disease severity, PCR, restriction endonuclease digestion and electrophoresis were performed from a sample of 209 individuals with moderate to severe CAL and individuals without periodontitis. The evaluation of the genotype and allele distributions were performed comparing individuals with periodontitis and individuals without disease, taking smoking and non-smoking into consideration. With regard to genotype distribution in non-smokers, a statistical difference was observed between the groups with severe and moderate periodontitis (P= 0·05, χ2 = 6·14), as well as between the groups with severe periodontitis and control (P= 0·04, χ2 = 6·68) (Table 2). We observed that individuals with severe periodontitis displayed a lower frequency of CC when compared to moderate periodontitis and control groups. No significant difference in the allele distribution was observed among groups, taking smoking and non-smoking into consideration (Table 3).
Table 2.
Distribution of the IL-6 (−174) genotypes in the study groups.*
| Healthy controls | Severe periodontitis | Moderate periodontitis | |
|---|---|---|---|
| Non-smokers | |||
| GG (%) | 30 (57·7) | 30 (63·8) | 36 (61) |
| GC (%) | 17 (32·7) | 17 (36·2) | 18 (30·5) |
| CC (%) | 5 (9·6) | 0 (0) | 5 (8·5) |
| Non-smokers + smokers | |||
| GG (%) | 30 (55·6) | 40 (59·7) | 58 (65·9) |
| GC (%) | 18 (33·3) | 24 (35·8) | 24 (27·3) |
| CC (%) | 6 (11·1) | 3 (4·5) | 6 (6·8) |
Statistical analysis (3 × 2 contingency table): non-smokers: P = 0·046, χ2 = 6·14 (likelihood ratio χ2 of severe periodontitis versus moderate periodontitis). P= 0·036, χ2 = 6·68 (likelihood ratio χ2 of severe periodontitis versus control).
Table 3.
Distribution of the IL-6 (−174) alleles in the study groups.*
| Healthy controls | Severe periodontitis | Moderate periodontitis | |
|---|---|---|---|
| Non-smokers | |||
| G (%) | 77 (74) | 77 (81·9) | 90 (76·3) |
| C (%) | 27 (26) | 17 (18·1) | 28 (23·7) |
| Non-smokers + smokers | |||
| G (%) | 78 (72·2) | 104 (77·6) | 140 (79·5) |
| C (%) | 30 (27·8) | 30 (22·4) | 36 (20·5) |
No statistical difference was observed (2 × 2 contingency table).
The presence of the IL-6 high-producer allele in the population, as evaluated by the frequency of G+ individuals, showed that the frequency of G+ versus G– individuals between the groups was significantly different when analysing non-smokers, but not when smokers were included (Table 4). We observed that non-smokers with moderate periodontitis, and also the control group, displayed a higher incidence of the G– genotype when compared to individuals with severe periodontitis (P= 0·05 and P = 0·04, respectively) (Table 4).
Table 4.
Distribution of the IL-6 (−174) genotypes in the study groups, considering the G+/G– genotypes.*
| Healthy controls | Severe periodontitis | Moderate periodontitis | |
|---|---|---|---|
| Non-smokers | |||
| G+ (%) | 47 (90·4) | 47 (100) | 54 (91·5) |
| G– (%) | 5 (9·6) | – | 5 (8·5) |
| Non-smokers + smokers | |||
| G+ (%) | 48 (88·9) | 64 (95·5) | 82 (93·2) |
| G– (%) | 6 (11·1) | 3 (4·5) | 6 (6·8) |
Statistical analysis (Fisher's exact test): non-smokers: P = 0·049 (severe periodontitis versus moderate periodontitis). P= 0·036 (severe periodontitis versus control).
Discussion
Periodontitis is an inflammatory disease, which is a major cause of tooth loss in adults [22]. Cytokines play a central role in the inflammatory process associated with the destruction in periodontitis and the nature of the host immune response to the components of the plaque varies significantly between individuals [12].
In our study, we evaluated the expression of IL-6 in nonsmoker Brazilians with periodontitis, considering the severity of disease. We observed a positive correlation between intensity of IL-6 expression and mean CAL in individuals with periodontitis. Furthermore, we observed that individuals with severe periodontitis displayed a higher intensity of IL-6 expression when compared to individuals with moderate periodontitis. Our data suggest that the severity of periodontitis may be associated with increased levels of IL-6. We used anti-CD3 and anti-CD28 as a polyclonal stimulus in order to amplify cytokine expression by all the activated cells, optimizing detection as perfomed extensively (reviewed by Gauduin [23]). It has been postulated that high-responder individuals produce high levels of inflammatory mediators and cytokines as part of their host inflammatory response to the presence of plaque [12,24] and, consequently, these individuals are more susceptible to periodontitis or severity of disease. Chen et al. [25] reported that the IL-1 and IL-6 are increased in CP, and there was a positive correlation between both mediators and clinical assessments of periodontal destruction. Evidence has indicated that patients with severe periodontitis have a perturbation of their systemic inflammatory status manifested by increased serum levels of IL-6, IL-1 and C-reactive protein when compared with unaffected populations [16]. Moreover, a decrease in serum C-reactive protein and IL-6 following successful periodontal therapy indicates an association between periodontal infections and the observed increased levels of serum inflammatory mediators [26–28].
While microbial and environmental factors are believed to initiate and modulate disease progression, evidence supports that genes play a role in the predisposition to and progression of periodontitis [29]. Studies have reported associations between cytokine gene polymorphisms and periodontitis in distinct populations [15,30–32], and contradictory results have been found among different ethnic groups, even in the same country [18]. Pontes et al. [22] concluded that IL-4 gene polymorphisms were not related to periodontitis susceptibility in the African American Brazilian population, while Scarel-Caminaga et al. [33] observed that the IL-4 gene was not associated with susceptibility to CP in Caucasoid, African American, Mulatto and Asian Brazilians. Few other studies have evaluated cytokine gene polymorphism in Brazilian individuals with periodontitis. It has been shown that specific haplotypes and single nucleotide polymorphisms (SNPs) in IL-10 and the IL-1B (+3954) gene polymorphisms are associated with susceptibility to CP [34,35]. Considering that little is known about the genetic basis of susceptibility to periodontitis in the Brazilian population, analysis of polymorphisms in Brazilian individuals represents important information.
Because of the association observed between the high expression on IL-6 and severity of periodontitis, we evaluated the association of the IL-6 (−174) polymorphism with severity of periodontitis in Brazilian individuals. We observed that an allele distribution was similar among groups not showing an association between IL-6 (−174) gene polymorphism and severity of periodontitis. However, a lower incidence of CC genotype was observed in the severe periodontitis group when compared to the moderate periodontitis group and also to the control groups. These results suggest that the CC genotype may display a protector role in relation to the severity of periodontitis. In the literature, associations between this polymorphism and clinical forms of periodontitis are contradictory. An association was not observed between this polymorphism with CP in Japanese descent and in Czech patients [36,37]. However, an association of the GG genotype with CP in the Brazilian population has been observed [15]. The explanation for these different results may be due to the analysis of different ethnic groups. Although our purpose was not to evaluate the occurrence of polymorphism with different clinical forms of disease, we also grouped individuals into chronic and aggressive periodontitis groups and did not see an association between the IL-6 polymorphism with different forms of the disease (data not shown).
In our study, organization of the sample into ethnic groups was not performed due to the strong miscegenation present among Brazilians. Parra et al. [38] does not recommend grouping Brazilians into ethnic groups based on colour and other physical characteristics associated with racial divisions. Brazilian individuals classified as ‘white’ or ‘black’ have significantly overlapping genotypes considering race-associated loci for Caucasians, Africans and Indigenous people, due probably to miscegenation [38]. In the present study, all the patients and controls were selected from the same geographical area and were of the same socio-economic status. Thus, individuals analysed from Minas Gerais State are as representative as possible of the Brazilian population, as shown by Parra et al. [38].
With regard to allele distribution, we observed that the C allele frequency in the control group was 28·6%, while the frequency of the same allele reported in literature was 33–55% in Caucasians, 9% in African Americans, 5% in Afro-Caribbeans and less than 1% in Eastern Asians [39]. These data show that the allelic distribution may vary among ethnic groups, and emphasize the importance of the investigation of polymorphisms in different populations.
When we evaluated the severity of periodontitis stratifying the groups according to mean CAL, we observed that non-smokers with severe periodontitis displayed only the G+ genotype. The same was not observed in non-smokers with moderate periodontitis and controls, where a frequency of 8·5% and 9·6% of the G– genotype was observed, respectively. This result suggests that the presence of G+ genotype may be associated with severity, while the G– genotype may represent a protective function. This suggestion is based on the fact that the presence of the G allele has been associated with higher expression levels of IL-6 when compared with the C allele [7]. In our study we observed that genetic association with severity of periodontitis was evident only when smokers were excluded from the study group, confirming the importance of this risk factor, and suggesting that its effect is strong even in subjects who are not genetically susceptible to disease. These data suggest that the smoking-related risk may obscure the polymorphism-related risk, as described previously [31].
Although it was not possible to make a direct comparison between the IL-6 (−174) polymorphism and phenotypic expression due to the small number of individuals evaluated in our phenotypic analysis, our data show that increased expression of IL-6 and the polymorphism studied may be involved with the severity of periodontitis. However, this is contradictory to the findings of D'Aiuto et al. [28], who observed that, in patients with severe periodontal infections, higher serum IL-6 levels were associated with the carriage of allele C for the IL-6 (−174) polymorphism. One possible explanation for this is based on the fact that differences in the IL-6 promoter haplotype may have an important role in determining levels of transcription for the IL-6 gene [2]. Terry et al. [2] have reported that the transcriptional control of this gene is complex, and subtle variations in the promoter influence the regulation of the system.
Besides the −174 polymorphism, other polymorphisms in the IL-6 gene have been evaluated in periodontitis. The IL-6-373 A9T11 allele was associated with a reduced susceptibility to CP among Japanese subjects and decreased IL-6 serum levels [36]. The −572 C allele was suggested to be a protective factor against CP in the Czech population [37]. Study of other functional polymorphisms in the IL-6 gene will contribute to a better understanding of the pathogenesis of periodontitis providing information that may have diagnostic and therapeutic value in the future.
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Brazil.
References
- 1.Klein W, Tromm A, Griga T, et al. The polymorphism at position −174 of the IL-6 gene is not associated with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2001;13:45–7. doi: 10.1097/00042737-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Terry CT, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;24:18138–44. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 3.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol 2000. 1997;14:112–43. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 4.Hughes FJ, Howells GL. Interleukin-6 inhibits bone formation in vitro. Bone Miner. 1993;21:21–8. doi: 10.1016/s0169-6009(08)80117-1. [DOI] [PubMed] [Google Scholar]
- 5.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL-6 gene in primary Sjögren's syndrome and correlate with the clinical manifestations of the disease. Rheumatology. 2001;40:656–61. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 6.Licastro F, Pedrini S, Caputo L, et al. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 7.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman R, Krueger J, Yourish D, et al. Interleukin-6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367–71. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin CR, Myrillas TT. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998;4:43–7. doi: 10.1111/j.1601-0825.1998.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 10.Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–66. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 11.McCauley LK, Nohutcu RM. Mediators of periodontal osseous destruction and remodeling. principles and implications for diagnosis and therapy. J Periodontol. 2002;73:1377–91. doi: 10.1902/jop.2002.73.11.1377. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JJ, Preshaw PM, Donaldson PT. Cytokine gene polymorphism and immunoregulation in periodontal disease. Periodontol 2000. 2004;35:158–82. doi: 10.1111/j.0906-6713.2004.003561.x. [DOI] [PubMed] [Google Scholar]
- 13.Iacopetta B, Grieu F, Joseph D. The −174 G/C gene polymorphism in interleukin 6 is associated with an aggressive breast cancer phenotype. Br J Cancer. 2004;90:419–22. doi: 10.1038/sj.bjc.6601545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licastro F, Grimaldi LM, Bonafe M, et al. Interleukin-6 gene alleles affect the risk of Alzheimer's disease and levels of the cytokine in blood and brain. Neurobiol Aging. 2003;24:921–6. doi: 10.1016/s0197-4580(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 15.Trevilatto PC, Scarel-Caminaga RM, Brito RB, Jr, Souza AP, Line SR. Polymorphism at position −174 of IL-6 gene is associated with susceptibility to chronic periodontitis in a Caucasian Brazilian population. J Clin Periodontol. 2003;30:438–42. doi: 10.1034/j.1600-051x.2003.20016.x. [DOI] [PubMed] [Google Scholar]
- 16.D'Aiuto F, Parkar M, Andreou G, Brett PM, Ready D, Tonetti MS. Periodontitis and atherogenesis: causal association or simple coincidence? J Clin Periodontol. 2004;31:402–11. doi: 10.1111/j.1600-051X.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 17.Hughes FJ, Turner W, Belibasakis G, Martusceli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol 2000. 2006;41:48–72. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenstein G, Hart TC. A critical assessment of interleukin-1 (IL-1) genotyping when used in a genetic susceptibility test for severe chronic periodontitis. J Periodontol. 2002;73:231–47. doi: 10.1902/jop.2002.73.2.231. [DOI] [PubMed] [Google Scholar]
- 19.Holla LI, Buckova D, Fassmann A, Halabala T, Vasku A, Vacha J. Promoter polymorphisms in the CD14 receptor gene and their potencial association with the severiry of chronic periodontitis. J Med Genet. 2002;39:844–8. doi: 10.1136/jmg.39.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottrel RL, Dutra WO, Martins FA, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69:3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boom R, Sol CJ, Salimans MM, et al. Rapid and simple method for purification nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pontes CC, Gonzales JR, Novaes AB, Jr, et al. Interleukin-4 gene polymorphism and its relation to periodontal disease in a Brazilian population of African heritage. J Dent. 2004;32:241–6. doi: 10.1016/j.jdent.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Gauduin MC. Intracellular cytokine staining for the characterization and quantitation of antigen-specific T lymphocyte responses. Methods. 2006;38:263–73. doi: 10.1016/j.ymeth.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Offenbacher S, Heasman PA, Collins JG. Modulation of host PDGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432–44. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Chang KL, Huang JF, Huang JS, Tsai CC. Correlation of interleukin-1 beta, interleukin-6 and periodontitis. Kaohsiung J Med Sci. 1997;13:609–17. [PubMed] [Google Scholar]
- 26.D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–60. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 27.Mattila K, Vesanen M, Valtonen V, et al. Effect of treating periodontitis on C-reactive protein levels: a pilot study. BMC Infect Dis. 2002;2:30. doi: 10.1186/1471-2334-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Aiuto F, Parkar M, Brett PM, Ready D, Tonetti M. Gene polymorphisms in pro-inflammatory cytokines are associated with systemic inflammation in patients with severe periodontal infections. Cytokine. 2004;28:29–34. doi: 10.1016/j.cyto.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–49. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- 30.Walker SJ, Van Dyke TE, Rich S, Kornman KS, di Giovine FS, Hart TC. Genetic polymorphisms of the IL-1α and Il−1β genes in African-American LJP patients and an African-American control population. J Periodontol. 2000;71:723–8. doi: 10.1902/jop.2000.71.5.723. [DOI] [PubMed] [Google Scholar]
- 31.Kornman KS, Crane A, Wang HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–7. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 32.Gore EA, Sanders JJ, Pandey JP, Palesch Y, Galbraith GM. Interleukin-beta +3953 allele 2: association with disease status in adult periodontitis. J Clin Periodontol. 1998;25:781–5. doi: 10.1111/j.1600-051x.1998.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 33.Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Jr, Line SR. Investigation of IL-4 gene polymorphism in individuals with different levels of chronic periodontitis in a Brazilian population. J Clin Periodontol. 2003;30:341–5. doi: 10.1034/j.1600-051x.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 34.Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Jr, Camargo LE, Line SR. Interleukin 10 gene promoter polymorphisms are associated with chronic periodontitis. J Clin Periodontol. 2004;31:443–8. doi: 10.1111/j.1600-051X.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 35.Moreira PR, de Sá AR, Xavier GM, et al. A functional interleukin-1β gene polymorphism is associated with chronic periodontitis in a sample of Brazilian individuals. J Periodont Res. 2005;40:306–11. doi: 10.1111/j.1600-0765.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 36.Komatsu Y, Tai H, Galicia JC, et al. Interleukin-6 (IL-6) −373, A9T11 allele is associated with reduced susceptibility to chronic periodontitis in Japanese subjects and decreased serum IL−6 level. Tissue Antigens. 2005;65:110–14. doi: 10.1111/j.1399-0039.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 37.Holla LI, Fassmann A, Stejskalova A, Znojil V, Vanek J, Vacha J. Analysis of the interleukin-6 gene promoter polymorphisms in Czech patients with chronic periodontitis. J Periodontol. 2004;75:30–6. doi: 10.1902/jop.2004.75.1.30. [DOI] [PubMed] [Google Scholar]
- 38.Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA. 2003;100:177–82. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim CS, Zheng S, Kim YS, et al. The −174 G to C polymorphism of interleukin-6 gene is very rare in Koreans. Cytokine. 2002;19:52–4. doi: 10.1006/cyto.2002.1951. [DOI] [PubMed] [Google Scholar]