Abstract
Systemic administration of islet-derived antigens has been shown to protect against diabetes in the non-obese diabetic (NOD) mouse by the induction of antigen-specific regulatory T cells. Bystander regulation to related and unrelated islet-derived antigens (intramolecular and intermolecular recognition) in this context is recognized. We tested if intranasal administration of glutamic acid decarboxylase 65 (GAD 65)-derived peptides could protect against both autoimmune and, through bystander regulation, alloimmune responses in a NOD mouse model. Spontaneously diabetic female NOD mice underwent islet transplantation from either C57Bl/6 or NOD islet donors. Islet recipients were treated with intranasal GAD 65-derived peptides or control (ovalbumin) peptide pre- and post-transplantation. In-vitro analysis of the effect of inhalation was defined using lymph node proliferation assays and supernatant analysis for cytokines. GAD 65-derived peptide inhalation resulted in significant protection against recurrent autoimmune disease, with the generation of an interleukin (IL)-10-producing immune phenotype in a syngeneic islet transplant model. This phenotype, however, was not robust enough to protect against alloimmune responses. Inhalation of GAD-derived peptides induces an immunoregulatory response that protects against recurrent autoimmune, but not alloimmune responses in the NOD mouse.
Keywords: bystander regulation, GAD 65, islet transplantation, NOD mice, peptide inhalation, recurrent autoimmunity
Introduction
Animal models of autoimmune disease indicate that following initial recognition of single/few autoantigen-derived epitope/s, there is a proinflammatory cascade of T cell responses with subsequent expansion to additional target epitopes and antigens: a process termed determinant spreading [1]. By the time of presentation the immune response in clinical autoimmune disease, including type 1 diabetes, will have undergone this process. Thus immunotherapy for autoimmune diseases must control this expanded T cell repertoire. Autoantigen-specific immunotherapy prevents or reverses established autoimmune disease in a number of animal models [2–6] by recruitment and enhancement of antigen-specific regulatory T cells. These regulatory T cells suppress effector T cells locally, regardless of their antigenic specificity, by the process of bystander regulation or linked suppression. Importantly, bystander regulation allows a limited number of ‘therapeutic’ peptides to control an autoimmune response in which multiple autoantigenic determinants are being recognized. This has been demonstrated elegantly in the NOD mouse model by the administration of β chain of insulin and glutamate decarboxylase 65 (GAD 65)-derived peptides. It was shown that peptide administration not only prevents disease onset [7–10], but can also protect against recurrent autoimmunity in a syngeneic islet transplant setting [11,12]. This showed that peptide administration in a tolerogenic regimen can inhibit major histocompatibility complex (MHC) class II-restricted CD4+ T cell recognition and MHC class I-restricted CD8+ T cell effector responses to islet autoantigens, against the islets transplanted under the kidney capsule.
The phenomenon of bystander regulation proposes that responses to any antigen in this microenvironment may be similarly suppressed. Thus, following peptide administration in a tolerogenic fashion, protective T helper 2 (Th2) responses may spill over to involve other epitopes derived from the same antigen (intramolecular spread) and unrelated antigens (intermolecular spread) [9]. In a murine islet transplant model, we hypothesized that auto- and alloantigeneic epitopes will be presented by the same antigen-presenting cells, and potentially the process of intermolecular spread of bystander regulation could also extend to protect against alloresponses. We tested if inhalation of GAD 65-derived peptides could initiate a regulatory T cell phenotype, and whether such cells could protect a syngeneic and/or allogeneic islet transplant in NOD mice.
Even though a number of different routes of administration of autoantigenic peptides have been demonstrated, we chose to use the intranasal route, as it had demonstrated success previously in the NOD mouse model and we felt that this route had the best potential for translation to clinical medicine.
Materials and methods
Mice
NOD/Caj mice were obtained from the colony maintained in the specific pathogen-free (SPF) facility at Bristol University. Diabetes incidence in female mice was 65% by 30 weeks of age. Diabetic mice were maintained with intraperitoneal injections of 5–10 IU of Insulatard (Novo-Nordisk A/S, Bagsvaerd, Denmark) on alternate days. C57Bl/6 mice were obtained from Harlan UK (Bicester, UK). All experimental mice were housed in the SPF facility under standard conditions. All animal experiments complied with the United Kingdom Home Office regulations regarding use of laboratory animals and the principles of laboratory animal care (NIH) were followed throughout.
Peptides
GAD 65 peptides chosen were NMYAMLIARYKMFPEVKEKG (peptide 17), VPPSLRTEDNEERMSRLSK (peptide 34) and SRLSKVAPVIKARMMEYGTT (peptide 35) and the insulin β chain-derived peptides (9–23), SHLVEALYLVCGERG. The amino acid sequences for the GAD 65 peptides were obtained by referring to the original sequence analysis of murine cDNA coding for GAD [13]. Peptides were synthesized by the small-scale peptide synthesis unit at the W. M. Keck Biotechnology Resource Laboratory (http://keck.med.yale.edu/ssps/ Yale University, CT, USA). Whole ovalbumin (Sigma, Gillingham, Dorset, UK) was used as control peptide.
Treatment
Peptides were dissolved in phosphate-buffered saline (PBS) to achieve a final concentration of 10 µg/µl. Thirty µl of the peptide solution was administered intranasally as the mice recovered from light ether anaesthesia. Treatment was administered on days − 14, − 10, − 7 and − 4, with either islet transplantation or immunization with peptide mixture performed on day 0. Post-transplantation, the peptides were administered intranasally every 7 days until graft failure was confirmed.
Proliferation assay
Up to five NOD mice, 8–10 weeks old, were treated with GAD 65-derived peptides or control peptide as per the above protocol. Fourteen days after treatment, the mice received 100 µg of GAD 65 peptide mix in complete Freund's adjuvant (CFA; 100 µl in total) subcutaneously at the base of the tail. Ten days after injection, the animals were killed and the inguinal lymph nodes harvested and cell suspensions prepared. The cells were plated at 1 × 106 cells per well in a 96-well plate in Dulbecco's modified Eagle's medium (DMEM; Sigma) enriched with 0·5% murine serum and glutamine. Proliferative recall responses to each of the three GAD 65-derived peptides and β chain of insulin was assessed in triplicate, at varying concentrations, with appropriate positive and negative controls. During the last 16 h of a 72-h culture period, 50 µl of the supernatant was harvested for cytokine analysis and 0·5 µCi [3H]thymidine was added to each well. Thymidine incorporation was measured using liquid scintillation counting for beta emissions at the end of the 72-h culture period.
Cytokine analysis
Supernatants harvested as described above were tested using a BD cytometric bead array (BD™ CBA; BD Biosciences, San Diego, CA, USA) and the results read using a BD FACSCalibur™ flow cytometer as per the manufacturer's recommendations. Interleukin (IL)-10 and interferon (IFN)-γ was assayed for each sample.
Islet isolation and murine islet transplantation
Donor mice were killed and the pancreas was distended with up to 3 ml of 1 mg/ml collagenase P (Roche Diagnostics, Burgers Hill, UK), followed by a period of stationary digestion. The islets were then purified by density gradient centrifugation on Ficoll (Sigma) and handpicked under a dissecting microscope [14].
Female NOD mice were monitored by urine glucostix (Chemstrip™; Roche Diagnostics) for onset of glycosuria. Hyperglycaemia in glycosuric mice was confirmed by measurement of blood glucose following a tail vein bleed using an Accu-Check II BM meter (Roche Diagnostics). Blood glucose levels > 15 mmol/l on two measurements within 3 days were taken as diagnostic of diabetes mellitus, and such mice selected as recipients for islet transplantation. Recipient mice were anaesthetized by intraperitoneal (i.p.) injections of a mixture of fluanisone (10 mg/ml; Hypnorm, Janssen, UK) and midazolam (5 mg/ml) (Hypnovel; Roche, Welwyn Garden City, UK). The left kidney was buttonholed through a subcostal incision, and 600 islets transplanted under the kidney capsule. Mice were monitored for recurrence of hyperglycaemia at least three times weekly. Animals in which hyperglycaemia was sustained were considered to have lost their grafts, and the day of graft loss was defined as the first day of recurrent hyperglycaemia (blood glucose > 15 mmol).
Statistical analysis
Data were analysed using GraphPad™ 4·0 prism software (GraphPad, San Diego, CA, USA). Proliferation assay and cytokine data are presented as mean ± standard error of the mean (s.e.m.). Appropriate parametric tests after testing for distribution were used to compare proliferation and cytokine data. Log-rank comparisons of the groups were used to calculate P-values from Kaplan–Meier curves of islet graft survival.
Results
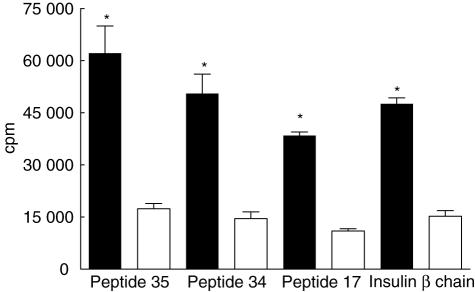
Intranasal administration of GAD 65-derived peptides inhibited proliferative responses when recalled with GAD 65 or insulin β chain-derived peptides. This inhibition was maximal when treatment was with the mix of GAD peptides (Fig. 1). Intranasal treatment with peptides 34 and 35 individually produced a slightly weaker but statistically significant response, and there was also a trend towards diminished proliferation after inhalation of peptide 17 (data not shown) that did not reach statistical significance. The positive control used for the proliferation assays was purified protein derivative (PPD), with medium alone serving as negative control.
Fig. 1.
T cell proliferation in glutamic acid decarboxylase 65 (GAD 65)-derived peptides (17, 34 and 35) and β chain of insulin (9–23) after pretreatment with intranasal control ovalbumin peptide shown in filled bars or GAD 65 mixture shown in open bars. *P < 0·001. The standard errors for each triplicate were < 10%. Mean counts per million for positive and negative controls were 87 000 and 7700, respectively. The cpm is an average of three experiments with four to five animals in each group/experiment.
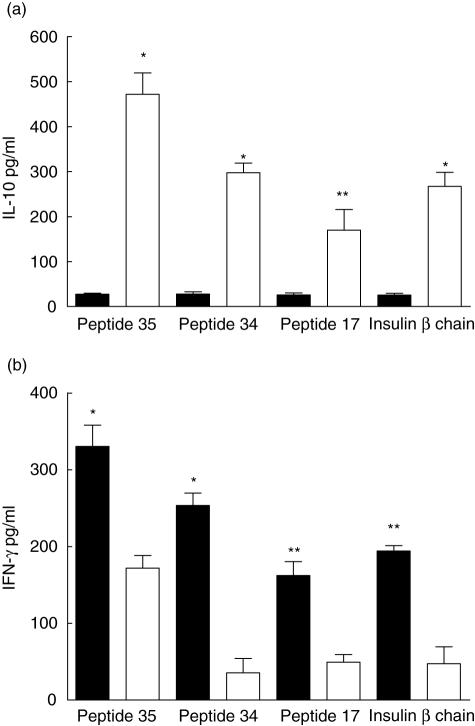
GAD 65 treatment induced a skewing to type 2 phenotype with increased IL-10 production and diminished IFN-γ production. This effect was seen not only when challenged with the same or related peptide, but also when recalled against B9–23 peptides of insulin. Once again, this response was maximal when the animals were treated with the mix of GAD 65 peptides (Fig. 2), with significant responses in animals which had inhaled peptides 34 or 35 individually, with a less significant response to inhalation of peptide 17 alone (data not shown).
Fig. 2.
Lymphocytes from glutamic acid decarboxylase 65 (GAD 65) peptide mix-treated animals exhibit a type 2 phenotype. Supernatants from lymph node proliferation assays showed animals treated with intranasal GAD 65 peptide mix, shown in open bars, had significantly greater interleukin-10 production (a) and diminished interferon-γ production (b) in comparison with peptide-treated animals shown in filled bars. Levels are a mean of three experiments with four to five animals/group/experiment. *P < 0·001; **P < 0·01.
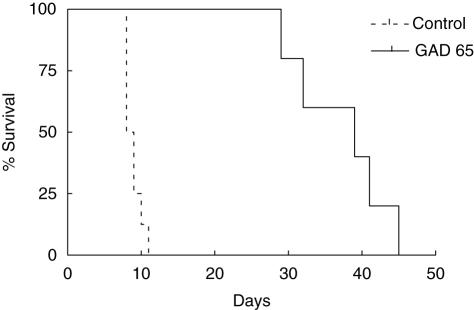
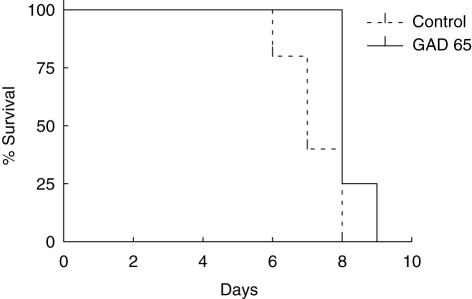
Intranasal administration of GAD 65-derived peptides significantly prolonged syngeneic graft survival in diabetic female NOD mice (Fig. 3). This showed that GAD 65 peptide inhalation could protect against recurrent autoim munity. However, the same treatment was unable to protect an allograft derived from C57Bl/6 donors (Fig. 4). In this experiment the protective influence of GAD 65 inhalation was tested against the combined effects of alloimmune and recurrent autoimmune responses. All islet recipients in both control and experimental groups had normal blood glucose levels for a minimum of 72 h post-transplantation, indicating successful completion of the procedure.
Fig. 3.
Kaplan–Meier survival curves for spontaneously diabetic female non-obese diabetic mice treated intranasally with glutamic acid decarboxylase 65 (n = 5) and control peptide (n = 7) followed by syngeneic islet transplant under the left kidney capsule. P = 0·006.
Fig. 4.
Kaplan–Meier survival curves for spontaneously diabetic female non-obese diabetic mice treated intranasally with glutamic acid decarboxylase 65 (n = 5) and control peptide (n = 7) followed by allogeneic islet transplant from C57B1/6 donors under the left kidney capsule. P > 0·05.
To test whether the inability of GAD 65 intranasal treatment to protect against rejection of an allotransplant was as a result of the combination of allogeneic and autoimmune responses, representing too great an immunological challenge to suppress, we transplanted streptozotocin-induced diabetic male NOD mice with C57Bl/6 islets. Peptide inhalation was also unable to prevent graft rejection in this experimental model (data not shown).
Discussion
Islet transplantation has emerged as offering a reasonable hope of significantly improving glycaemic control in type 1 diabetes in suitable recipients, but current conventional techniques and immunosuppression have made long-term islet survival a yet-unachieved goal in most patients. Non-specific blood-mediated inflammatory responses immediately post-transplantation [15] and, to a lesser extent, recurrent autoimmune and alloimmune antigen-specific injury together pose a considerable challenge if successful engraftment and function of transplanted human islets is to be achieved. Conventional immunosuppression is also well recognized to have significant systemic and β cell-specific side effects. Any therapeutic intervention that may alleviate these potentially deleterious side effects would obviously be welcome.
Peptide inhalation is safe and has been proved to modify immune responses favourably in animal models of autoimmune disease and transplantation. However, induction of transplantation tolerance in an alloimmune setting with this method is more difficult, due to the very high precursor frequency of responding T cells. Equally importantly, immunomodulatory MHC-derived epitopes will be difficult to define in an outbred population.
However, islet transplantation offers a unique opportunity, as patients with type 1 diabetes are preselected as ‘responders’ to islet auto-antigens. Therefore, their proven responsiveness to islet auto-antigens could potentially be turned to our advantage when trying to induce antigen-specific tolerance to an islet graft. Because autoantigen and alloantigen recognition post-transplantation would involve the same antigen-presenting cells (APC) and microenvironment, immunomodulation induced by administration of known peptide sequences in such ‘responders’ could, potentially, protect against unrelated alloantigeneic epitopes, resulting in antigen-specific tolerance.
We have demonstrated that GAD 65-derived peptide inhalation can induce a regulatory T cell phenotype, even in mice that have established autoimmune diabetes. This phenotype is sufficiently robust to delay significantly recurrent autoimmune disease when challenged subsequently with a syngeneic islet graft. However, the bystander regulation thus generated is not sufficiently robust to also protect against alloimmune responses. This may be because the high precursor frequency of MHC-responsive T cells overwhelms any regulatory T cells generated by peptide inhalation. In our model there was complete mismatch between donor and recipient in the allotransplant recipients. It is possible that survival of less MHC mismatched may have been prolonged. However, such a scenario of less mismatched grafts is not viable in the clinical setting, as most patients will need more islets from more than one MHC mismatched donor. Finally, intranasal peptide administration can only influence the indirect pathway of alloantigen recognition. Although inhibition of direct alloresponses has been demonstrated, peptide inhalation may be less efficient at inhibiting such responses, or direct responses alone may be sufficiently robust to cause graft destruction [16]. However, islet transplants are inherently skewed towards indirect recognition, making them ideal for attempts to modify alloresponses using peptide inhalation. It is thus disappointing that, despite clear inhibition of recurrent autoimmune disease, we were not able to suppress allogeneic responses.
One potential criticism of our study would be that a regulatory T cell phenotype is implied by the in-vitro proliferation and cytokine experiments plus prolonged syngeneic graft survival, but not proved conclusively by histology or T cell transfer experiments. Existence of T cells with a regulatory phenotype protecting against recurrent autoimmune responses has been demonstrated previously in similar settings, and we felt that further animal killing in order to re-establish this fact would not be justified. If the alloimmune responses had also been inhibited, transfer experiments would have been essential as they would, for the first time, establish conclusively the principle of bystander regulation in this setting. As intranasal peptide inhalation did not prolong allotransplant survival we elected not to pursue with transfer experiments.
We conclude that inhalation of GAD 65-derived peptides results in an immunomodulatory response that can protect a NOD mouse against recurrent autoimmunity but not alloimmunity in a murine islet transplant model. Harnessing the potential of this regulatory response may still be worthwhile in an islet transplant setting, as it may prevent recurrent autoimmune disease and opens the way for studies looking at the possibility for decreasing the amount of systemic immunosuppression that may be required.
Acknowledgments
The research in Professor N. G. Morgan's laboratory is sponsored by Diabetes UK. S. F. Wong is a Wellcome Trust Senior Fellow in Clinical Science.
References
- 1.Tian J, Olcott AP, Hanssen LR, Zekzer D, Middleton B, Kaufman DL. Infectious Th1 and Th2 autoimmunity in diabetes-prone mice. Immunol Rev. 1998;164:119–27. doi: 10.1111/j.1600-065x.1998.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 2.Myers LK, Stuart JM, Seer JM, Kang KH. Identification of an immunosuppressive epitome of type II collagen that confers protection against collagen induced arthritis. J Exp Med. 1989;170:1999–2010. doi: 10.1084/jem.170.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaur A, Weirs B, Liu A, Rothbard J, Fatima CG. Amelioration of autoimmune encephalomyelitis by myelin basic protein synthetic peptide induced anergy. Science. 1992;258:1491–4. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- 4.Mukasa A, Itoh M, Tokunaga Y, Hiramine C, Hojo K. Inhibition of a novel model of murine experimental autoimmune orchitis by intravenous administration with a soluble testicular antigen: participation of regulatory CD8 T cells. Clin Immunol Immunopathol. 1992;62:210–19. doi: 10.1016/0090-1229(92)90074-x. [DOI] [PubMed] [Google Scholar]
- 5.Sasamoto Y, Kawano YI, Bouligny R, Wiggert B, Chader GJ, Gery I. Immunomodulation of experimental autoimmune uveoretinitis by intravenous injection of uveitogenic peptides. Invest Ophthalmol Vis Sci. 1992;33:2641–9. [PubMed] [Google Scholar]
- 6.Fuller BE, Okayasu I, Simon LL, Giraldo AA, Kong YM. Characterisation of resistance to murine experimental autoimmune thyroiditis: duration and afferent action of thyroglobulin and TSH-induced suppression. Clin Immunol Immunopathol. 1993;69:60–8. doi: 10.1006/clin.1993.1150. [DOI] [PubMed] [Google Scholar]
- 7.Daniel D, Wegman DR. Protection of nonobese diabetic mice from diabetes by intranasal or sub-cutaneous administration of insulin peptide B-{9–23} Proc Natl Acad Sci USA. 1996;93:956–60. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisch R, Liblau RS, Yang XD, Liblau P, McDevitt HO. Induction of GAD 65 specific regulatory T-cells inhibits ongoing auto-immune diabetes in nonobese diabetic mice. Diabetes. 1998;47:894–9. doi: 10.2337/diabetes.47.6.894. [DOI] [PubMed] [Google Scholar]
- 9.Tian J, Atkinson MA, Clare-Salzler M, et al. Nasal administration of Glutamate decarboxylase {GAD65} peptides induces Th2 responses and prevents murine insulin dependent diabetes. J Exp Med. 1996;183:1561–7. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock WW, Polanski M, Zhang J, Blogg N, Weiner HL. Suppression of insulitis in nonobese diabetic mice by oral insulin administration is associated with selective expression of interleukin-4 and -10, transforming growth factor-β, and prostaglandin E. Am J Pathol. 1995;147:1193–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Tian J, Clare-Slazler M, Herschenfeld A, et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes prone mice. Nat Med. 1996;2:1348–53. doi: 10.1038/nm1296-1348. [DOI] [PubMed] [Google Scholar]
- 12.Rayat GR, Singh B, Korbutt GS, Rajotte RV. Single injection of insulin delays the recurrence of diabetes in syngeneic islet transplanted diabetic NOD mice. Transplantation. 2000;70:976–9. doi: 10.1097/00007890-200009270-00016. [DOI] [PubMed] [Google Scholar]
- 13.Lee DS, Tian J, Phan T Kaufman DL. Cloning and sequence analysis of a murine cDNA encoding glutamate decarboxylase [GAD65] Biochem Biophys Acta. 1993;1216:157–60. doi: 10.1016/0167-4781(93)90056-j. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh M, Maki T, Satomi S, Porter J, Monaco AP. Immunological characteristics of purified pancreatic islet grafts. Transplantation. 1986;42:387–90. doi: 10.1097/00007890-198610000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–45. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 16.Makhlouf L, Yamada A, Ito T, et al. Allorecognition and effector pathways of islet allograft rejection in normal versus nonobese diabetic mice. J Am Soc Nephrol. 2003;14:2168–75. doi: 10.1097/01.asn.0000079041.15707.a9. [DOI] [PubMed] [Google Scholar]