Abstract
The mechanism of action of methotrexate (MTX) in autoimmune diseases (AID) is unclear. A pro-apoptotic effect has been demonstrated in mitogen-stimulated peripheral blood mononuclear cells (PBMC), but studies employing conventional antigens have disputed a pro-apoptotic effect. CD4+ T helper (Th) cells play a significant role in most AID. We therefore examined directly, by flow cytometry, the uptake of MTX by the T helper (Th) cells stimulated for 6 days with Candida albicans (CA) or tetanus toxoid (TT), and its consequences with respect to induction of apoptosis. While none of the resting Th cells took up MTX, nearly all the dividing Th cells did, and this abrogated further cell division. Among dividing Th cells, MTX induced an approximately sixfold increase over baseline levels in the proportion of apoptotic cells. This proportion could be reverted to baseline by the addition of folic acid. Exposure of CA-stimulated PBMC to MTX significantly increased their level of cleaved poly(ADP-ribose) polymerase (PARP), and a similar tendency was observed in TT-stimulated cells. Unlike CA and TT, the mitogen phytohaemagglutinin (PHA) induced proliferation of both CD4– and CD4+ T cells, and induced apoptosis in both undivided and divided Th cells. PHA-induced apoptosis involved activation of caspase-3 and the anti-apoptotic protein Bcl-2 in addition to PARP cleavage, suggesting that PHA induces apoptosis via different pathways than CA and TT. We suggest that the latter are more representative of stimulation with self-antigens in AID, and that a pro-apoptotic effect of MTX on self-antigen-stimulated Th cells contributes to the effect of MTX in the treatment of AID.
Keywords: apoptosis, CD4+ T cells, methotrexate
Introduction
Methotrexate (MTX) is one of the most widely used drugs for the treatment of rheumatoid arthritis (RA) [1] and other chronic inflammatory diseases. While known to act as a folic acid antagonist in the dosages administered in malignant disease, the mechanisms underlying the effect of MTX, in the much smaller dosages used for treatment of rheumatic disease, are less well understood.
It has been proposed that MTX inhibits enzymes involved in purine metabolism, resulting in accumulation of extracellular adenosine [2]. Adenosine interacts with receptors on neutrophils, the engagement of some of which inhibits adherence to endothelial cells and decreases the generation of reactive oxygen species [2,3]. MTX has also been suggested to induce apoptosis of activated T cells [4].
Previous studies addressing the apoptosis-inducing effect of MTX have been conducted using peripheral blood mononuclear cells (PBMC) or lymphocytes as a bulk [4,5]. Hence, these studies have not allowed assessment of differential effects of MTX on minor lymphocyte subsets. An influence of MTX on apoptosis in PBMC has been demonstrated only under non-physiological conditions, for example after stimulation with mitogens [4,5]. Employing stimulation with conventional antigens, others have failed to demonstrate an apoptotic effect of MTX on PBMC, and instead attributed the effect of MTX on T cells to suppression of T cell activation and reduced expression of T cell adhesion molecules [5].
Because CD4+ T helper cells play a pivotal role in the pathogenesis of a number of autoimmune diseases, including RA, in this study we have focused upon the uptake of MTX by dividing and non-dividing subsets of CD4+ T cells stimulated for proliferation in a physiologically relevant manner, i.e. by foreign recall antigens. Moreover, we have examined whether MTX induces apoptosis in dividing and/or resting CD4+ T cells and CD4– T cells. We show that MTX is taken up solely by cells undergoing cell division, and that this results in apoptosis in a proportion of dividing CD4+ T cells. This MTX-induced apoptosis appears to involve cleavage of poly(ADP-ribose) polymerase (PARP), but not activation of caspase-3.
Materials and methods
Cells and serum
Upon informed consent, venous blood was harvested from 16 healthy blood donors using sodium citrate as anti-coagulant. Whole blood cells, washed twice in phosphate-buffered saline (PBS), PBMC isolated by density centrifugation in lymphocyte separation medium (Axix-Shield, Oslo, Norway), were used. Serum preparations were derived from venous blood of the same donors collected without anti-coagulant. The study was approved by the local ethics committee.
Antibodies
A phycoerythrin–cyanin 5- (PC5-) conjugated monoclonal antibody (MoAb), APO-2·7 (Beckman-Coulter, Fullerton, CA, USA), recognizing a 38-kDa mitochondrial membrane protein (7A6 antigen) exposed to the cell surface at the early stage of apoptosis [6] was used for identification of apoptotic cells. Phycoerythrin (PE)–anti-CD4 MoAbs (Becton-Dickinson, Copenhagen, Denmark) and allophycocyanin (APC)–anti-CD3 MoAbs (Becton-Dickinson) were used in combination with PC5–APO-2·7 for identification of apoptotic CD4+ and CD4– T cells. In experiments involving PKH-26-stained cells (to detect cell division), peridinin chlorophyll (PerCP)-conjugated anti-CD4 MoAbs (Dako, Copenhagen, Denmark) was used as a marker for CD4+ T cells.
Antigens
The foreign recall antigens tetanus toxoid (TT), a kind gift from Claus Koch (State Serum Institute, Copenhagen, Denmark) or Candida albicans (CA), kindly provided by Else Svejgaard (Department of Dermatology, Bispebjerg Hospital, Copenhagen, Denmark), were used for stimulation of PBMC. CA was a lyophilized whole cell extract, which was dissolved in water before use, to a concentration of ca. 10 mg/ml.
Labelling of PBMC with PKH-26 or 5-carboxy-2′,7′-dichlorofluorescein diacetate succinimidyl ester (CFSE)
PKH-26 (PKH26 Red Fluorescent Cell Linker Kit; Sigma, Brondby, Denmark) or CFSE (Molecular Probes, Eugene, OR, USA) were used as markers for proliferation as described [7–9], and stored as stock solutions of 1 mM in the manufacturer's diluent C (PKH26 Red Fluorescent Cell Linker Kit; Sigma) and 5 mM in dimethylsulphoxide (DMSO), respectively. PBMC purified from 7 ml blood were suspended in diluent C and incubated with PKH-26 (2 µM) for 3 min at room temperature. The reaction was stopped by addition of an equal volume of fetal calf serum (FCS). Alternatively, the same amount of PBMC were suspended in RPMI-1640 and incubated with sterile filtered CFSE (2 µM) for 10 min at 37°C. After both dying procedures, PBMC were washed thrice in RPMI-1640 (Gibco/Invitrogen, Taastrup, Denmark) before cultivation.
Addition of MTX to stimulated PBMC cultures
Fluorescence-labelled or blank PBMC were suspended in RPMI-1640 containing 30% (v/v) serum, and distributed in flat-bottomed Nunclon™ MicroWell™ microtitre plates (Gibco/Invitrogen) with 2·5 × 105 PBMC per well in the presence of TT, phytohaemagglutinin (PHA) (both at final concentrations of 10 µg/ml), CA (100 µg/ml) or no antigen. At day 6, fluorescein isothiocyanate (FITC)-MTX (Invitrogen, Eugine, OR, USA) was added to PKH-26-stained cells, and unlabelled MTX (Wyeth, Taplow, Bucks, UK) was added to CFSE-stained or blank cells; the final MTX-concentrations being 0, 0·1, 1 or 10 µg/ml (clinically relevant doses as 0·1 µg/ml equalize 5 mg in a person of 70 kg). In some experiments, fluorescein-5-carboxamide lysine (5-FAM-lysine) (MW: 619; CPC Scientific, http://www.cpcscientific.com) coupled to FITC (final concentrations 1 and 10 µg/ml) was used as a control for unspecific uptake of MTX (MW: 454) coupled to FITC. In other cases, Isovorine® (Wyeth) was added simultaneously with MTX (10 µg/ml) to final Isovorine concentrations of 1, 10 or 100 µg/ml.
Apoptosis
Apoptotic cells among dividing and non-dividing CD4+ T cells and CD4– T cells were identified by means of staining with PC5- APO 2·7 MoAb (Beckman-Coulter, Fullerton, CA, USA) or PE–annexin V (Becton-Dickinson). Moreover, the cellular content of cleaved PARP, activated caspase-3 and the anti-apoptotic protein Bcl-2 was assessed by cytometric bead array (CBA, Becton-Dickinson) according to the manufacturer's instructions.
Determination of absolute CD4+ T cell and CD4–T cell counts in culture wells
Immediately before flow cytometric acquisition, a known number of beads (ca. 5000) from TruCOUNT tubes (Becton-Dickinson) were added to the samples. During analysis, the beads were gated on the basis of their high FL-1/FL-2 emission.
Flow cytometry
Analyses were performed using a fluorescence activated cell sorter (FACScalibur) flow cytometer (Becton-Dickinson). Cellular staining was performed in the combinations FITC-MTX/PE–PHK-26/PerCP–anti-CD4/APC–anti-CD3 or CFSE/PE–anti-CD4/PC-5–APO-2·7/APC–anti-CD3 or CFSE/PE–annexin-V/PerCP–anti-CD4/APC–anti-CD3. CellQuest software (Becton-Dickinson) was used, and the CBA (Becton-Dickinson) was employed for analyses of PARP, caspase-3 and Bcl-2 data.
Statistics
Paired t-tests were used, the null hypothesis being no difference between the data sets compared; P < 0·05 was considered significant.
Results
Uptake of MTX by dividing CD4+ T cells
In order to track cell divisions, we labelled PBMC with the proliferation markers PKH-26 or CFSE, the contents of which are halved upon each cell division. When FITC-labelled MTX was added to PBMC cultures grown for 6 days in the absence or presence of the foreign antigens CA and TT, we observed that a day later unstimulated CD4+ lymphocytes had failed to take up MTX (Fig. 1a), while only the proliferating subset of antigen-stimulated CD4+ lymphocytes exhibited uptake (Fig. 1b). By contrast, FITC-labelled 5-FAM-lysine, with a molecular weight resembling that of FITC–MTX, was not taken up by dividing CD4+ lymphocytes (Fig. 1c).
Fig. 1.
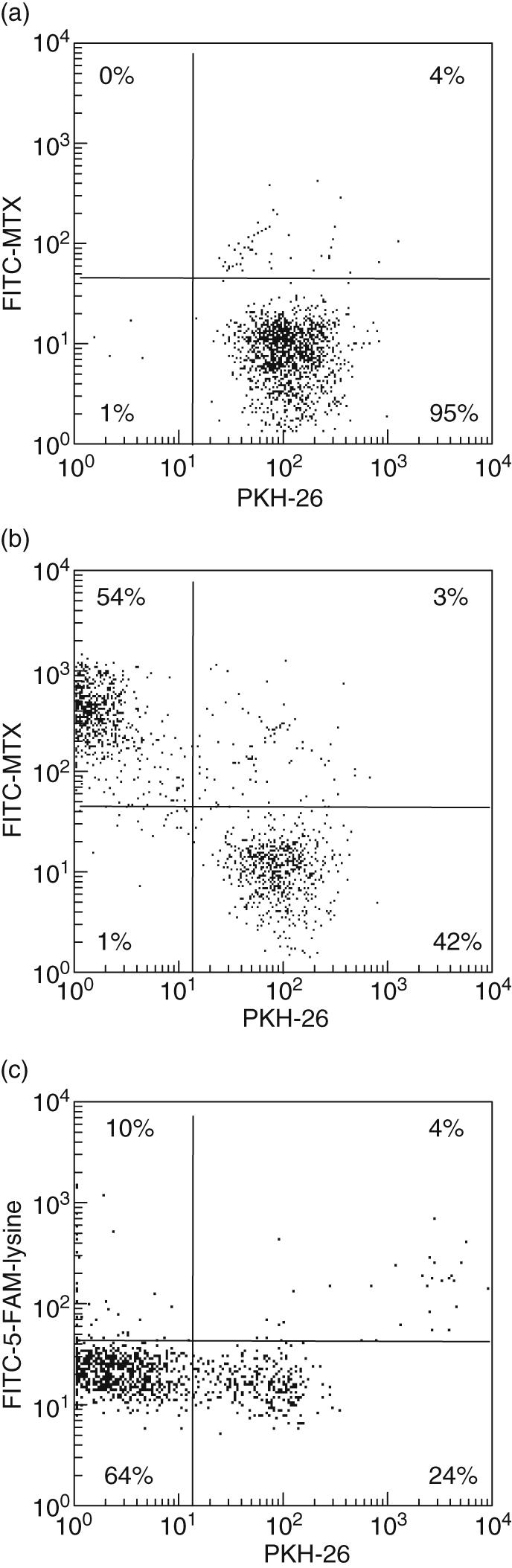
Uptake of methotrexate (MTX) by dividing CD4+ T cells. Peripheral blood mononuclear cells were stained with the proliferation marker PKH-26 and cultured for 7 days in the presence or absence of Candida albicans as stimulating antigen. At day 6, fluorescein isothiocyanate (FITC)-labelled MTX or FITC-labelled fluorescein-5-carboxamide lysine (5-FAM-lysine) was added to final concentrations of 1 ìg/ml. (a) The low uptake of FITC–MTX by undivided cells CD4+ lymphocytes in the absence of stimulation is seen. (b) Upon CA-stimulation, divided CD4+ lymphocytes exhibit uptake of FITC–MTX (upper left quadrant), while non-dividing cells do not (lower right quadrant). (c) The corresponding uptake of FITC-5-FAM-lysine by CA-stimulated CD4+ lymphocytes is shown. Data are representative of five experiments.
Influence of MTX on T cell proliferation
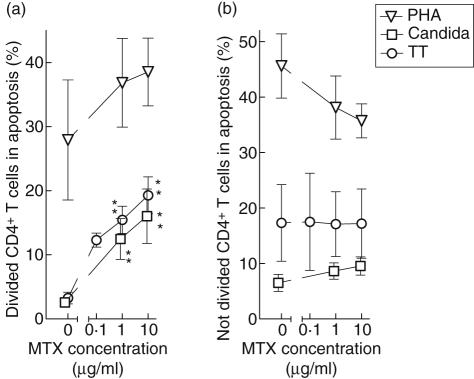
Incubation of PBMC with MTX for the latest 24 h significantly reduced, in a dose-dependent manner, the number of CD4+ T cells having divided upon stimulation with CA or TT for 7 days (Fig. 2a). By contrast, the number of divided, PHA-stimulated CD4+ T cells was not affected significantly.
Fig. 2.
The influence of methotrexate (MTX) on T cell proliferation. Peripheral blood mononuclear cells were cultured in the presence of tetanus toxoid (circles), Candida albicans (squares) or phytohaemaglutinin (triangles) for 7 days, with or without MTX, at the given concentrations, between days 6 and 7. The resulting number of divided CD4+ T cells (a) and CD4– T cells (b) per well are shown as mean ± s.e.m. of six experiments. *P < 0·05 (*)P = 0·06.
Reductions of the number of divided CD4+ T cells by 59% and 66%, on average, upon incubation with CA- and TT-stimulated cultures, respectively, indicate that MTX had prevented the entire population of proliferating CD4+ T cells from going through one cell cycle, and possibly a fraction of cells from going through two divisions during the 24 h of incubation. Alternatively or additionally, MTX had caused cell death and disintegration of a substantial fraction of the proliferating cells.
CD4– T cells, on the other hand, did not proliferate in response to CA and TT, while PHA also induced MTX-independent proliferation of this T cell subset (Fig. 2b).
MTX-induced apoptosis of proliferating T cells
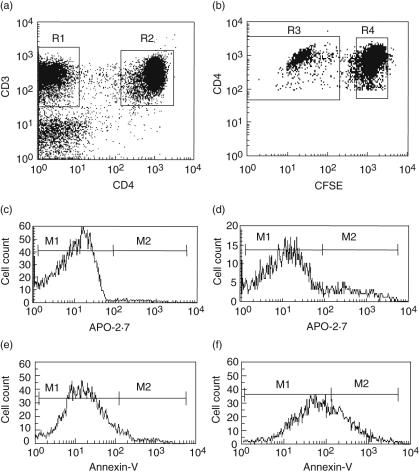
We further examined whether the MTX-mediated prevention of CD4+ T cells from proliferation was related to apoptosis. To that end, we co-stained CD4+ T cells (Fig. 3a) stimulated for proliferation with antigen (Fig. 3b) with a MoAb against APO-2·7, a mitochondrial antigen exposed to the cell surface at the early stage of apoptosis [6,10] (Fig. 3c,d). As a control marker for early apoptosis, annexin-V was employed (Fig. 3e,f).
Fig. 3.
Measurement of apoptosis by APO-2·7 monoclonal antibody (MoAb) or annexin-V. 5-Carboxy-2′,7′-dichlorofluorescein diacetate succinimidyl ester-stained peripheral blood mononuclear cells were incubated with Candida albicans (CA) for 7 days, with or without methotrexate (MTX) (1 µg/ml) during the last day. (a) CD4– T cells (R1) and CD4+ T cells (R2) were identified. (b) CD4+ T cells were subdivided into proliferating (R3) and non-proliferating (R4) cells. (c) Histogram showing the binding of APO-2·7 MoAb dividing CD4+ T cells not exposed to MTX. (d) The corresponding binding upon exposure to MTX. (e) Histogram showing the binding of annexin-V to dividing CD4+ T cells not exposed to MTX. (f) The corresponding annexin-V binding upon exposure to MTX. M1 and M2 indicate cell fractions interpreted as non-apoptotic and apoptotic, respectively. (a–d) Representative of six experiments; (e–f) of three experiments.
At concentrations of 1 µg/ml and 10 µg/ml, MTX induced significant increases in the apoptotic fraction of CD4+ T cells stimulated for proliferation by CA or TT (Fig. 4a). In CA-stimulated cultures, this fraction rose from a baseline level of 2·5 ± 0·5% (mean ± standard error of the mean) to 15·2 ± 4·0% with 10 µg/ml MTX (P < 0·02). Correspondingly, an increase from 3·3 ± 0·9% to 19·0 ± 2·4% apoptotic cells occurred in TT-stimulated cultures (P < 0·009). PHA induced per se a high degree of apoptosis, presumably as a result of proliferation-induced cell death. In the absence of MTX, 27·9 ± 9·4% of the proliferating CD4+ T cells were apoptotic, while this fraction increased non-significantly to 37·6 ± 5·5% on exposure to 10 µg/ml MTX.
Fig. 4.
Methotrexate (MTX)-induced apoptosis in a subset of dividing CD4+ T cells. 5-Carboxy-2′,7′-dichlorofluorescein diacetate succinimidyl ester-labelled peripheral blood mononuclear cells were stimulated with tetanus toxoid (circles), Candida albicans (squares) or phytohaemagglutinin (triangles) for 7 days. At day 6, MTX was added to the cultures at the given concentrations. The fraction of apoptotic cells among divided (a) and resting (b) CD4+ T cells are shown as mean ± s.e.m. of six experiments. **P < 0·01.
In accordance with these findings, staining with annexin-V (employing a conservative definition of positivity) revealed that MTX increased the proportion of apoptotic divided CD4+ T cells from 12·0 ± 3·5% to 26·5 ± 10·9% in CA-stimulated cultures (Fig. 3e.f) and from 14·3 ± 2·4% to 22·2 ± 2·0% in TT-stimulated cultures.
The baseline proportion of apoptotic cells was slightly higher in resting CD4+ T cells than in dividing cells, and was largely unaffected by MTX in cultures stimulated with CA or TT (Fig. 4b). In PHA-stimulated cultures a non-significant decrease occurred upon incubation with MTX.
Rescue from MTX-induced apoptosis by folic acid
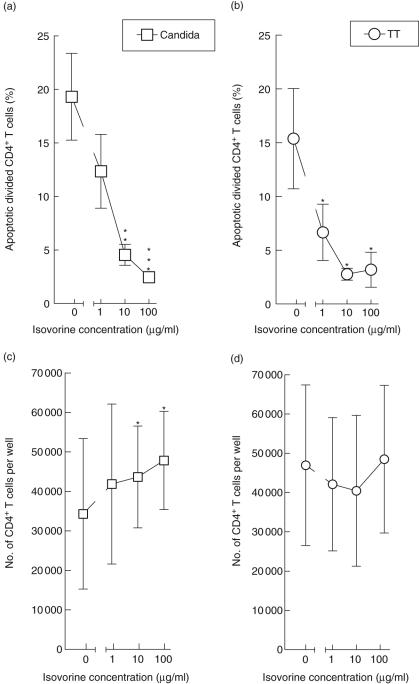
We further investigated whether proliferating CD4+ T cells could be rescued from apoptosis by folic acid. To this end, Isovorine® was added to CA- or TT-stimulated PBMC cultures at day 6, concomitantly with MTX. At concentrations of 10 µg/ml and 100 µg/ml, folic acid protected against MTX-induced apoptosis of CD4+ T cells stimulated for proliferation by CA (Fig. 5a) as well as TT (Fig. 5b), and similar tendencies were observed with 1 µg/ml folic acid. This rescue was reflected by a dose-dependent increase in the total number of CD4+ T cells in PBMC cultures incubated with CA (Fig. 5c) but not with TT (Fig. 5d). Taken together, these findings indicate that induction of apoptosis by MTX is related to its action as a folic acid antagonist.
Fig. 5.
Rescue of dividing CD4+ T cells from methotrexate (MTX)-induced apoptosis by folic acid. Peripheral blood mononuclear cells were stimulated for 6 days with Candida albicans (a,c) or tetanus toxoid (b,d) before addition of MTX (10 µg/ml) along with various concentrations of folic acid (Isovorine). At day 7, the fraction of apoptotic (APO-2·7-positive) cells among divided CD4+ T cells was measured by flow cytometry (a,b), as were the absolute CD4+ T cell counts in the culture wells (c,d). Mean ± s.e.m. of five experiments are shown. *P < 0·05, **P < 0·01, ***P < 0·005.
MTX-induced PARP, caspase-3 and Bcl-2 in antigen-stimulated PBMC
The apoptosis-inducing effect of MTX in subsets of PBMC was confirmed by an overall increase in cleaved PARP upon addition of MTX (1 or 10 µg/ml) to CA-stimulated PBMC (P < 0·02 at both concentrations), while a similar tendency was observed upon stimulation with TT (P < 0·09 and P < 0·14, respectively) (Fig. 6a). No MTX-induced increase was observed in PHA-stimulated or unstimulated PBMC (‘-Ag’ in Fig. 6).
Fig. 6.
Methotrexate (MTX)-induced changes in intracellular apoptosis-related proteins. Peripheral blood mononuclear cells were grown for 7 days in the presence or absence of stimulating agents Candida albicans, tetanus toxoid or phytohaemaglutinin, with or without MTX for the last day. The cellular content of cleaved poly(ADP-ribose) polymerase, active caspase 3 and the anti-apoptotic protein Bcl-2 was subsequently measured by cytometric bead array. The results are shown as mean ± s.e.m. (n = 5). (*)P < 0·07, *P < 0·02, **P < 0·006 for no change induced by MTX.
MTX did not induce any significant changes in the levels of active caspase-3 (Fig. 6b), nor in the levels of the anti-apoptotic protein Bcl-2 (Fig. 6c). The high proportions of apoptotic cells observed upon stimulation with PHA (Fig. 4) were reflected by high levels of PHA-induced cleaved PARP and activated caspase 3 and Bcl-2 (Fig. 6a–c).
Discussion
The effect of MTX on apoptosis in circulating lymphocytes has been a matter of controversy [4,5]. Thus, MTX-induced apoptosis has been demonstrated in PBMC stimulated with mitogens [4]. The pro-apoptotic effect of MTX was present only at intermediate doses of the drug, while low or high doses simply inhibited cell proliferation. Johnston and colleagues confirmed a pro-apoptotic effect of MTX on PHA-stimulated lymphocytes, but an MTX-induced decrease in the number of apoptotic lymphocytes stimulated with conventional antigens such as streptococcal extract or superantigens [5]. They argued that the pro-apoptotic effect of MTX is restricted to lymphocytes exposed to potent, non-physiological stimuli, such as mitogens that induce detrimental ‘cytokine storms’ in PBMC cultures.
In the present study, we demonstrate that MTX is taken up by human CD4+ T cells stimulated for proliferation by the antigens, TT and CA, stimulating T cells in a physiologically relevant manner via antigen-presenting cells. By contrast, MTX was not taken up by non-proliferating CD4+ T cells. Exposure of MTX for 1 day significantly inhibited proliferation by the majority of CD4+ T cells (Fig. 2). Staining with APO-2·7 [10], a marker for early apoptosis, revealed that MTX induced apoptosis in ca. 15% of CD4+ T cells proliferating after stimulation with CA, and ca. 19% after stimulation with TT. These data were confirmed by staining with annexin-V. By contrast, MTX did not induce apoptosis in resting CD4+ T cells, nor in CD4– T cells. Flow cytometric detection of apoptosis in small proportions of cells, such as the subset of proliferating CD4+ T cells examined here, is presumably not possible without simultaneous staining for CD3, CD4, proliferation and apoptosis, as the signal is likely to fall below the detection limit if the bulk of lymphocytes or mononuclear cells is examined as in the study by Johnston and colleagues [5].
The occurrence of MTX-induced apoptosis was confirmed by an MTX-dependent increase in amount of cleaved PARP in CA-stimulated, and with borderline significance in TT-stimulated, PBMC. Cleavage of PARP is one of the of the earliest detectable protein-degradation events following fragmentation of chromatin DNA, but before internucleosomal DNA fragmentation [11]. By contrast, MTX did not induce active caspase-3 in antigen-stimulated cells. The increase in cleaved PARP induced by MTX supports a pro-apoptotic effect of MTX, and suggests that the apoptosis observed in CD4+ T cells is caspase-independent. It may involve the release of apoptosis-inducing factors from the mitochondria, induced by elevated poly(ADP-ribose) levels following activation of PARP [12,13]. MTX did not significantly affect Bcl-2, an anti-apoptotic protein associated with the endoplasmic reticulum- and mitochondria membranes [14,15].
MTX is known to act as a folic acid antagonist in the dosages administered in malignant diseases. The mechanisms underlying its effect in rheumatic disease, on the other hand, are less well understood. In keeping with a previous report of an anti-apoptotic effect of folic acid [4], we found that addition of Isovorine to cultures stimulated with CA or TT significantly reduced the proportion of apoptotic CD4+ T cells in a dose-dependent manner (Fig. 5a,b). Accordingly, Isovorine significantly increased the number of CD4+ T cells in MTX-treated cultures stimulated with CA. However, a similar effect of Isovorine on the overall CD4+ T cell number was not demonstrated in TT-stimulated cultures. In a previous study, Genestier et al. showed that MTX-induced apoptosis in mitogen activated PBMC was inhibited by folic acid [4].
In contrast with CA and TT, the mitogen PHA stimulated CD4– T cells as well as CD4+ T cells for proliferation in an MTX-independent manner. A large proportion of non-proliferating as well as proliferating CD4+ T cells were apoptotic after incubation with PHA for 7 days, and the size of this proportion was not affected by MTX. This finding is in contrast with that of others [5]. Moreover, the apoptotic pathways involved seemed to differ from those induced by MTX, as PHA induced activation of caspase-3 in addition to cleavage of PARP.
Taken together, our findings demonstrate that MTX inhibits proliferation in CA- and TT-stimulated CD4+ T cells, and induces apoptosis in a subset of stimulated CD4+ T cells. The apoptotic pathways involved seem to be PARP-dependent and caspase-3 independent. PHA, on the other hand, stimulates CD4+ T cells and CD4– T cells indiscriminately for proliferation, by a pathway involving activation of caspase-3 in addition to PARP cleavage. We suggest that stimulation of T cells with the conventional antigens CA and TT more probably resembles stimulation with self-antigens occurring in autoimmune diseases, including RA, and the anti-proliferative and pro-apoptotic effects of MTX demonstrated here reflect the actions of MTX in the treatment of rheumatic diseases.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Ms Anne Ambjørnsen and Ms Winnie Hansen.
References
- 1.Yazici Y, Sokka T, Kautiainen H, Swearingen C, Kulman I, Pincus T. Long term safety of methotrexate in routine clinical care: discontinuation is unusual and rarely the result of laboratory abnormalities. Ann Rheum Dis. 2005;64:207–11. doi: 10.1136/ard.2004.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4:266–73. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cronstein BN. Molecular mechanism of methotrexate action in inflammation. Inflammation. 1992;16:411–23. doi: 10.1007/BF00918968. [DOI] [PubMed] [Google Scholar]
- 4.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322–8. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 2005;114:154–63. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Ao Z, Seth A, Schlossman SF. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol. 1996;157:3980–7. [PubMed] [Google Scholar]
- 7.Givan AL, Fisher JL, Waugh MG, Bercovici N, Wallace PK. Use of cell-tracking dyes to determine proliferation precursor frequencies of antigen-specific T cells. Meth Mol Biol. 2004;263:109–24. doi: 10.1385/1-59259-773-4:109. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen CH, Leslie RG, Jepsen BS, Kazatchkine MD, Kaveri SV, Fischer E. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. Eur J Immunol. 2001;31:2660–8. doi: 10.1002/1521-4141(200109)31:9<2660::aid-immu2660>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen CH, Hegedus L, Leslie RG. Autoantibodies in autoimmune thyroid disease promote immune complex formation with self antigens and increase B cell and CD4+ T cell proliferation in response to self antigens. Eur J Immunol. 2004;34:263–72. doi: 10.1002/eji.200324413. [DOI] [PubMed] [Google Scholar]
- 10.Koester SK, Roth P, Mikulka WR, Schlossman SF, Zhang C, Bolton WE. Monitoring early cellular responses in apoptosis is aided by the mitochondrial membrane protein-specific monoclonal antibody APO2.7. Cytometry. 1997;29:306–12. [PubMed] [Google Scholar]
- 11.Greidinger EL, Miller DK, Yamin TT, Casciola-Rosen L, Rosen A. Sequential activation of three distinct ICE-like activities in Fas-ligated Jurkat cells. FEBS Lett. 1996;390:299–303. doi: 10.1016/0014-5793(96)00678-3. [DOI] [PubMed] [Google Scholar]
- 12.Gagne JP, Hendzel MJ, Droit A, Poirier GG. The expanding role of poly(ADP-ribose) metabolism: current challenges and new perspectives. Curr Opin Cell Biol. 2006;18:145–51. doi: 10.1016/j.ceb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–98. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 14.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–36. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 15.Daniel PT, Schulze-Osthoff K, Belka C, Guner D. Guardians of cell death: the Bcl-2 family proteins. Essays Biochem. 2003;39:73–88. doi: 10.1042/bse0390073. [DOI] [PubMed] [Google Scholar]