Abstract
Recently we demonstrated that in inflammatory bowel disease (IBD) macrophage-oxidative burst activity is increased and NADPH oxidase mRNA is induced. The herbal phenylethanoid acteoside isolated from Plantago lanceolata L. was shown to exhibit anti-oxidative potential. Using the dextran sulphate sodium (DSS)-induced colitis model, in this study we have assessed whether systemic application of acteoside affects colitis. Colitis was induced by DSS in Balb/c mice. Treatment with acteoside (120, 600 µg/mouse/day) was performed intraperitoneally. The colon lengths were determined. Colonic tissue was scored histologically (max. score 8) by a blinded investigator. T cells isolated from mesenteric lymph nodes (MLN) were stimulated with anti-CD3 antibody in the presence of interleukin (IL)-2 (final concentration 10 U/ml). After incubation for 24 h, IL-1β, IL-6, IL-12 tumour necrosis factor (TNF)-α and interferon (IFN)-γ levels in supernatants were analysed by the beadlyte® cytokine detection system. Histological scoring of colonic tissue revealed that application of acteoside was followed by a significantly improved histological score. In acute colitis the histological score was 3·2 with acteoside versus 5·2 with phosphate-buffered saline (PBS) (P < 0·02). In chronic colitis both 120 µg (3·3 versus 5·2) or 600 µg acteoside (3·0 versus 5·2) significantly ameliorated colitis (both P < 0·02). Stimulated MLN from mice with chronic DSS-induced colitis treated with acteoside showed a significant down-regulation of IFN-γ secretion (195 pg/ml with 600 µg acteoside versus 612 pg/ml with PBS, P < 0·02). Inhibition of oxidative burst activity with acteoside reduced mucosal tissue damage in DSS colitis and could be a therapeutic alternative for IBD treatment. Further studies of this agent are warranted.
Keywords: acteoside, DSS, monocytes/macrophages, oxidative burst, Plantago lanceolata L.
Introduction
Recently we performed a subtractive screening method to study mRNA expression in purified intestinal macrophages (IMAC) from Crohn's disease patients. Surprisingly, in inflammation-associated intestinal macrophages only few cytokines were among the strongest induced genes; on the other hand, mainly tissue-degrading enzymes and pathways were induced. Among those we demonstrated that the cytochrome b subunit gp91 is up-regulated in IMAC from Crohn's disease patients [1]. Cytochrome b is the functional subunit of the membrane-associated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, responsible for production of reactive oxidants in phagocytic cells during respiratory burst.
This led us to investigate differences in oxidative burst activity in isolated IMAC from control and inflamed mucosa from patients with inflammatory bowel disease (IBD). We could show that in IBD macrophages oxidative burst activity is increased and NADPH oxidase mRNA is induced [1].
The production of superoxide and other reactive oxygen intermediates responsible for microbicidal, tumoricidal and inflammatory activities in phagocytes is mediated by NADPH oxidase [2,3]. Superoxide anion is the starting-point for the formation of other reactive oxidants such as hydrogen peroxide, which is converted further by a lysosomal myeloperoxidase (MPO) to hypochlorous acid.Secretion of these oxidants can be associated with inflammatory tissue destruction in IBD [4].
In relation to oxidative stress, the colon may be subjected to the production of reactive oxygen species from phagocytic leucocytes during inflammation; the colonic mucosa contains relatively small amounts of antioxidant enzymes [5]. The colon may be overwhelmed with reactive oxygen during times of active inflammation, which could result in intestinal injury.
Antioxidants have been shown to reduce mucosal inflammation in IBD and could become part of anti-inflammatory therapy in the future. It was suggested that nutritional anti-oxidants have potential as therapeutic and chemopreventive agents [6] to settle the imbalance in the formation of reactive oxygen species and antioxidant micronutrients in the pathogenesis and/or perpetuation of the tissue injury in IBD [7].
The plantain herb, Plantago lanceolata L. (Plantaginaceae), is used in traditional medicine. Folk medicine recommends the leaf juice for treating blisters, ulcers, insect stings and bites, conjunctival congestion and to reduce the heat and pain of inflammation. Despite frequent application, the active substances from plantain herb are not exactly known. Plantago species have led to the isolation of various natural products including glycosides (e.g. aucubin and catalpol) and five phenylethanoids (acteoside, cistanoside F, lavandulifolioside, plantamajoside and isoacteoside). Aucubin and acteoside have to be considered to be the main constituents with pharmacological effects. In vitro bacteriostatic and bactericidal activity have been shown for aucubin [8–11]. In contrast, for acteoside, impressing anti-inflammatory properties have been reported on the basis of a clear antioxidant effect [12–15], the inhibition of protein kinase C (PKC) [16], a down-regulated expression of intercellular adhesion molecule-1 (ICAM-1) [17,18] and inhibition of 5-hydroxy-6,8,11,14-eicosatetraenoic acid and leukotriene B4-induced inflammation [19,20].
We were particularly interested in the antioxidative potential of the phenylethanoid acteoside isolated from P. lanceolata L. Using the dextran sulphate sodium (DSS)-induced colitis model, we have now assessed whether systemic application of acteoside affects colitis in a mouse model.
Materials and methods
Isolation of acteoside
The herbal drug of P. lanceolata L. was extracted exhaustively with methanol. The methanol was removed on a rotary evaporator at 40°C in vacuo. The dried extract was dissolved in methanol : water = 40 : 60 (v/v). The solution was partioned with ethylacetate three times. The ethylacetate phases were combined and the solvent was removed on a rotary evaporator, as mentioned previously. The residue was dissolved in methanol and the final purification was performed using a preparative high performance liquid chromatography (HPLC)-system; the following conditions were employed: column: Eurospher 100-C18, 16 × 250 mm, 10 µm, Knauer, solvent system: acetonitrile : water = 23 : 77 (v/v), flow rate: 4 ml/min, detection: UV 340 nm. The fractions containing acteoside were pooled and the acetonitrile was removed on a rotary evaporator at 40°C in vacuo. The aqueous residue was lyophilized.
The identity of acteoside [C29H35O15, molecular weight (MW) = 624·61] was proved by its respective nuclear magnetic resonance (NMR)-spectra ([1H], [13C], DEPT-135, DEPT-90, COSY, HETCOR) and FAB-MS-spectra. Solvents used were of p.A. or HPLC grade. The purity of acteoside was > 98% and was determined via thin layer chromatography and HPLC, using the methods specified in the European pharmacopeia.
Mice
Female Balb/c mice weighing 20–22 g (Charles River, Sulzfeld, Germany) were used for the experiments and housed during the experiments in a conventional facility. Animals obtained food and water ad libitum. The animal studies were approved by the local Institutional Review Board.
Induction and treatment of DSS colitis
Acute colitis was induced by feeding 3% DSS over 7 days. Acteoside was extracted and isolated from the plantain herb as described by Paper and Marchesan [21]. Animals were treated with acteoside dissolved in sterile phosphate-buffered saline (PBS) or with sterile PBS alone (no acteoside). Treatment was performed intraperitoneally from days 3–7. Mice not fed with DSS and not treated with acteoside were used as control. DSS colitis is not established fully on day 3; however, the beginning of colitis is indicated by weight loss and has been confirmed by histological analysis in previous experiments. Mice were killed on day 8 as described previously [22].
For induction of chronic colitis, mice received four cycles of DSS treatment. One cycle consisted of feeding 3% DSS in drinking water for 7 days followed by a period of 10 days drinking water without DSS. Treatment with acteoside was performed intraperitoneally over 5 days, 2 weeks after the last cycle of colitis induction.
Assessment of histological score in mice
From the distal third of the colon 1 cm of colonic tissue was removed and used for histological analysis, as described previously [22,23]. Three sections, each obtained at 100-µm distance, were evaluated. Mice were scored individually, each score representing the mean of three sections. Histological examination was performed by an independent investigator blinded to the type of treatment.
Histology was scored as follows:
Epithelium (E) 0: normal morphology; 1: loss of goblet cells; 2: loss of goblet cells in large areas; 3: loss of crypts; 4: loss of crypts in large areas.
Infiltration (I) 0: no infiltrate; 1: infiltrate around crypt basis; 2: infiltrate reaching to L. muscularis mucosae; 3: extensive infiltration reaching the L. muscularis mucosae and thickening of the mucosa with abundant oedema; 4: infiltration of the L. submucosa.
The total histological score represents the sum of the epithelium and infiltration score, and thus ranges from 0 to 8 (total score = E + I). Further, the number of lymphoid follicles was counted in each section. As each section is different in length, the number of counted lymphoid follicles is expressed as number/cm. An increased number of lymphoid follicles counted in histological sections may not be a consequence of de novo generation, but due to more frequent hits in the section caused by bigger size.
Isolation and cytokine enzyme-linked immunosorbent assay (ELISA) of mesenteric lymph node cells (MLN)
MLN (pooled from each group of mice) were collected under sterile conditions in cold cell-culture medium [RPMI-1640, 10% fetal calf serum (FCS), 100 U/ml penicillin and 100 µg/ml streptomycin from Gibco-BRL (Eggenstein, Munich, Germany) and β-mercaptoethanol (3 × 10−5 M, Sigma)]. MLN were collected under sterile conditions in ice-cold medium, disrupted mechanically, and the cell suspension was filtered through a cell strainer (70 µm). Tissue culture plates were coated with monoclonal anti-CD3 antibody (BD Pharmingen, Heidelberg, Germany; 2·5 µg/well); 2 × 105 cells/well were incubated in 200 µl complete medium and stimulated for 24 h with 10 U/ml interleukin (IL)-2 (Chiron, Munich, Germany). Cytokine levels were measured in the supernatant by ELISA (all from Endogene, Woburne, MA, USA) according to the manufacturer's instructions, using four wells per condition.
Demasking of paraffin-embedded sections
Paraffin-embedded sections used for microscopy were cut (5 µm), floated on demineralized water, placed on slides and baked for 30 min at 60°C. Slides were dewaxed for 10 min with xylene and rehydrated in a graded ethanol series (99%, 95%, 70% ethanol and PBS pH 7·4 for 5 min each). For demasking sections were incubated for 30 min with target retrieval solution (S3307; Dako, Hamburg, Germany) at 95°C in a microwave oven.
Immunofluorescence
To block unspecific binding sites PBS with bovine serum albumin (BSA) (5% final concentration) was added. The following monoclonal antibody was used for the identification of macrophages in mouse mucosa: rat anti-mouse F4/80 antigen (clone F4/80, #ab6640, IgG2b, final concentration 2 µg/ml; Abcam, Cambridge, UK; isotype control: rat IgG1 κ, clone R3-34 final concentration 2 µg/ml; BD Biosciences Pharmingen, San Jose, CA, USA). For green staining goat anti-rat secondary antibody (A11006, final concentration 0·2 µg/ml; Molecular Probes, Leiden, the Netherlands) tagged with Alexa Fluor® 488 complex was applied. Reactions were stopped with PBS. Finally the sections were mounted with Vectashield mounting medium with diamidino phenyl indole (DAPI) (H-1500, Vector Laboratories, Burlingame, CA, USA).
MPO activity assay
Colon specimens were rinsed with PBS and homogenized mechanically in 50 mM phosphate buffer with 0·5% hexadecyltrimethylammonium bromide (H-5882, Sigma) with a tissuelyser (#69982, Qiagen, Hilden, Germany). Three freeze-and-thaw cycles were performed. Homogenates were centrifuged for 2 min at maximum speed; 20 µl of the supernatant were transferred to a 96-well plate in duplicate and mixed with 280 µl of 0·02% dianisidine (#D-3252 in 50 mM phosphate buffer and 0·0005% H2O2; Sigma). After 20 min absorbance was measured at 460 nm.
Protein concentration of the supernatant (protein fraction) was determined by Bradford protein assay (Bio-Rad). MPO activity was calculated as mean absorbance (460 nm)/incubation time/protein concentration.
Statistical analysis
Statistical analysis was performed using the paired Student's t-test (cytokine levels, quantification of immunohistochemistry) or the Mann–Whitney rank sum test (histological score, lymphoid follicles). Data are expressed as mean ± s.d. Differences were considered significant at a P-value of < 0·05.
For semiquantitative analysis of macrophage migration in the intestinal mucosa, cells were counted in four high-power fields (hpfs) at a magnification of 200×. The number of migrated macrophages was calculated from four hpfs.
Curve progression of weight loss in acute colitis was calculated with spss version 13·0.1 (Apache Software Foundation, Forest Hill, MD, USA). Data are expressed as mean ± s.d. Differences were considered significant at a PGLM-value of < 0·001.
Results
Effects of acteoside treatment in acute colitis
Using the acute DSS-induced colitis model the effect of systemic application of acteoside was assessed. Mice with established acute DSS-induced colitis received either acteoside (600 µg/mouse a day, n = 19) or PBS (no acteoside, n = 19) per mouse over 5 days. Treatment was performed intraperitoneally from days 3 to 7. Water consumption was not reduced in mice treated with acteoside.
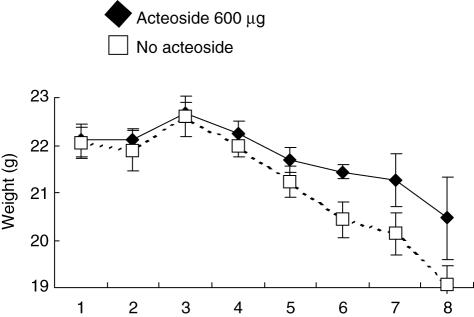
The effect of acteoside treatment on the weight loss over 8 days in acute colitis was determined (Fig. 1). The curve progression of the group treated with acteoside was significantly different (PGLM < 0·001) from the PBS-treated no-acteoside group in three independent experiments with five mice per group.
Fig. 1.
Effect of acteoside treatment on weight loss in acute colitis. Mice with established acute dextran sulphate sodium (DSS)-induced colitis received either acteoside (600 µg a day, black diamonds) or phosphate-buffered saline (PBS) (no acteoside, white squares) per mouse over 5 days. The weight loss was determined over 8 days. The data presented are representative for three independent experiments with five mice per group each. Bars represent mean ± s.e.m. The curve progression of the acteoside-treated group is significantly different from the no-acteoside group (PGLM < 0·001). Statistics were calculated with spss, as described in Material and methods.
The epithelial damage and the inflammatory infiltrate was assessed in four independent experiments with five mice per group. According to decreased inflammation the total histological score for acteoside-treated mice was significantly reduced compared to the PBS-treated no-acteoside mice (Fig. 2a). Numbers of lymphoid follicles were not significantly different between both groups: 1·2 ± 1·0/cm versus 1·0 ± 0·9/cm (Table 1). Colonic sections taken from animals treated with acteoside showed intact crypts in large areas without extensive infiltration or thickening of the mucosa (Figs 2b,c).
Fig. 2.
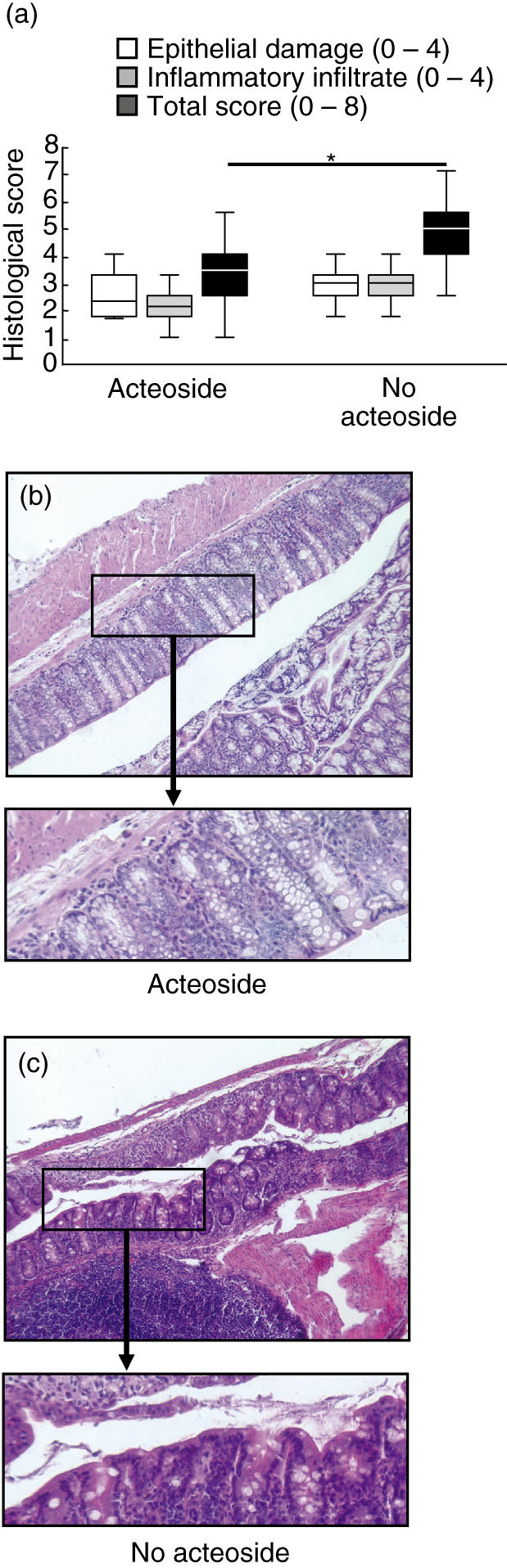
Effect of acteoside treatment on histological parameters in acute colitis. Mice with established acute dextran sulphate sodium (DSS)-induced colitis received either acteoside (600 µg a day) or phosphate-buffered saline (PBS) (no acteoside) per mouse over 5 days. The epithelial damage (white bars), inflammatory infiltrate (grey bars) and total histological score (black bars, a) were determined as described in Materials and methods. The data presented are representative for four independent experiments with five mice per group. The boundary of the box indicates the 25th and the 75th percentiles; the line within the box marks the median. Whiskers indicates the 90th and 10th percentile. Whiskers including < 6 data points were not computed. *Significantly different from the total histological score of the no-acteoside group (a). Two representative colonic haematoxylin and eosin sections from animals treated with acteoside (b) or without acteoside (c) are shown (magnification 100×). From the marked areas, enlarged (three- to fourfold) areas are presented.
Table 1.
Effect of acteoside treatment in acute colitis. Mice with established acute dextran sulphate sodium (DSS)-induced colitis received either acteoside (600 µg a day) or phosphate-buffered saline (PBS) (no acteoside) per mouse over 5 days. The number of lymphoid follicles were determined as described in Materials and methods. The data presented are representative for four independent experiments with five mice per group.
| Acteoside | No acteoside | |
|---|---|---|
| No. of lymphoid follicles | 1·3 ± 1·0 | 1·2 ± 1·1 |
| Length of analysed section (cm) | 1·1 ± 0·2 | 1·2 ± 0·2 |
| No./cm | 1·2 ± 1·0 | 1·0 ± 0·9 |
Effects of acteoside treatment on chronic DSS colitis
For induction of chronic colitis, mice received four cycles of DSS treatment. One cycle consisted of feeding DSS in drinking water for 7 days followed by a period of 10 days drinking water without DSS. Treatment with acteoside was performed intraperitoneally over 5 days, 2 weeks after the last cycle of colitis induction.
Using the chronic DSS-induced colitis model the effectof systemic application of acteoside was assessed. Micewith established chronic DSS-induced colitis received either acteoside (600 µg/mouse, n = 19 or 120 µg/mouse, n = 19) or PBS (no acteoside, n = 19) per mouse over 5 days.
The total histological score was assessed in two independent experiments with seven mice per group. The score from both groups of mice treated with 600 µg or 120 µg/mouse acteoside were improved significantly compared to the PBS-treated no-acteoside group (Fig. 3a). Numbers of lymphoid follicles were not significantly different between the 600 µg, 120 µg/mouse acteoside and the no-acteoside group: 1·8 ± 1·4/cm and 2·5 ± 1·5/cm versus 2·0 ± 1·4/cm, respectively (Table 2) without evidence of altered dimension and distribution. Colonic sections taken from animals treated with 600 µg/mouse acteoside showed intact crypts in large areas without extensive infiltration or thickening of the mucosa (Fig. 3b–d).
Fig. 3.
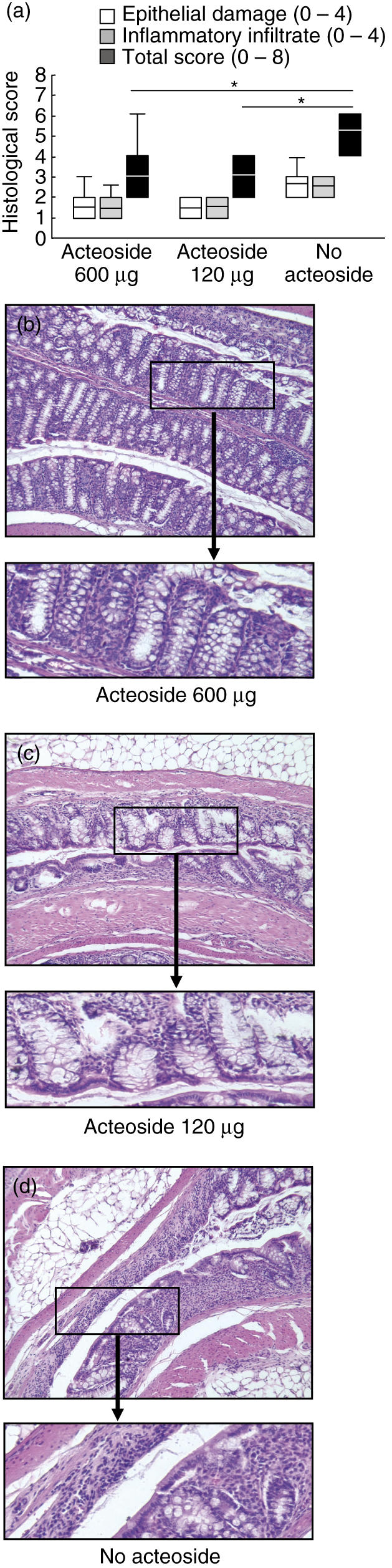
Effect of acteoside treatment on histological parameters in chronic colitis. Mice with established chronic dextran sulphate sodium (DSS)-induced colitis received either acteoside (600 or 120 µg a day) or phosphate-buffered saline (PBS) (no acteoside) per mouse over 5 days. The data presented are representative for two independent experiments with seven mice per group. Box indicates 25th and 75th percentiles and median. Whiskers indicates 90th and 10th percentiles. *Significantly different from the total histological score of the no-acteoside group (a). Three representative colonic haematoxylin and eosin sections from animals treated with 600 µg acteoside (b), 120 µg acteoside (c) or without acteoside (d) are shown (magnification 100×). From the marked areas, enlarged (three- to fourfold) areas are presented.
Table 2.
Effect of acteoside treatment in chronic colitis. Mice with established chronic dextran sulphate sodium (DSS)-induced colitis received either acteoside (600 or 120 µg a day) or phosphate-buffered saline (PBS) (no acteoside) per mouse over 5 days. The data presented are representative for two independent experiments with seven mice per group.
| 600 μg/mouse acteoside | 120 μg/mouse acteoside | No acteoside | |
|---|---|---|---|
| No. of lymphoid follicles | 2·3 ± 1·9 | 3·0 ± 1·6 | 2·2 ± 1·5 |
| Length of analysed section (cm) | 1·3 ± 0·2 | 1·2 ± 0·2 | 1·1 ± 0·2 |
| No./cm | 1·8 ± 1·4 | 2·5 ± 1·5 | 2·0 ± 1·4 |
Effects of acteoside on proinflammatory cytokine secretion from MLN cells and colonic cytokine expression in acute and chronic colitis
The decrease of colonic inflammation as demonstrated by the above macroscopic and microscopic parameters (Figs 1–3) was accompanied by a decreased cytokine secretion from stimulated MLN cells. Lymph node cells were stimulated with anti-CD3 antibodies in the presence of interleukin (IL)-2, and cytokine concentrations in supernatants were determined 24 h later.
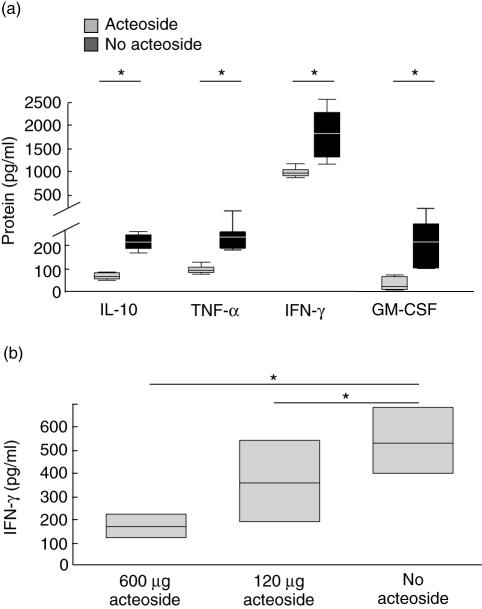
IL-10, tumour necrosis factor (TNF)-α, interferon (IFN)-γ and granulocyte–macrophage colony-stimulating factor (GM-CSF) secretion was decreased significantly in acute colitis in acteoside-treated animals (Fig. 4a).
Fig. 4.
Effect of acteoside treatment on cytokine secretion in acute (a) and chronic (b) colitis. Mesenteric lymph nodes (MLN) were stimulated with anti-CD3 antibody in the presence of interleukin (IL)-2. After incubation for 24 h cytokine levels in supernatants were analysed by beadlyte cytokine detection system, as described in Materials and methods. Cytokine secretion of stimulated MLN from mice with acute dextran sulphate sodium (DSS)-induced colitis treated with acteoside (600 µg per day, grey bars) or phosphate-buffered saline (PBS) (no acteoside, black bars) per mouse over 5 days (a). The data presented are the mean value of four independent experiments with five mice per group each. Interferon (IFN)-γ secretion of stimulated MLN from mice with chronic DSS-induced colitis treated with acteoside (600 or 120 µg per day) or without acteoside per mouse over 5 days. Box indicates 25th and 75th percentiles and median. Whiskers indicates 90th and 10th percentiles (b). The data presented are the mean value of two independent experiments with seven mice per group. Whiskers were not computed. *Significantly different from cytokine secretion of the no-acteoside group.
IFN-γ secretion showed a significant and dose-dependent decrease in lymph node cells from animals with chronic colitis treated with either 600, 120 µg/mouse acteoside versus PBS-treated no-acteoside animals (Fig. 4b).
Effects of acteoside on frequency and distribution of intestinal macrophages and MPO activity in acute and chronic colitis
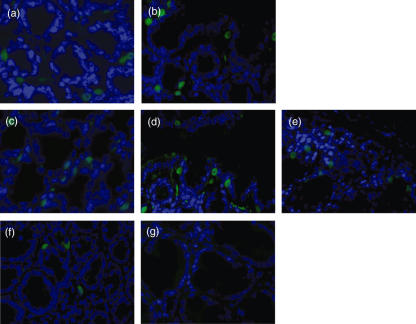
Immunofluorescence staining of paraffin-embedded and demasked sections obtained from intestinal mucosa was performed to localize F4/80 antigen in the intestinal mucosa. F4/80+ macrophages were detected in the lamina propria in a typical inhomogeneous pattern of distribution. In the intestinal mucosa of control mice (without DSS treatment) not treated with acteoside 3·3 ± 1·4 cells stained positive for the macrophage-marker F4/80 (Fig. 5, calculated from four hpfs).
Fig. 5.
Localization of macrophages in the intestinal mucosa. Paraffin-embedded sections were cut and the antigens unmasked for immunostaining. Detection of F4/80 (green) in mice with established acute dextran sulphate sodium (DSS)-induced colitis received either (a) acteoside (600 µg a day) or (b) phosphate-buffered saline (PBS) (no acteoside) per mouse over 5 days. Immunostaining in mice with established chronic DSS-induced colitis received either (c) 600, (d) 120 µg acteoside per day or (e) PBS (no acteoside) per mouse over 5 days. (f) Detection of F4/80 in control mice not fed with DSS and not treated with acteoside. (g) Isotype control (original magnification × 200). No evidence was found for an enhanced recruitment of monocytes into the mucosa in both acute and chronic colitis. Cells were counterstained with diamidino phenyl indole (DAPI). The figures are representative for five independent experiments each.
In acute DSS-induced colitis the recruitment of monocytes/macrophages into the mucosa was enhanced. However, no significant difference was found between the acteoside-treated group (6·9 ± 2·4 cells) and the group treated with only PBS (no acteoside) 7·4 ± 1·2 cells. Similarly, in chronic DSS-induced colitis accumulation of F4/80+ intestinal macrophages was also unchanged and did not depend upon treatment with the phenylethanoid acteoside: 6·9 ± 2·6 cells with 600 µg/day acteoside, 7·4 ± 2·1 cells with 120 µg/day acteoside and 6·8 ± 2·1 cells without acteoside.
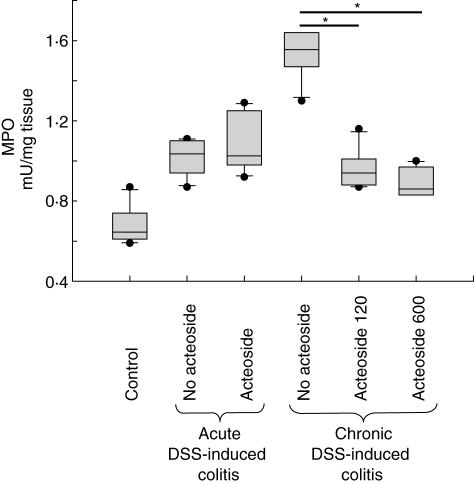
To quantify the formation of reactive oxygen species we applied a MPO activity assay. MPO is a prototypic enzyme activated during oxidative burst reaction. In the mucosa homogenates of control mice (not fed with DSS) and not treated with acteoside 0·7 ± 0·1 mU/mg tissue enzyme activity was detectable (Fig. 6). In both acute and chronic DSS-induced colitis MPO activity was increased. In mucosa homogenates of acute DSS-induced colitis no difference was found between the acteoside-treated group (1·1 ± 0·1 mU/mg tissue) and the group treated with PBS (no acteoside, 1·0 ± 0·1 mU/mg tissue). However, in chronic DSS-induced colitis treatment with phenylethanoid acteoside caused significantly reduced MPO activity compared to PBS (no acteoside): 0·9 ± 0·1 mU/mg tissue with acteoside treatment (600 µg a day), 1·0 ± 0·1 mU/mg tissue with acteoside treatment (120 µg a day) and 1·5 ± 0·1 mU/mg tissue with PBS, no-acteoside (P < 0·05 both).
Fig. 6.
Myeloperoxidase (MPO) activity assay. Enzyme activity in mucosa homogenates of control mice not fed with dextran sulphate sodium (DSS) and not treated with acteoside (control) compared to both acute and chronic DSS-induced colitis treated or not treated with acteoside. Significantly increased MPO activity was detected in chronic DSS-induced colitis (P < 0·3 10−5versus control). Treatment with acteoside significantly reduced MPO activity in the mucosa (P < 0·05 both); n = five mice per group. The assay was performed in duplicate.
Discussion
The generation of toxic oxygen species is instrumental to the tissue damage of diverse conditions, including infection, ischaemic injury, arthritis and other chronic inflammatory and autoimmune disorders [24], and contribute to mutation and carcinogenesis [25].
In previous work we demonstrated an increased oxidative burst activity in intestinal macrophages from patients with IBD [1]. The production of superoxide and other reactive oxygen intermediates responsible for microbicidal, tumoricidal and inflammatory activities in phagocytes is mediated by a membrane-associated NADPH oxidase [25]. We could show an increased expression for two subunits of NADPH oxidase. The enzyme system is responsible for superoxide generation and forms a small transmembrane electron transport system that results in the oxidation of NADPH on the cytoplasmic surface and the generation of superoxide on the outer surface of the membrane.
For the present study we used the herbal phenylethanoid acteoside, isolated from P. lanceolata L., that was shown to exhibit anti-oxidative potential. Our data suggest that in vivo treatment with acteoside ameliorates intestinal inflammation in both acute and chronic DSS-induced colitis. Mice with acute or chronic DSS-induced colitis which received acteoside treatment exhibited a significantly diminished histological score. Significantly diminished weight loss as a result of acteoside treatment in acute colitis confirmed these data. A decrease in production of several cytokines with important immunoregulatory and proinflammatory activities has been demonstrated during acteoside treatment. These cytokines, including IFN-γ, TNF-α and GM-CSF, have important roles in the initiation and amplification of inflammatory responses that lead to intestinal injury. Earlier reports have shown that the critical proinflammatory mediators TNF-α and IFN-γ contribute to tissue damage in chronic DSS-induced colitis [22,26]. Activated T cells are known to secrete growth factors such as GM-CSF, and these growth factors have been shown to stimulate the proliferation of haematopoietic cells, followed by differentiation to granulocytes or macrophages [27]. As a result of acteoside treatment in acute colitis IL-10, TNF-α, IFN-γ and GM-CSF cytokine expression was significantly down-regulated in mesenteric lymph nodes. In chronic colitis IFN-γ was the predominant cytokine, probably reflecting a difference between the two colitis models or different stages of the disease.
Oxidative burst activity leading to the generation of toxic oxygen species is increased in macrophages in inflamed mucosa [1]. Therefore, we studied the effect of acteoside treatment on the number and distribution of macrophages being the main toxic oxygen producer in the mucosa. Inflammation-induced recruitment of macrophages was determined in both acute and chronic DSS-induced colitis with and without acteoside treatment and compared to non-DSS-exposed controls. The phenylethanoid acteoside did not influence the colitis-induced recruitment of mononuclear cells into the mucosa indicating that it had no influence on the generation of chemokines or the expression of proteins involved in cell evasion from the bloodstream, such as vascular cell adhesion molecule (VCAM), mucosal adressin cell adhesion molecule 1 (MadCAM) or liver cell adhesion molecule (LCAM).
MPO activity is a useful indicator for the generation of reactive oxygen species contributing to local tissue damage during inflammation. Acteoside significantly decreased MPO activity in mice with chronic DSS-induced colitis. As the number of F4/80+ cells was unchanged by acteoside as well as the number of neutrophils, it is unlikely that the reduction of MPO activity was caused by an effect of acteoside on the cellular composition of the inflammatory infiltrate. The elimination of reactive oxygen species may be an important factor for the amelioration of tissue damage seen after application of acteoside in DSS-induced colitis. Our data do not allow us to postulate that the reduction of MPO activity by the herbal agent is crucial for and exclusively responsible for its activity in DSS-induced colitis. The remarkable benefit of the acteoside treatment and the down-regulation of IFN-γ expression could result from other unknown properties of the substance.
Several reports have already indicated that acteoside is able to suppress important proinflammatory mediators. It inhibits the enzymatic activity of (1) inducible nitric oxide synthase (iNOS) [28] expressed in both macrophages and neutrophils and (2) 5-lipoxygenase [19,20]. (1) Nitric oxide (NO) is released in the immune system by iNOS, where it facilitates the killing of invading microorganisms [29]. However, high NO production by iNOS was shown to provoke inflammatory diseases [30]. iNOS is under control of the transcriptional regulation activator protein-1 [31]. The effect of acteoside on iNOS and NO production could be comfirmed in in vitro experiments by blocking activator protein-1 activation in macrophages [28]. Thus the anti-inflammatory effect of acteoside is dependent upon blocking transcriptional activation of genes associated withinflammation (e.g. iNOS). (2) Inhibition of 5-lipoxygenase results in the inhibition of 5-hydroxy-6,8,11,14-eicosatetraenoic acid and leukotriene B4-induced inflammation [19,20].
In addition to the interference with enzyme activity, the inflammatory potential of immune cells invading from the peripheral blood system to the inflamed bowel could be down-regulated at an earlier time: at their recruitment. Acteoside treatment was shown to decrease significantly the up-regulation of ICAM-1 expression in nephritic glomeruli during the development of glomerulonephritis in an in vivo model [17,18]. Further, it prevented the up-regulation of ICAM-1 expression mediated by inflammatory cytokines or phorbol 12-myristate 13-acetate on human umbilical vein endothelial cells and rat mesangial cells. Interestingly, the regulatory effect of acteoside on ICAM-1 expression is explained by its inhibition of PKC [16]: PKC activated by TNF-α or phorbol 12-myristate 13-acetate was shown to induce ICAM-1 [32]. However, recruitment of murine macrophages was not altered in our experiments.
Taken together, these findings provide evidence that acteoside treatment ameliorates intestinal inflammation in DSS-induced colitis and in the future could become part of anti-inflammatory therapy.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Ro 1236/3–2, SFB 585, Projekt A6, BMBF Kompetenznetz CED).
References
- 1.Hausmann M, Spottl T, Andus T, et al. Subtractive screening reveals up-regulation of NADPH oxidase expression in Crohn's disease intestinal macrophages. Clin Exp Immunol. 2001;125:48–55. doi: 10.1046/j.1365-2249.2001.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–22. [PubMed] [Google Scholar]
- 3.Henderson LM, Chappel JB. NADPH oxidase of neutrophils. Biochim Biophys Acta. 1996;1273:87–107. doi: 10.1016/0005-2728(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 4.Allgayer H. Clinical relevance of oxygen radicals in inflammatory bowel disease − facts and fashion. Klin Wochenschr. 1991;69:1001–3. doi: 10.1007/BF01645146. [DOI] [PubMed] [Google Scholar]
- 5.Yamada T, Grisham MB. Role of neutrophil-derived oxidants in the pathogenesis of intestinal inflammation. Klin Wochenschr. 1991;69:988–94. doi: 10.1007/BF01645144. [DOI] [PubMed] [Google Scholar]
- 6.Bulger EM, Helton WS. Nutrient antioxidants in gastrointestinal diseases. Gastroenterol Clin North Am. 1998;27:403–19. doi: 10.1016/s0889-8553(05)70010-8. [DOI] [PubMed] [Google Scholar]
- 7.Lih-Brody L, Powell SR, Collier KP, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 8.Ellich J. FU Berlin: 1996. Antibacterial activity of plantain herb (Antibakterielle Aktivität einiger Plantago-Arten) Dissertation. [Google Scholar]
- 9.Haensel R. Bitter compounds of the monoterpen series (Glykosidische Bitterstoffe der Monoterpenreihe) Dtsch Apoth Ztg. 1996;106:1761–7. [Google Scholar]
- 10.Ellich J. The antibacterial principle of plantain herb (Das antibakterielle Prinzip einiger einheimischer Plantago-arten) Dtsch Apoth Ztg. 1966;106:428. [Google Scholar]
- 11.Ellich J. The antibacterial principle of plantain herb (Das antibakterielle Prinzip unserer einheimischen Plantago-Arten) Dtsch Apoth Ztg. 1961;101:1387. [Google Scholar]
- 12.Pan N, Hori H. Antioxidant action of acteoside and its analogs on lipid peroxidation. Redox Rep. 1996;2:149–54. doi: 10.1080/13510002.1996.11747042. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Kang J, Zheng R, et al. Scavenging effects of phenylpropanoid glycosides from Pedicularis on superoxide anion and hydroxyl radical by the spin trapping method (95)02255–4. Biochem Pharmacol. 1996;51:687–91. doi: 10.1016/s0006-2952(95)02255-4. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Zheng R, Su B, et al. Repair of dGMP hydroxyl radical adducts by verbascoside via electron transfer: a pulse radiolysis study. Int J Radiat Biol. 1996;69:481–5. doi: 10.1080/095530096145779. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wang PF, Zheng R, Liu ZM, Jia Z. Protection of phenylpropanoid glycosides from Pedicularis against oxidative hemolysis in vitro. Planta Med. 1993;59:315–17. [PubMed] [Google Scholar]
- 16.Herbert JM, Maffrand JP, Taoubi K, Augereau JM, Fouraste I, Gleye J. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J Nat Prod. 1991;54:1595–600. doi: 10.1021/np50078a016. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Nagamatsu T, Ito M, Hattori T, Suzuki Y. Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (2): effect of acteoside on leukocyte accumulation in the glomeruli of nephritic rats. Jpn J Pharmacol. 1994;66:47–52. doi: 10.1254/jjp.66.47. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Nagamatsu T, Ito M, Yagita H, Suzuki Y. Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (3): effect of aceteoside on expression of intercellular adhesion molecule-1 in experimental nephritic glomeruli in rats and cultured endothelial cells. Jpn J Pharmacol. 1996;70:157–68. doi: 10.1254/jjp.70.157. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Okuda H, Nishibe S, Arichi S. Effects of caffeoylglycosides on arachidonate metabolism in leukocytes. Planta Med. 1987;53:148–53. doi: 10.1055/s-2006-962658. [DOI] [PubMed] [Google Scholar]
- 20.Ravn H, Nishibe S, Sasahara M, Xuebo L. Phenolic compounds from Plantago asiatica. Phytochemistry. 1990;29:3627–31. [Google Scholar]
- 21.Paper DH, Marchesan M. Plantain herb [Spitzwegerich (Plantago lanceolata L.)] Z Phytother. 1999;20:231–8. [Google Scholar]
- 22.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–45. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitisby Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis. 1995;54:505–10. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojouharoff G, Hans W, Obermeier F, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark SC, Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987;236:1229–37. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Woo ER, Kang KW. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J Ethnopharmacol. 2005;97:561–6. doi: 10.1016/j.jep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 29.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 30.Blantz RC, Munger K. Role of nitric oxide in inflammatory conditions. Nephron. 2002;90:373–8. doi: 10.1159/000054723. [DOI] [PubMed] [Google Scholar]
- 31.Kristof AS, Marks-Konczalik J, Moss J. Mitogen-activated protein kinases mediate activator protein-1-dependent human inducible nitric-oxide synthase promoter activation. J Biol Chem. 2001;276:8445–52. doi: 10.1074/jbc.M009563200. [DOI] [PubMed] [Google Scholar]
- 32.Mattila P, Majuri ML, Mattila PS, Renkonen R. TNF alpha-induced expression of endothelial adhesion molecules, ICAM-1 and VCAM-1, is linked to protein kinase C activation. Scand J Immunol. 1992;36:159–65. doi: 10.1111/j.1365-3083.1992.tb03087.x. [DOI] [PubMed] [Google Scholar]