Abstract
Deficiency of both mannan-binding lectin (MBL) and complement components C4 and C2 has been associated with increased risk of systemic lupus erythematosus (SLE). MBL can activate the complement system either through C4 and C2 or directly through C3. Circulating immune complexes (CICs) are believed to play a pathogenic role in SLE and MBL has been shown to bind certain forms of immunoglobulins, including IgM, IgG and IgA. Thus, MBL might promote CIC clearance. In order to evaluate this, six individuals with non-functional classical pathway due to the rare homozygous C2 deficiency were chosen, as the classical pathway is known to have a fundamental role in CIC clearance. Four of the six C2-deficient individuals had SLE, two of whom also had MBL deficiency. MBL serum levels and genotypes were compared with the serum levels of CICs, as measured by their content of kappa, lambda, IgM, IgA, IgG and C3 opsonization. The C2-deficient individuals had higher serum levels of CICs than 16 healthy controls (P < 0·0001). Furthermore, an inverse association was observed between MBL and CIC levels in the C2-deficient individuals, which was strongest for IgM-CICs (r = − 0·84, P = 0·037). Moreover, C3 opsonization of the CICs correlated positively with MBL levels in the C2-deficient individuals (r = 0·89, P = 0·017). In conclusion, individuals with C2 deficiency have increased levels of CICs and MBL may facilitate their clearance. Defective CIC clearance might partly explain the increased risk of SLE associated with low MBL.
Keywords: clearance, complement C2, deficiency, immune complexes, mannan-binding lectin, systemic lupus erythematosus
Introduction
Complement activation is a double-edged sword in systemic lupus erythematosus (SLE). While complement activation locally in tissues can be harmful, the complement system has an important role in continuous non-inflammatory clearance of circulating immune complexes (CICs). This has been demonstrated in a C2-deficient SLE patient, who for many years has shown a consistent clinical improvement and lowered CIC levels after regular infusions of fresh frozen plasma to resubstitute C2 [1,2]. However, complement-mediated clearance of CICs in vivo has been analysed hitherto only in the context of the classical pathway, without taking into account the possibility that the lectin pathway of complement activation may also be involved. Little is known about the significance of the lectin pathway when other components of the complement system are impaired [3]. Both C1q of the classical pathway and mannan-binding lectin (MBL) of the lectin pathway can activate the complement system through C4 and C2, but it has been shown recently that MBL can also activate C3 directly [4]. MBL activates the lectin pathway after binding to surface carbohydrate residues on various microorganisms and outworn self-components, including apoptotic debris [5]. MBL has also been reported to bind agactosylated IgG [6] and IgG complexes from rheumatoid arthritis (RA) patients [7] and furthermore it has been shown to bind polymeric IgA [8]. Recently, MBL was also shown to bind around 20% of human IgM glycoforms [9]. Thus, MBL might have a role in CIC clearance. Depending on definition, 10–30% of Caucasians have MBL deficiency [10], which is a similar proportion to the C2-deficient individuals who suffer from SLE [3]. We have recently reported an association between low MBL and SLE in multi-case SLE families [11], and low MBL together with partial C4 deficiency may have an additive effect on the risk of SLE [11,12]. Notably, the C2-deficient SLE patient mentioned above [1,2] also has undetectable MBL levels (unpublished observation). We hypothesized that if MBL is low, CICs may accumulate to a threshold level that triggers or augments SLE symptoms and flare-ups. This may be particularly important in individuals who also have a deficiency of C2 and/or C4. As the classical pathway is known to have a fundamental role in CIC clearance, individuals with a non-functional classical pathway due to C2 deficiency were chosen to evaluate whether MBL might also have a role in CIC clearance.
Patients and methods
The study was carried out in accordance with the Helsinki Declaration, and was approved by the Ethics Committee of the University Hospital in Iceland and the Icelandic Computer Database Committee. All participants provided informed consent.
Subjects
The study subjects were six individuals with total C2 deficiency, of whom four had SLE. The remaining two had recurrent infections without symptoms of SLE. Of the SLE patients, two also had a history of recurrent or severe infections. The SLE patients all had positive anti-nuclear antibodies (ANA), malar rash and arthritis. In addition, three had other antibodies included in the SLE classification criteria (immunological disorder), two had photosensitivity and the following criteria were fulfilled in one patient each: serositis (pericarditis), discoid rash, oral ulcers and haematological disorder (thrombocytopenia). All six C2-deficient individuals had normal C4 levels. For comparison, sera were used from 16 healthy controls with normal C2 and MBL.
MBL serum levels and genotypes
MBL serum levels were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) system, as described previously [11], and MBL was genotyped by a real-time–polymerase chain reaction (RT–PCR) technique performed with the LightCycler™ Instrument (Roche Diagnostics, Mannheim, Germany), as described previously by Steffensen et al. [13]. Using temperature curve analysis, the wild-type A allele and the three mutant structural alleles [at codons 54(B), 57(C) and 52(D)] within exon 1, that are associated with low MBL serum levels, were detected in a single reaction.
Levels of CICs
CICs were measured in serum by a novel ELISA system that does not require an initial isolation step (Proceptor™, ProgenBiologics, Wildwood, MO, USA). The ELISA plates were precoated with ProGen's Proprietary Receptors™ isolated from lymphoid cell lines. These purified receptors selectively bind complexed immunoglobulins. Briefly, sera diluted 1/200 in a washing buffer were incubated for 90 min at room temperature (RT). A serial dilution of aggregated human IgG (30 µg/ml) was used as a standard for the IgG content of the CICs as recommended by the manufacturer. For IgM and IgA content of the CICs, control samples containing CICs with high levels of IgM or IgA isotypes were also provided by the manufacturer. These undiluted control samples were given a value of 100 arbitrary units (AU) and standard curves were obtained by serial dilutions. Horseradish peroxidase (HRP)-conjugated polyclonal antibody to kappa and lambda and to each of the three Ig isotypes (µ, ã, α) was added for 60–90 min at RT in order to estimate the total amount of CICs and their relative content of IgM, IgG and IgA, respectively. The degree of complement C3 opsonization of the complexes was also assessed using other ELISA plates containing the same Proceptor™ coating but HRP-conjugated detection antibodies against C3. An attempt was also made to detect MBL in the complexes that bound to the Proceptor plates by adopting the monoclonal antibodies (clone 131–1, Statens Serum Institute, Copenhagen, Denmark) used in the sandwich ELISA system for measuring serum MBL [11].
After incubation with tetramethylbenzidine (approximately 7 min for C3-, IgM- and IgG-CIC; 20 min for IgA-CIC), the reaction was stopped with 0·5 N sulphuric acid and the absorbance read at 450 nm. The microtitre wells were washed three times with phosphate-buffered saline (PBS)/Tween-20 after each step. The total CIC levels measured by this novel assay were also compared to two more cumbersome assay systems that have previously been used extensively in our laboratory [1].
Statistical analysis
Unpaired t-test was used to compare two groups, analysis of variance (anova) for comparison of three groups and repeated-measures anova for changes in continuous variables over time. Pearson's correlation was used for comparison of two continuous variables. Statistical analysis was performed using Prism 4 (GraphPad) software. All tests were two-sided and the level of significance was set at P < 0·05.
Results
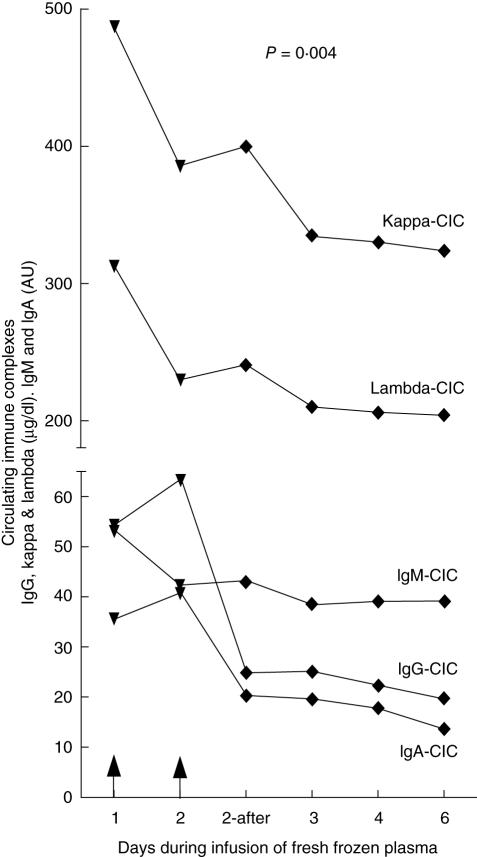
In order to validate the Proceptor™ ELISA system, CICs were measured in serial serum samples taken during an infusion of fresh frozen plasma to a SLE patient with both C2 and MBL deficiency, thus reconstituting both C2 and MBL. As seen in Fig. 1, the serum CIC levels in this patient were lowered following the infusions in a very similar fashion, as had been reported previously after infusion of fresh frozen plasma into the same patient using other, more cumbersome, CIC detection methods. These assay systems, referred to as the complement consumption assay and the immune complex red cell binding assay, have been described previously [1]. The CIC levels in the C2-deficient subjects, and also in the patient after plasma infusion measured by the novel Proceptor™ system, were compared to CIC levels measured by these methods and showed a very good correlation. The levels of kappa and lambda chains were assumed to reflect the total CIC levels. Based on these findings, we decided to use this simple assay for measurement of CICs in this study.
Fig. 1.
Changes in the total level of circulating immune complexes (CICs) and their relative contents of the IgG, IgA and IgM isotypes, before and after infusions of fresh frozen plasma to one systemic lupus erythematosus (SLE) patient who is both C2- and mannan-binding lectin (MBL)-deficient. Arrows indicate timing of the plasma infusions (4 units on day 1 and 3 units on day 2). The MBL concentration in the patient's blood rose from < 20 µg/l before the infusion to 174 µg/l after the infusion on day 2, and then fell to 63 µg/l on day 4 and was < 20 µg/l on day 6 after the infusion.
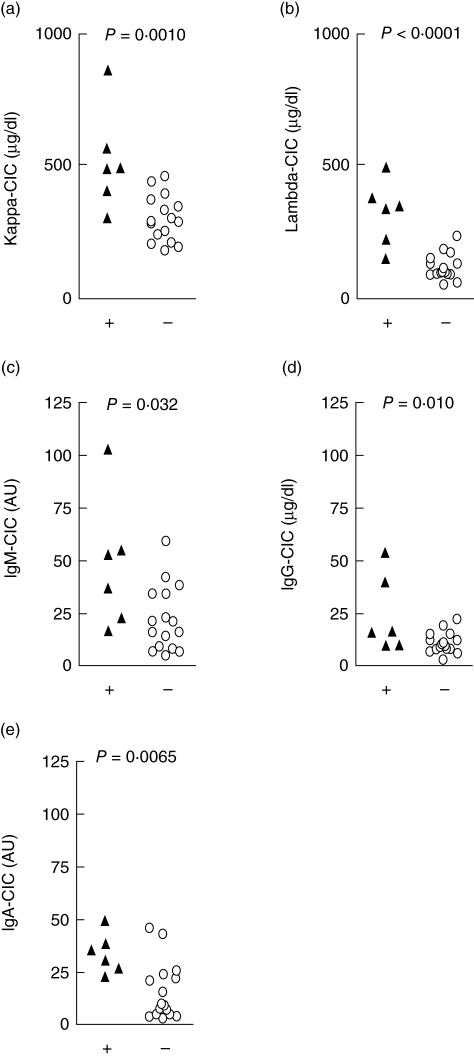
The levels of CICs, as judged by their content of kappa and lambda chains and the three main immunoglobulin isotypes (IgG, IgM and IgA) in the six individuals with a complete C2 deficiency, were compared to the healthy controls (Fig. 2). The C2-deficient patients had higher levels of CICs than the controls, as judged both by the amount of kappa and lambda light chains (P = 0·001 and P < 0·0001, respectively, Fig. 2a,b), and their content of IgM, IgG and IgA (P = 0·032, P = 0·010 and P = 0·0065, respectively, Fig. 2c–e). Notably, the two C2-deficient individuals who had the highest levels of IgG-CIC both had SLE. Moreover, two of the six C2-deficient individuals did not have a history of recurrent infections and they had the lowest IgM-CIC levels.
Fig. 2.
Comparison of the levels of circulating immune complexes (CICs) measured by their content of kappa (a) and lambda (b) chains and also the individual Ig isotypes (c–e: IgM, IgG and IgA) in the six C2-deficient patients (black triangles) compared to controls with normal C2 and mannan-binding lectin (MBL) (open circles).
Two of the C2-deficient individuals also had unmeasurable MBL levels. One was the SLE patient who has been receiving plasma infusions for many years (Fig. 1), and she is homozygous for the C variant structural MBL allele. The other patient was heterozygous for the D variant structural MBL allele. Neither of the two C2-deficient individuals who only had infections had low MBL levels and they were both homozygous for the wild-type structural MBL alleles.
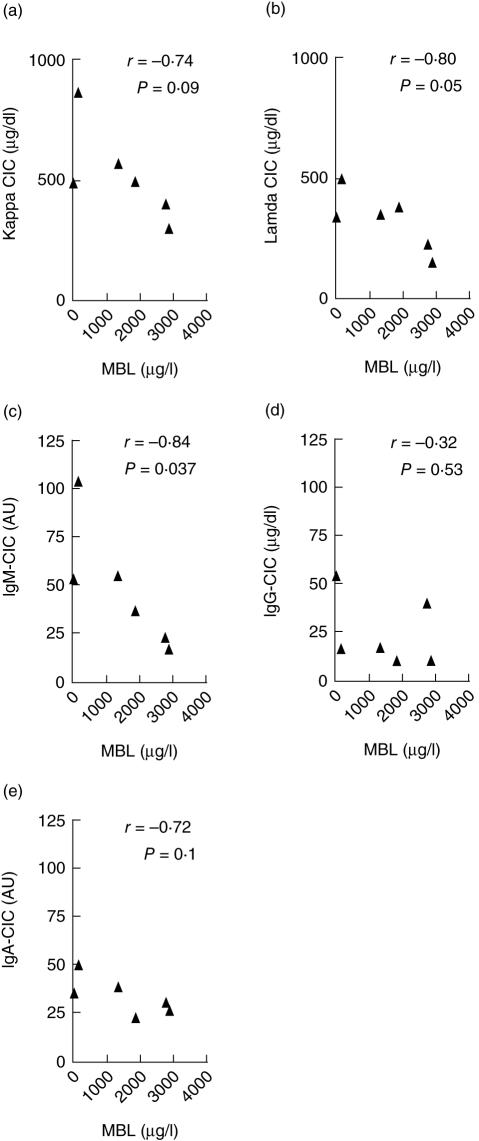
As the clearance of CICs from the circulation via the classical complement pathway is largely blocked in C2-deficient individuals, it was of interest to study the association between the levels of MBL and CICs in the context of C2 deficiency. An inverse association was observed between the levels of MBL and the CICs (Fig. 3), as detected both by their content of lambda (r = −0·80, P = 0·05) and kappa chains (r = −0·74, P = 0·09). This also applied to the IgM content of the CICs (r = −0·84, P = 0·037) and a similar trend was seen for the IgA isotype (r = −0·72, P = 0·1) but not for IgG (r = −0·32, P = 0·5).
Fig. 3.
On a background of total C2 deficiency, an inverse trend association was observed between mannan-binding lectin (MBL) and circulating immune complex (CIC) levels as measured by their content of kappa (a) and lambda (b) chains and the IgM (c), IgG (d) and IgA (e) isotypes.
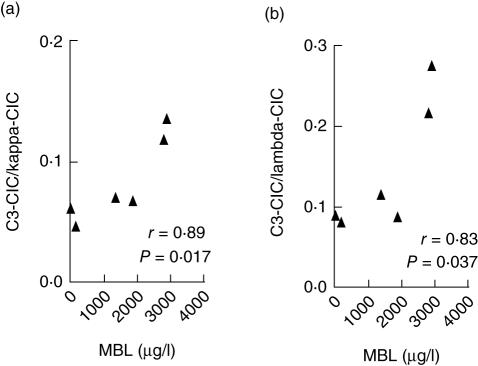
Deposition of the complement component C3 (C3d) indicates an ongoing complement activation. Supporting the notion that MBL helps the clearance of CICs, the relative amount of C3 deposited onto the CICs correlated fairly strongly with the levels of MBL in the C2-deficient individuals (r = 0·89, P = 0·017 and r = 0·83, P = 0·037; Fig. 4). However, MBL could not be detected in the CICs captured by the Proceptor plates.
Fig. 4.
The proportion of circulating immune complexes (CICs) with C3 deposition is associated directly with the mannan-binding lectin (MBL) levels in the individuals with a total C2 deficiency (a: kappa content; b: lambda content).
Discussion
To our knowledge, this is the first study analysing MBL in relation to CIC levels, thus addressing the question of whether MBL may, like the classical complement pathway, have a role in the clearance of immune complexes. Individuals with non-functional classical pathway due to complement C2 deficiency were chosen to evaluate independently the role of MBL.
Not surprisingly, the C2-deficient individuals had markedly higher CICs levels than the healthy controls, highlighting the role of the classical pathway in CIC clearance. However, an inverse association was observed between MBL and CIC levels in the C2-deficient sera, and this was particularly evident for CICs with a high IgM content. Furthermore, we observed an inverse association between the relative amount of C3 on the CICs and MBL levels in the individuals with C2 deficiency. This is in accordance with the recent finding that MBL can bypass C2 by directly activating C3 [4]. Taken together, these findings indicate that MBL may have a role in the clearance of CICs at least in C2-deficient individuals. This is in accordance with recent studies, where MBL has been shown to bind certain immunoglobulin isotypes in vitro [6–9]. Our study is the first attempt to evaluate the potential effect of such MBL binding on the overall levels of CICs in vivo. However, we were not able to detect MBL in CICs bound to the Proceptor™ plates. This might be due to steric hindrance or that MBL bound to CICs was below the detection limit of the method we used. Furthermore, it can be envisaged that CICs with relatively high MBL content are cleared preferentially from the circulation. We are currently studying whether MBL can bind CICs in vitro under conditions that mimic the in vivo microenvironmental scenario as far as possible.
The simple CIC detection assay used correlated well with more complex assays that have been used for measuring CIC levels [1]. It also distinguished well between patients and controls and has a potential utility for routine application. Thus, changes in serum levels of CIC during plasma infusions to a C2-deficient SLE patient measured with the new assay were very similar to those measured previously with the more complex assays. A homozygous C2 deficiency is rare, and this study is based on all the six individuals in Iceland known to have C2 deficiency. However, the results are significant despite the small size of this precious study group, and the same mechanisms are likely to also apply to individuals without C2 deficiency.
It is concluded that MBL may promote the clearance of CICs in vivo, probably via direct activation of C3 [4]. Low MBL levels or associated genotypes have been associated with the risk of SLE both in multi-case family studies [11] and a recent meta-analysis [12], and this might be due partly to defective CIC clearance. A phase I clinical trial of MBL infusion to healthy volunteers has now been conducted both for purified [14] and recombinant MBL [15] without any adverse effects. It remains to be elucidated whether infusions of MBL may have a therapeutic role in patients with immune complex mediated diseases such as SLE.
Acknowledgments
We wish to thank Thora Vikingsdottir for assisting with control samples and her, along with Kristin Traustadottir, Kristjan Erlendsson, Gudmundur J. Arason and Raghildur Kolka, Landspitali University Hospital, Iceland for their methodological advice. The study received funding from the Icelandic Research Fund for Graduate Students and the Science Fund of Landspitali University Hospital. The authors have no conflicts of interest.
References
- 1.Erlendsson K, Traustadottir K, Freysdottir J, Steinsson K, Jonsdottir I, Valdimarsson H. Reciprocal changes in complement activity and immune-complex levels during plasma infusion in a C2-deficient SLE patient. Lupus. 1993;2:161–5. doi: 10.1177/096120339300200306. [DOI] [PubMed] [Google Scholar]
- 2.Steinsson K, Erlendsson K, Valdimarsson H. Successful plasma infusion treatment of a patient with C2 deficiency and systemic lupus erythematosus: clinical experience over forty-five months. Arthritis Rheum. 1989;32:906–13. [PubMed] [Google Scholar]
- 3.Sjoholm AG, Jonsson G, Braconier JH, Sturfelt G, Truedsson L. Complement deficiency and disease: an update. Mol Immunol. 2006;43:78–85. doi: 10.1016/j.molimm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Selander B, Martensson U, Weintraub A, et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116:1425–34. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saevarsdottir S, Vikingsdottir T, Valdimarsson H. The potential role of mannan-binding lectin in the clearance of self-components including immune complexes. Scand J Immunol. 2004;60:23–9. doi: 10.1111/j.0300-9475.2004.01437.x. [DOI] [PubMed] [Google Scholar]
- 6.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1:237–43. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 7.Garred P, Madsen HO, Marquart H, et al. Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J Rheumatol. 2000;27:26–34. [PubMed] [Google Scholar]
- 8.Royle L, Roos A, Harvey DJ, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–53. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 9.Arnold JN, Wormald MR, Suter DM, et al. Human serum IgM glycosylation: identification of glycoforms which can bind to mannan binding lectin. J Biol Chem. 2005;280:29080–7. doi: 10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick DC. Mannan-binding lectin: clinical significance and applications. Biochim Biophys Acta. 2002;1572:401–13. doi: 10.1016/s0304-4165(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 11.Saevarsdottir S, Kristjansdottir H, Grondal G, Vikingsdottir T, Steinsson K, Valdimarsson H. Mannan binding lectin and complement C4A in Icelandic multicase families with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:1462–7. doi: 10.1136/ard.2005.046086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YH, Witte T, Momot T, et al. The mannose-binding lectin gene polymorphisms and systemic lupus erythematosus: two case–control studies and a meta-analysis. Arthritis Rheum. 2006;52:3966–74. doi: 10.1002/art.21484. [DOI] [PubMed] [Google Scholar]
- 13.Steffensen R, Hoffmann K, Varming K. Rapid genotyping of MBL2 gene mutations using real-time PCR with fluorescent hybridisation probes. J Immunol Meth. 2003;278:191–9. doi: 10.1016/s0022-1759(03)00190-x. [DOI] [PubMed] [Google Scholar]
- 14.Valdimarsson H, Vikingsdottir T, Bang P, et al. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand J Immunol. 2004;59:97–102. doi: 10.1111/j.0300-9475.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 15.Petersen KA, Matthiesen F, Agger T, et al. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J Clin Immunol. 2006;26:465–75. doi: 10.1007/s10875-006-9037-z. [DOI] [PubMed] [Google Scholar]