Abstract
Innate immune molecules such as lung collectins and serum pentraxins have evolved as important host defence proteins against Aspergillus fumigatus, a medically important opportunistic fungal pathogen. Mannan-binding lectin (MBL), an opsonin and lectin complement pathway activator, constitutes another vital player of innate immunity against several pathogenic organisms in the serum. Studies have reported significant binding of MBL to A. fumigatus; however, the protective role of MBL against A. fumigatus-mediated invasive disease remains elusive. Henceforth, we investigated the contribution of externally administered recombinant human (rh) MBL towards anti-fungal defence in invasive pulmonary aspergillosis (IPA) by in vivo and in vitro studies. In murine models of IPA with corticosteroid-induced immunosuppression, rhMBL-treated mice showed 80% survival compared to untreated IPA mice with no survivors. Treated IPA mice also showed a marked increase in tumour necrosis factor (TNF)-α and interleukin (IL)-1α and a significant decrease in pulmonary fungal hyphae and IL-10. In vitro, rhMBL-bound A. fumigatus conidia showed a dose-dependent increase in the deposition of C4b, the first product of the lectin pathway. There was an enhanced uptake of A. fumigatus conidia by the polymorphonuclear cells (PMNs) in the presence of rhMBL that increased further in the presence of MBL supplemented with MBL-deficient serum. However, an increase in the oxidative burst of PMNs and A. fumigatus killing were observed only when MBL was supplemented with MBL-deficient serum. The study suggests a therapeutic role of ex vivo-administered MBL in host defence against aspergillosis, possibly through MBL-mediated complement activation and other protective mechanisms aimed both directly at the pathogen, and indirectly through modulation of the host inflammatory responses.
Keywords: Aspergillus fumigatus, invasive pulmonary aspergillosis, mannan-binding lectin
Introduction
Aspergillus fumigatus is an airborne, opportunistic fungal pathogen that presents itself in a wide range of allergic and invasive clinical manifestations depending on the immune status of the host [1]. Invasive pulmonary aspergillosis (IPA), caused by A. fumigatus, is an important cause of morbidity and mortality in immunocompromised hosts [2,3]. The disease is manifested by colonization of the fungus in the lungs, hyphal invasion and destruction of the pulmonary tissue [2,3]. Although amphotericin B (AmB) has been the traditional treatment of choice for IPA, the previously inert spectrum of anti-fungal choices is changing rapidly, with newer anti-fungals and newer targets. However, even these newer therapies have been able to elevate the clinical response rates only to approximately 50% [4], and invasive aspergillosis is still associated with a high mortality rate that ranges from 30% to 90% [5].
Non-specific or natural immunity plays a major role in defence against A. fumigatus by recognition and clearance of the organism in immunocompetent hosts [1]. Effector cells such as pulmonary alveolar macrophages (PAMs), polymorphonuclear cells (PMNs), platelets and innate immune molecules such as anti-microbial peptides, Toll-like receptors, pentraxins, complement proteins and collectins conspire to restrict the initial spread of this fungal pathogen [6,7]. Hence, one way of expanding the current armamentarium for IPA is to hunt for plausible molecules and mechanisms that strengthen initial host immune responses against A. fumigatus. For example, the anti-fungal and immunomodulatory role of lung collectins such as surfactant proteins A and D, and serum pentraxins in host defence against A. fumigatus has been well established in recent years [8–10]. Another important host defence molecule of innate immunity, sharing close structural similarity with SP-A and SP-D, is the serum protein mannan-binding lectin (MBL) [11,12]. MBL being a soluble pattern recognition molecule, similar to the lung collectins, exhibits a number of anti-microbial activities after binding to carbohydrate moieties of pathogenic organisms [13]. Besides binding and opsonizing its target, MBL also activates the lectin complement pathway utilizing the MBL-associated serine proteases (MASPs) and induces the production of proinflammatory cytokines [14]. Serum concentration of MBL in most individuals is within the range of 0·2–2 μg/ml [12]. Low levels of MBL in the serum, attributed to the presence of certain single nucleotide polymorphisms (SNPs) in exon 1 (encoding the collagen region of the protein) and the promoter region of its gene, are associated with a defect of opsonization and hence constitute one of the important factors predisposing the individuals to multiple and recurrent infections [11].
Recent studies have reported important in vitro and in vivo roles of MBL in first-line defence against Candida albicans, another opportunistic fungal pathogen [15,16]. A large number of association studies between MBL deficiency and susceptibility to pulmonary diseases in humans, such as tuberculosis, cystic fibrosis and chronic necrotizing pulmonary aspergillosis (a sublethal pulmonary infection caused by A. fumigatus), suggest an inevitable role of MBL in pulmonary host defence [17–20]. Gomi et al. [19] have shown that MBL levels rise during infection/inflammation in the lungs and thus play an important role in pulmonary defence. The case for this collectin playing a plausible role in pulmonary defence against A. fumigatus is supported further by in vitro studies that have indicated significant binding of MBL to A. fumigatus [21].
Henceforth, in the present study we evaluated the protective role of MBL in host defence by its ex vivo administration in murine models of IPA. The mechanisms underlying the anti-fungal effect of MBL in the murine model were delineated further by in vitro interaction studies of MBL with A. fumigatus conidia and hyphae.
Materials and methods
Preparation of the organisms
A. fumigatus conidia and hyphae
Conidia were prepared from the 285 strain of A. fumigatus, suspended in Ca2 ± Mg2+ phosphate-buffered saline (PBS) and labelled with fluorescein isothiocyanate (FITC), as described in an earlier study [22]. A. fumigatus hyphae were prepared by plating a suspension of 105 conidia/ml in 96-well plates for 24 h [23].
Preparation of native and recombinant human (nhMBL, rhMBL) and MBL-deficient serum
Purified nhMBL and rhMBL were prepared and assessed for purity as described previously [24]. The amount of endotoxin was estimated as described previously [25] and was observed to be 25 pg of endotoxin per µg of MBL. MBL-deficient serum was obtained from three healthy MBL-deficient donors (serum MBL levels of 52 ng/ml, 23 ng/ml and 26 ng/ml, respectively).
Preparation of murine model of IPA and treatment with MBL
Male BALB/c mice (National Institute of Nutrition, Hyderabad, India), each weighing an average 20 g, housed in polycarbonate shoebox cages bedded with material from dried corncobs, were given a standard laboratory rodent diet and sterile water ad libitum. All procedures that involved the handling of mice were approved by the institutional animal use and care committee.
The mice were divided into eight groups, four IPA and four control groups, with 16 mice in each. The IPA and control mice were immunosuppressed by three intradermal injections of 2·5 mg of hydrocortisone acetate (Wycort, Wyeth Ltd, Mumbai, India) per mouse per day (125 mg per kg of body weight) 1 day before (day 0), the day of (day 1), and the day after spore challenge [25]. The IPA murine models were prepared by intranasal administration of 108 conidia of A. fumigatus in 50 µl of sterile PBS to the immunosuppressed mice on day 1 of the study, as described previously [25]. The control mice were given 50 µl of PBS after immunosuppression.
All preparations for treatment were administered intranasally in 50 µl of PBS on day 1 after the spore challenge. rhMBL was given in two concentrations, 0·05 mg/kg (1 µg/20 g of mice) and 0·25 mg/kg (5 µg/20 g of mice) in 50 µl of sterile PBS to different IPA and control mice groups. The selected doses of rhMBL are based on the therapeutically effective concentrations of lung surfactant proteins in the IPA murine model, as used previously [25]. The AmB-treated mice served as the positive control. AmB was prepared and used for treatment as reported previously [25].
Survival rate, pulmonary fungal load and histopathological analysis
Eight mice from each group were predesignated for the survival study, which was monitored for 15 days. The remaining eight animals were designated for determination of pulmonary fungal load, cytokine levels and lung histopathology. For this, four animals were killed on day 3 and four on day 5 from each control and IPA mice groups. Because in the untreated IPA mice group all the mice were dead by day 5, the analysis was carried out on both categories, the naturally dead (immediately after death) and the killed mice. No significant difference was observed in these categories with respect to histopathology, colony-forming unit (CFU) counts and cytokine data. For estimating the pulmonary fungal load, lung suspensions from homogenized tissue were diluted serially and incubated in duplicate on Sabouraud dextrose agar (SDA) plates at 37°C for 24 h and the colonies were counted visually. For histological analysis, excised lungs were fixed immediately in 10% buffered formalin. Sections (3–4 µm) of paraffin-embedded tissues were stained by haematoxylin and eosin and visualized at × 40.
Cytokine assays
Spleen cells collected from the mice killed on days 3 and 5 of the study were suspended in culture medium (2 × 106 cells/well) and allowed to proliferate in RPMI-1640 with 10% fetal calf serum (FCS). The supernatant was collected after 72 h and assayed for levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-10 and interferon (IFN)-γ by enzyme-linked immunosorbent assay (ELISA) (Bio-Rad Laboratories Inc, CA, USA).
MBL-mediated C4b deposition by A. fumigatus conidia and hyphae
The efficacy of the fungal conidia and hyphae to activate the MBL-mediated lectin pathway of complement was evaluated by an in vitro C4b deposition assay [26]. The 96-well plates were coated with A. fumigatus conidia (106/ml) or hyphae (from 105 conidia/ml) instead of mannan. Wells containing conidia alone were negative controls.
Isolation of human PMNs
PMNs were isolated from 10 ml of heparinized venous blood from healthy donors using 6% dextran and Ficoll–Hypaque density gradient centrifugation. Cellular viability, estimated by trypan blue exclusion test, exceeded 95%.
Uptake of A. fumigatus conidia by PMNs
To evaluate the uptake of conidia by PMNs, FITC-labelled conidia (106/ml) were preincubated in the absence or presence of 10% MBL-deficient serum supplemented with or without rhMBL in Ca2 ± Mg2+ PBS. After washing the unbound conidia and incubation with the PMNs (106/ml), the cells were fixed in 2% paraformaldehyde [22]. At the end of the assay, 1 mg/ml trypan blue was added for 10 min to the samples to quench the extracellular fluorescence due to adherent fungal conidia. The phagocytosis of FITC-labelled A. fumigatus conidia by the PMNs was evaluated using BD CellQuest™ Pro, version 5·1.1. PMNs with conidia alone were the negative controls.
Oxidative burst of PMNs
The ability of the A. fumigatus conidia (106/ml) bound by rhMBL to trigger superoxide release by PMNs was measured by a hydroethidine assay [22] in the absence or presence of 10% MBL-deficient serum supplemented with or without 5 µg/ml rhMBL. The fluorescence of ethidium bromide resulting from oxidation of dihydroethidine by reactive oxygen intermediates (ROIs) within the stimulated PMNs was measured with BD CellQuest™ Pro, version 5·1.1. PMNs with conidia alone were the negative controls.
Anti-Aspergillus activities of PMNs
The metabolic activity (MTT) [3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide] colorimetric assay was performed to evaluate the effect of MBL on the anti-fungal activity of PMNs against A. fumigatus hyphae, as reported previously [27]. Briefly, PMNs (106/ml) were added to A. fumigatus hyphae preincubated for 2 h with rhMBL, 10% MBL-deficient serum supplemented with or without 5 µg/ml rhMBL in 96-well plates. After lysis of the PMNs with sodium deoxycholate (0·5%), the wells were stained with MTT to determine hyphal viability. Wells containing hyphae alone were taken as positive controls while wells with PMNs alone were taken as negative controls. Mean percentage hyphal damage was calculated as:
OD of the positive control wells at 562 nm −OD of the test wells at 562 nm/OD of the positive control wells at 562 nm
The conidicidal activity of the PMNs in the presence of MBL was determined by the CFU plate assay. PMNs were treated with rhMBL and/or 10% MBL-deficient serum similar to as described in the MTT assay. After the lysis of PMNs, the number of surviving conidia was determined by plating different dilutions of the suspension from these wells on SDA plates and the number of colonies was counted after 24 h.
Statistical analysis
Data are expressed as mean ± standard deviation (s.d.). Wilcoxon's rank sum test and log-rank method were performed for data analysis of the Kaplan–Meier survival curves using jmp software version 3·2.1 (SAS Institute, North Carolina, USA). Comparative analysis of the CFU counts and cytokine levels in treated and untreated groups was performed by the Mann–Whitney U-test. The statistical significance of differences in the in vitro assays among various groups was determined by Student's unpaired t-test. Significance was defined as a P-value of < 0·05. The data reported were pooled from three experiments, unless specified otherwise. All analysis except the survival rate analysis was performed using online SISA (http://home.clara.net/sisa).
Results
rhMBL enhances survival of the IPA mice
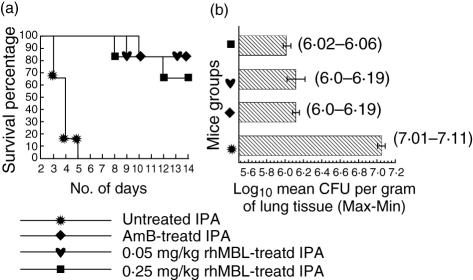
A significantly enhanced survival of 80% and 70% was shown in 0·05 and 0·25 mg/kg rhMBL-treated IPA mice, respectively, similar to the AmB-treated IPA mice compared to the untreated IPA mice that showed 0% survival by day 5 of the study (P < 0·0001, Fig. 1a). All the control mice, treated only with PBS, AmB or rhMBL, showed 100% survival.
Fig. 1.
Intranasal administration of recombinant human (rh) mannan-binding lectin (rhMBL) to invasive pulmonary aspergillosis (IPA) and control mice. Each group consisted of eight mice. (a) Kaplan–Meier survival curves in untreated, amphotericin B (AmB)-treated, 0·05 and 0·25 mg/kg rhMBL-treated mice groups infected intranasally with 108Aspergillus fumigatus conidia/50 µl of phosphate-buffered saline (PBS). (b) Mean colony-forming unit (CFU) counts represented as log10 geometric mean CFU units/g lung tissue in untreated and treated IPA mice groups on day 5 of the study. Data range (maximum–minimum) values are given.
rhMBL reduces pulmonary fungal load
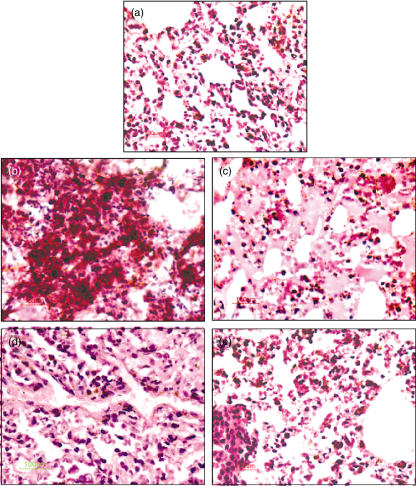
There was a significant reduction in the CFU counts in the rhMBL-treated and AmB-treated IPA mice compared to the untreated IPA mice on day 5 (Fig. 1b, P = 0·004). Histological analysis of the lung sections of the untreated IPA mice showed dense growth of A. fumigatus hyphae infiltrating the lung parenchymal cells on day 5. rhMBL and AmB-treated IPA mice showed considerably reduced fungal growth (Fig. 2). The control mice in all the groups had no evidence of fungal growth.
Fig. 2.
Histological features of lung tissues stained with haematoxylin and eosin from (a) control, (b) untreated, (c) amphotericin B-treated, (d) 0·05 mg/kg recombinant human (rh) mannan-binding lectin (rhMBL)-treated and (e) 0·25 mg/kg rhMBL-treated invasive pulmonary aspergillosis (IPA) mice groups on day 5 of the study (×40). Extensive fungal growth and tissue damage are evident in the untreated IPA mice but not in the treated mice.
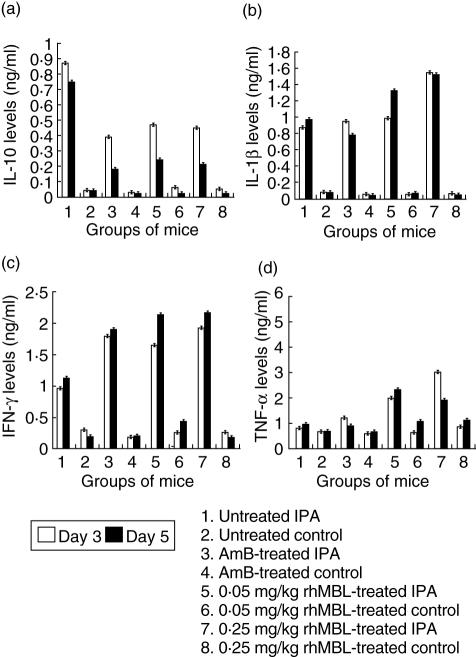
rhMBL enhances levels of TNF-α, IL-1β and IFN-γ in IPA mice
The IPA mice from all the groups showed significantly high levels of IL-1β, TNF-α, IL-10 and IFN-γ in comparison to the control mice groups (Fig. 3, P < 0·05). The rhMBL-treated IPA mice showed enhanced production of TNF-α (3·4-fold), IL-1β, IFN-γ (twofold) and suppressed production of IL-10 (twofold) in comparison to the untreated IPA mice on day 3 (Fig. 3, P < 0·05 for both 0·05 and 0·25 mg/kg rhMBL-treated mice). AmB-treated IPA mice showed a significant decrease in IL-10 (twofold), a non-significant increase in TNF-α (1·4-fold) and a significant increase in IFN-γ (twofold) levels in comparison to the untreated IPA mice on day 3 (Fig. 3, P < 0·05).
Fig. 3.
Cytokine levels in splenic suspensions of untreated, amphotericin B-treated, 0·05 and 0·25 mg/kg recombinant human (rh) mannan-binding lectin (rhMBL)-treated control and invasive pulmonary aspergillosis (IPA) mice groups on days 3 and 5 of the study. Each group consisted of eight animals.
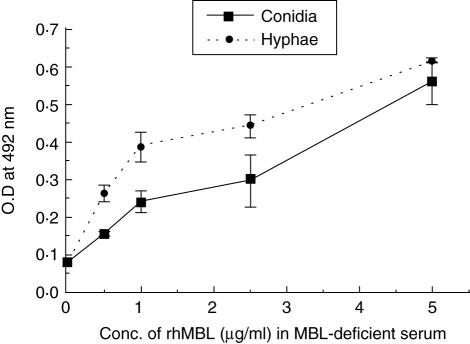
Dose-dependent C4b deposition
There was an increasing deposition of C4b on A. fumigatus conidia and hyphae with increasing concentrations of exogenous rhMBL (0–5 µg/ml) in MBL-deficient serum (Fig. 4). A negligible deposition of C4b on A. fumigatus conidia was observed in the absence of rhMBL (Fig. 4).
Fig. 4.
Percentage C4b deposition on Aspergillus fumigatus conidia (106/ml)-coated microtitre plates in the presence of mannan-binding lectin (MBL)-deficient (MBL-def) serum with recombinant human (rh)MBL added to the indicated concentrations. Results represent average value from three different experiments. Error bars indicate the mean standard deviation.
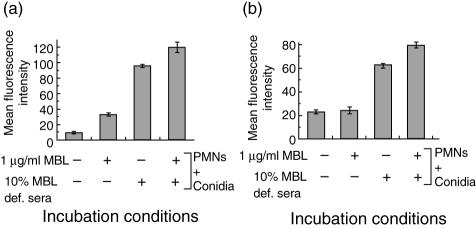
Increased uptake of MBL-bound A. fumigatus conidia by PMNs
The presence of 1 µg/ml rhMBL in serum-free conditions resulted in a significant increase in the uptake of fungal conidia by the PMNs compared to PMNs and conidia alone (P < 0·05, Fig. 5a). The presence of 1 µg/ml rhMBL supplemented with 10% MBL-deficient serum led to a greater increase in the opsonization of conidia compared to that of PMNs and conidia alone (P < 0·05) and to that of PMNs and conidia with MBL-deficient serum alone (P < 0·05, Fig. 5a).
Fig. 5.
In vitro interaction of recombinant human (rh) mannan-binding lectin (rhMBL)-bound Aspergillus fumigatus conidia with polymorphonuclear cells (PMNs). (a) Opsonization of fluorescein isothiocyanate (FITC)-labelled A. fumigatus conidia (106/ml) by PMNs (106/ml) in the presence of rhMBL and MBL-deficient (MBL-def) serum supplemented with or without rhMBL. (b) Release of superoxide radicals by PMNs (106/ml) measured by the oxidation of dihydroethidine to fluorescent ethidine bromide in the presence of A. fumigatus conidia (106/ml) incubated with rhMBL and MBL-deficient serum supplemented with or without rhMBL. Data expressed in terms of mean fluorescence intensity of the PMNs from three different experiments. Error bars indicate the mean ± standard deviation.
Increased oxidative burst of PMNs in the presence of MBL-bound A. fumigatus conidia in the presence of MBL-deficient serum
A. fumigatus conidia preincubated with 5 µg/ml rhMBL alone showed a non-significant enhancement in oxidative burst of PMNs when compared to that observed with PMNs and conidia alone (P > 0·05, Fig. 5b). The presence of both rhMBL and MBL-deficient serum resulted in a significant increase in the oxidative burst of PMNs in comparison to that of PMNs and conidia (P < 0·0001) and also to that of PMNs and conidia with MBL-deficient serum alone (P < 0·05, Fig. 5b). Similar doses of rhMBL alone or even supplemented with serum had no effect on the oxidative burst of PMNs in absence of conidia (results not shown).
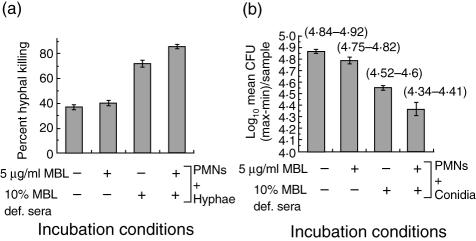
rhMBL increased anti-Aspergillus activities of PMNs in presence of MBL-deficient serum
In the presence of 5 µg/ml rhMBL alone, there was no significant increase in PMN-mediated hyphal damage compared to that observed with PMNs and hyphae alone (Fig. 6a). However, pretreatment with 5 µg/ml rhMBL supplemented with MBL-deficient serum resulted in profound hyphal damage (2·2-fold), in comparison to that observed with PMNs and hyphae (Fig. 6a, P < 0·05) and also in comparison to PMNs and hyphae with MBL-deficient serum alone (Fig. 6a, P < 0·05).
Fig. 6.
Anti-Aspergillus activity of polymorphonuclear cells (PMNs) in the presence of recombinant human (rh) mannan-binding lectin (rhMBL). (a) PMN-mediated killing of Aspergillus fumigatus hyphae in the presence of rhMBL and/or MBL-deficient (MBL-def) serum supplemented with or without rhMBL measured by the colorimetric MTT assay. (b) PMN-mediated killing of A. fumigatus conidia in the presence of rhMBL and/or MBL-deficient serum (MBL-def) supplemented with or without rhMBL measured by the colony-forming unit (CFU) count assay. The amount of CFUs per sample is presented as log10 geometric mean CFU units. Data range (maximum–minimum) values are given. Results represent average values from triplicate determinations. Error bars indicate the mean standard deviation.
The conidicidal effect of rhMBL observed in the metabolic MTT assays was confirmed further by the CFU count assays, showing that the fungal conidia preincubated with 5 µg/ml rhMBL and MBL-deficient serum resulted in a significant reduction in the number of viable conidia when compared to that observed with PMNs and conidia alone (Fig. 6b, P = 0·02), with PMNs and conidia in the presence of rhMBL alone (Fig. 6b, P = 0·02) and with PMNs and conidia in the presence of MBL-deficient serum alone (Fig. 6b, P = 0·02).
Discussion
The present study implicates an important therapeutic and immunomodulatory role of ex vivo-administered MBL in a murine model of invasive pulmonary aspergillosis.
A significant increase in the survival rate and a significant reduction in pulmonary fungal hyphae density and pulmonary fungal load after the administration of rhMBL in an immunocompromised IPA murine model that closely mimics human IPA demonstrates a therapeutic effect of MBL against IPA. Besides displaying anti-fungal activities, the administration of MBL had a profound effect on the modulation of cytokine responses in the IPA mice. Increased production of TNF-α in the rhMBL-treated IPA mice indicates that the protective effect of MBL may be mediated through enhanced production of TNF-α in a manner similar to that of AmB and lung collectins [28]. Several studies show that TNF-α augments the phagocytic activity of PAMs against A. fumigatus conidia, recruits PMNs into the lung and increases their capacity to damage Aspergillus hyphae through enhanced oxidative mechanisms [23,29,30]. Besides an increase in TNF-α, treatment with rhMBL also led to a significant decrease in IL-10 levels in splenic supernatants, confirming earlier observations that the absence of IL-10 augments innate and acquired anti-fungal immunity [31,32]. Also similar to previous reports, the decrease in IL-10 levels in the rhMBL-treated IPA mice led to a shift towards protective Th1 immune responses, e.g. up-regulation of IFN-γ, which is known to play an essential role in host defence against IPA [32,33]. These observations suggest that MBL may be acting as an immunoregulatory molecule against A. fumigatus, restoring a fine balance between proinflammatory and anti-inflammatory cytokines.
To determine some of the mechanisms underlying these anti-fungal activities of MBL, we also undertook a series of in vitro studies. Because MBL exerts some of its effects through the activation of a complement pathway, in these studies we included both MBL alone and MBL in the presence of MBL-deficient serum.
rhMBL-bound A. fumigatus conidia showed an increased uptake by the PMNs both in the presence and absence of MBL-deficient serum. This is in agreement with earlier studies, that MBL has both an intrinsic opsonization effect independently of serum, possibly via its direct receptors on PMNs, and a serum or C3b/iC3b-dependent opsonization effect via complement receptors on PMNs [13,34–37]. An increased deposition of C4b on A. fumigatus conidia when increasing amounts of rhMBL were added to MBL-deficient serum raises the possibility that MBL-bound A. fumigatus may participate in the activation of lectin complement pathway in vivo, as has also been proposed for C. albicans [19]. MBL may also affect the efficiency of other opsonic pathways in the serum, such as FcR-mediated phagocytosis [37]. In serum-free conditions, the observed conidial opsonization by MBL was similar to that of lung collectins [22].
Despite the increased uptake of A. fumigatus conidia by the PMNs in the presence of rhMBL, rhMBL alone did not lead to an increase in the oxidative burst of PMNs or in PMN-mediated fungal killing, as observed by MTT and CFU assays. A significant increase in the oxidative burst of PMNs by A. fumigatus-bound rhMBL in the presence of serum that correlated with an increase in the conidial and hyphal damage indicates a cooperative role of MBL and serum components in strengthening host defence by PMNs against A. fumigatus. One possibility of increased fungal killing by PMNs in the presence of rhMBL-supplemented serum may be an increased production of ROIs through activation of the nicotinamide adenine dinucleotide phosphate (NADPH) system in the PMNs by C3b/iC3b produced by MBL-mediated complement activation in the serum [38]. However, as the assay was carried out in the presence of MBL-deficient serum, which besides the complement molecules also contains other serum factors, the role of these factors in activating the PMNs and thus inhibiting the fungal growth cannot be ruled out. An increase in both conidial and hyphal damage in the presence of MBL suggests that besides enhancing the uptake and killing of the fungal conidia by PMNs as an early event, MBL-mediated complement activity may also contribute to PMN-induced hyphal killing as a late event.
Although the rescue of 80% IPA mice after the intranasal administrations of MBL is similar to that observed in the case of lung collectins, PMN-mediated in vitro killing in the presence of MBL alone observed in this study is less than that observed by lung collectins [22]. This suggests a possible role of MBL-mediated complement activation in host defence against aspergillosis, along with other protective mechanisms similar to lung collectins, aimed both directly at the microorganism (opsonization, killing) and indirectly through modulation of the host inflammatory responses. Simultaneous and extensive experiments with these three collectins in IPA murine models may extend our understanding of their relative contributions in host defence against A. fumigatus and also lead to novel therapeutic paradigms for IPA.
A recent study by Hogaboam et al. [39] demonstrated that mMBL-A deficiency did not affect the survival of mice following intratracheal challenge with A. fumigatus conidia. However, the above study used non-immunocompromised mice, whereas in our study the mice were immunosuppressed by steroid treatment. The study by Hogaboam et al. [39], however, observed that MBL-A deficiency had an effect on the histological appearance of the lung, suggesting that the clearance of A. fumigatus from the lungs of non-sensitized MBL-A–/– mice was not as efficient as that in their wild-type counterparts [39]. These observations, along with those made in the present study, indicate that in a normal immunocompetent host MBL may be playing a non-redundant role in innate immune responses to A. fumigatus conidia. However, in conditions of immunodeficiency such as corticosteroid-induced immunodeficiency, used in the present study, the administration of MBL may strengthen the host defence mechanisms, thus indicating that MBL deficiency may be particularly important in the context of a co-existing immunodeficiency [40]. Therapeutic efficacy of ex vivo-administered rhMBL in murine models of IPA suggests exploration of rhMBL as a supportive therapy in patients of invasive aspergillosis in conjunction with lung surfactant proteins or the anti-fungal drugs already in clinical use.
Acknowledgments
This work was supported by the Council for Scientific and Industrial Research (COR0011). Ms Savneet Kaur is a recipient of Senior Research Fellowship, Council of Scientific and Industrial Research.
References
- 1.Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–9. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 3.Oren I, Goldstein N. Invasive pulmonary aspergillosis. Curr Opin Pulm Med. 2002;8:195–200. doi: 10.1097/00063198-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Steinbach WJ. Combination antifungal therapy for invasive aspergillosis: utilizing new targeting strategies. Curr Drug Targets Infect Disord. 2005;5:203–10. doi: 10.2174/1568005054880145. [DOI] [PubMed] [Google Scholar]
- 5.Brakhage AA. Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants. Curr Drug Targets. 2005;6:875–86. doi: 10.2174/138945005774912717. [DOI] [PubMed] [Google Scholar]
- 6.Shoham S, Levitz SM. The immune response to fungal infections. Br J Haematol. 2005;129:569–82. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 7.Israel-Biet D. Pulmonary anti-Aspergillus defences. Arch Pediatr. 2003;5:S563–8. doi: 10.1016/s0929-693x(03)90038-8. [DOI] [PubMed] [Google Scholar]
- 8.Kishore U, Madan T, Sarma PU, Singh M, Urban BC, Reid KB. Protective roles of pulmonary surfactant proteins, SP-A and SP-D,against lung allergy and infection caused by Aspergillus fumigatus. Immunobiology. 2002;205:610–18. doi: 10.1078/0171-2985-00158. [DOI] [PubMed] [Google Scholar]
- 9.Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–6. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 10.Gaziano R, Bozza S, Bellocchio S, et al. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother. 2004;48:4414–21. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick DC. Mannan-binding lectin and its role in innate immunity. Transfus Med. 2002;12:335–52. doi: 10.1046/j.1365-3148.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 12.Turner MW, Hamvas RM. Mannose-binding lectin: structure, function, genetics and disease associations. Rev Immunogenet. 2000;2:305–22. [PubMed] [Google Scholar]
- 13.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lillegard JB, Sim RB, Thorkildson P, Gates MA, Kozel TR. Recognition of Candida albicans by mannan-binding lectin in vitro and in vivo. J Infect Dis. 2006;193:1589–97. doi: 10.1086/503804. [DOI] [PubMed] [Google Scholar]
- 16.Ip WK, Lau YL. Role of mannose-binding lectin in the innate defense against Candida albicans: enhancement of complement activation, but lack of opsonic function, in phagocytosis by human dendritic cells. J Infect Dis. 2004;190:632–40. doi: 10.1086/422397. [DOI] [PubMed] [Google Scholar]
- 17.El Sahly HM, Reich RA, Dou SJ, Musser JM, Graviss EA. The effect of mannose binding lectin gene polymorphisms on susceptibility to tuberculosis in different ethnic groups. Scand J Infect Dis. 2004;36:106–8. doi: 10.1080/00365540310018860. [DOI] [PubMed] [Google Scholar]
- 18.Davies JC, Turner MW, Klein NJ, London MBL CF Study Group. Impaired pulmonary status in cystic fibrosis adults with two mutated MBL-2 alleles. Eur Respir J. 2004;24:798–804. doi: 10.1183/09031936.04.00055404. [DOI] [PubMed] [Google Scholar]
- 19.Gomi K, Tokue Y, Kobayashi T, et al. Mannose-binding lectin gene polymorphism is a modulating factor in repeated respiratory infections. Chest. 2004;126:95–9. doi: 10.1378/chest.126.1.95. [DOI] [PubMed] [Google Scholar]
- 20.Crosdale DJ, Poulton KV, Ollier WE, Thomson W, Denning DW. Mannose-binding lectin gene polymorphisms as a susceptibility factor for chronic necrotizing pulmonary aspergillosis. J Infect Dis. 2001;184:653–6. doi: 10.1086/322791. [DOI] [PubMed] [Google Scholar]
- 21.Neth O, Jack DL, Dodds AW, et al. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madan T, Eggleton P, Kishore U, et al. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997;65:3171–9. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roilides E, Dimitriadou-Georgiadou A, Sein T, Kadiltsoglou I, Walsh TJ. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect Immun. 1998;66:5999–6003. doi: 10.1128/iai.66.12.5999-6003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vorup-Jensen T, Sorensen ES, Jensen UB, et al. Recombinant expression of human mannan-binding lectin. Int Immunopharmacol. 2001;1:677–87. doi: 10.1016/s1567-5769(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 25.Madan T, Kishore U, Singh M, et al. Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect Immun. 2001;69:2728–31. doi: 10.1128/IAI.69.4.2728-2731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur S, Gupta VK, Shah A, et al. Plasma mannan-binding lectin levels and activity are increased in allergic patients. J Allergy Clin Immunol. 2005;116:1381–3. doi: 10.1016/j.jaci.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Levitz SM, Diamond RD. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–45. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 28.Becker MJ, de Marie S, Fens MH, Verbrugh HA, Bakker-Woudenberg IA. Effect of amphotericin B treatment on kinetics of cytokines and parameters of fungal load in neutropenic rats with invasive pulmonary aspergillosis. J Antimicrob Chemother. 2003;52:428–34. doi: 10.1093/jac/dkg367. [DOI] [PubMed] [Google Scholar]
- 29.Madan T, Reid KB, Singh M, Sarma PU, Kishore U. Susceptibility of mice genetically deficient in the surfactant protein (SP)-A or SP-D gene to pulmonary hypersensitivity induced by antigens and allergens of Aspergillus fumigatus. J Immunol. 2005;174:6943–54. doi: 10.4049/jimmunol.174.11.6943. [DOI] [PubMed] [Google Scholar]
- 30.Schelenz S, Smith DA, Bancroft GJ. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med Mycol. 1999;37:183–94. doi: 10.1046/j.1365-280x.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- 31.Clemons KV, Grunig G, Sobel RA, et al. Role of IL-10 in invasive aspergillosis: increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin Exp Immunol. 2000;122:186–91. doi: 10.1046/j.1365-2249.2000.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Sero G, Mencacci A, Cenci E, et al. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1999;1:1169–80. doi: 10.1016/s1286-4579(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 33.Cenci E, Perito S, Enssle KH, et al. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun. 1997;65:564–70. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–45. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polotsky VY, Belisle JT, Mikusova K, Ezekowitz RA, Joiner KA. Interaction of human mannose-binding protein with Mycobacterium avium. J Infect Dis. 1997;175:1159–68. doi: 10.1086/520354. [DOI] [PubMed] [Google Scholar]
- 36.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–6. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 37.Tenner AJ, Robinson SL, Ezekowitz RA. Mannose-binding protein (MBP) enhances mononuclear phagocyte function via a receptor that contains the 126,000 Mr component of the C1q receptor. Immunity. 1995;3:485–93. doi: 10.1016/1074-7613(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson M, Weineisen M, Andersson T, Truedsson L, Sjobring U. Critical role for complement receptor 3 (CD11b/CD18), but not for Fc receptors, in killing of Streptococcus pyogenes by neutrophils in human immune serum. Eur J Immunol. 2005;35:1472–81. doi: 10.1002/eji.200424850. [DOI] [PubMed] [Google Scholar]
- 39.Hogaboam CM, Takahashi K, Ezekowitz RA, Kunkel SL, Schuh JM. Mannose-binding lectin deficiency alters the development of fungal asthma: effects on airway response, inflammation, and cytokine profile. J Leukoc Biol. 2004;75:805–14. doi: 10.1189/jlb.0703325. [DOI] [PubMed] [Google Scholar]
- 40.Klein NJ. Mannose-binding lectin: do we need it? Mol Immunol. 2005;42:919–24. doi: 10.1016/j.molimm.2004.12.006. [DOI] [PubMed] [Google Scholar]