Abstract
A limited number of therapeutic strategies are currently available for patients with inflammatory bowel disease (IBD). In particular, the maintenance therapy after remission in Crohn's disease (CD) is not satisfactory and new approaches are needed. Interleukin-10 gene-deficient (IL-10–/–) mice, a well-characterized experimental model of CD, develop severe chronic colitis due to an aberrant Th1 immune response. Everolimus, an inhibitor of the mammalian target of rapamycin (mTOR), a new immunosuppressive reagent, has been used successfully in animal models for heart, liver, lung and kidney transplantation. In the present study, we examined the efficacy of everolimus in the treatment of chronic colitis in an IL-10–/– mouse model. Everolimus was administered orally for a period of 4 weeks to IL-10–/– mice with clinical signs of colitis. The gross and histological appearances of the colon and the numbers, phenotype and cytokine production of lymphocytes were compared with these characteristics in a control group. The 4-week administration of everolimus resulted in a significant decrease in the severity of colitis, together with a significant reduction in the number of CD4+ T cells in the colonic lamina propria as well as IFN-γ production in colonic lymphocytes. Everolimus treatment of established colitis in IL-10–/– mice ameliorated the colitis, probably as a result of decreasing the number of CD4+ T cells in the colonic mucosa and an associated reduction in IFN-γ production.
Keywords: everolimus, IFN-γ, inflammatory bowel disease, interleukin-10 knockout mice, mTOR inhibitor
Introduction
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) of the human gastrointestinal tract. The aetiology and pathogenesis of CD remain unknown, but increasing evidence suggests that the dysregulation of mucosal T cells may play an important role in the pathogenesis of CD, which results in the secretion of pro-inflammatory mediators, the accumulation of inflammatory cells, and tissue damage. Five-amino salicylic acid (5-ASA) and corticosteroid are commonly used as medical therapy for IBD [1–4]. Some new therapies have recently been aimed at drug delivery systems, the reduction or suppression of certain types of enteral flora, or the modulation of focal immunological targets [5–8].
Some new strategies for the induction of remission in the acute stage of CD have been reported. New strategies for cytokine antagonist therapy have also been developed. Anti-TNF-α monoclonal antibody (mAb) therapy (infliximab) [9,10] and anti-IL-6 receptor mAb therapy (MRA) [11,12] have proved to be effective in the treatment of CD. Granulocytapheresis (GCAP) has also been successfully used in the remission of acute-phase CD [13]. However, the maintenance therapy after the remission of CD is not satisfactory and new approaches are needed. Some immunosuppressive agents that are utilized in transplantation have been also applied to the treatment for CD, for example calcineurin inhibitors (CNIs) such as cyclosporin (CsA) [14–16] and tacrolimus (Tac) [17–19].
Everolimus, a structural analogue of sirolimus, is a new agent of the class of mTOR inhibitors that has been used in organ transplantation. Compared with sirolimus, the more hydrophilic everolimus has a shorter half-life and a higher bioavailability [20]. It blocks a growth-driven transduction signal (IL-2 signalling) in the T-cell response to alloantigens and thus functions at the later stage of T-cell proliferation, compared with CNIs [21,22]. Everolimus has the advantage of a much lower nephrotoxicity in comparison with the CNIs [20,23]. Furthermore, everolimus appears to protect against viral infections such as CMV [22] and has potential anti-tumour effects [24].
It was reported that immunoregulatory T cells prevent and cure intestinal inflammation in IBD [25,26]. Recently, there are some exciting reports regarding a potential role for sirolimus in the promotion of human regulatory T cells [27,28]. Interleukin-10, however, was necessary for the effector function of the regulatory T cell population [29]. It has never been reported whether everolimus expands immunoregulatory T cells in IL-10–/– mice.
In this study, we hypothesized that the administration of everolimus to IL-10–/– mice inhibits the proliferation of activated T cells, decreases the number of Th1 cells and the production of inflammatory cytokines by Th1 cells in the lamina propria, and eventually ameliorates chronic colitis. Furthermore, we examined the effect of everolimus on immunoregulatory T cells. This is the first report to demonstrate that everolimus is a potent therapeutic agent for the control of inflammatory enterocolitis.
Materials and methods
Mice
Breeding pairs of IL-10–/–mice with a C57BL/6 background were purchased from Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6 mice (wild type: WT) were purchased from Japan CLEA (Tokyo, Japan). Before the study began, the mice were maintained in a specifically pathogen-free animal facility at the Institute of Experimental Animal Science, Osaka University Graduate School of Medicine. Mice of both sexes, 16–20 weeks of age, were used. Preliminary experiments demonstrated that under standard laboratory conditions, IL-10–/– mice developed progressive colitis starting at 4 weeks of age. Clinical manifestations of the disease included the passage of mucus in the stool, diarrhoea, rectal prolapse, and weight loss of more than 5% of the total body weight.
Drug treatment of mice
Everolimus: RAD001 (Novartis Pharma AG, Basel, Switzerland) was dissolved in sterile distilled water. When the mice manifested signs of colitis, such as diarrhoea and anal prolapse, the drug was administered daily for 4 weeks by gavage at a dose of 3·0 mg/kg/day to 14–18-week-old IL-10–/– mice (mean ± s.d.: 16·3 ± 2·3 weeks). Control mice (mean ± s.d.: 16·8 ± 1·6 weeks) received only a placebo solution (Novartis Pharma AG). At weekly intervals, the general conditions and body weight of each mouse were observed. At the end of the experiment, the mice were killed by excessive anaesthesia with pentobarbital sodium after the daily administration of everolimus.
Isolation of lymphoid cells from spleen, mediastinal lymph nodes and colon
The spleen and mediastinal lymph nodes (MLN) were aseptically removed and single-cell suspensions were prepared by a standard procedure involving mechanical disruption as described previously [30–32]. All MLN were taken along the ileocecal vessels. The cells were briefly resuspended in distilled water to lyse the red blood cells. The cells were then washed three times in a large volume of RPMI.
The harvested colon tissue was cleared of faecal matter by flushing with phosphate-buffered saline (PBS), opened longitudinally, and cut into 5-mm pieces. Single cell suspensions of colonic lamina propria (CLP) lymphocytes were prepared by an enzymatic dissociation method using collagenase, as described elsewhere [30–32].
Flow cytometric analysis
Immunofluorescent analyses were performed, using FACScan flow cytometry or a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA, USA). Cells stained with single-colour reagent were used to set the appropriate compensation levels and at least 10 000 events were stored and analysed using the CellQuest software. The following mAbs from BD PharMingen (San Diego, CA, USA) were used: anti-CD4 (clone H129·19), anti-CD8 (53–6·7), anti-CD3 ε (145–2C11), anti-CD45R/B220 (RA3–6B2), anti-CD44 (IM7), and anti-CD25 (3C7). One million cells in 20 µL PBS containing 2% foetal calf serum (FCS) and 0·02% sodium azide were first incubated with anti-FC receptor mAb (PharMingen) to prevent non-specific staining, then stained with the appropriate FITC mAb and PE mAb for two-colour flow cytometry. Furthermore, for three-colour flow cytometry, cells were stained with FITC mAb, APC-conjugated mAb, and Cy-Chrome-conjugated mAb. Negative control samples were stained with irrelevant rat isotype IgG2a antibody (PharMingen) in parallel with the experimental samples.
In vitro functional analysis of CD4+ CD25+ immunoregulatory T cells
Spleen cells from C57BL/6 mice were separated into unfractioned CD4+ T cells as responder cells using the anti-CD4 (L3T4) MicroBeads (Miltenyi Biotec). Spleen cells from C57BL/6 mice or untreated IL-10–/– mice or treated IL-10–/– mice were separated into CD4+CD25+ T cells and CD4+CD25– T cells using CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). CD4+ T cells (5 × 104) were cultured with MMC-treated C57BL/6 CD4– cells (2 × 105) as APCs were for 72 h in round-bottom 96-well plates in RPMI 1640 supplemented with 10% fetal calf serum, 100 IU/ml penicillin, 100 µg/ml streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, and 50 µM 2-mercaptoethanol. Cells were also stimulated with 1 µg/ml qanti-CD3 mAb (PharMingen). In co-culture experiments, two-fold numbers of CD4+CD25+ T cells or CD4+CD25– T cells (1 × 105) from each group were simultaneously added into the wells. Incorporation of [3H]-thymidine (1 µCi/well) by proliferating cells was measured during the last 9 h of culture [33].
Culture condition for the analysis of cytokine production
Spleen and CLP lymphocytes from everolimus-treated and control mice were cultured in a medium consisting of RPMI 1640 supplemented with 3 mM l-glutamine, 10 mM Hepes buffer, 10 µg/ml gentamicin, 100 U/ml penicillin and streptomycin, 0·05 mM 2-ME, and 10% FCS (Hyclone Co., Salt Lake City, UT, USA). To measure cytokine production, CLP and spleen lymphocytes (1 × 106 cells/ml) were loaded into wells coated with murine anti-CD3 ε antibody (clone 145–2C11; PharMingen, San Diego, CA) and 1 µg/ml soluble anti-CD28 (clone 37·51; PharMingen). To fully activate T lymphocytes, an anti-CD28 antibody was used as the primary stimulator along with anti-CD3 ε and cultured for 48 h. The culture supernatants were then harvested and assayed by enzyme-linked immunosorbent assay (ELISA) for cytokine concentration. In this assay, we used 24-well plates (Costar Corp., Cambridge, MA, USA) that we coated with anti-CD3 ε antibody by adding a solution containing anti-CD3 ε antibody (10 µg/ml) in carbonate buffer (pH 9·6) overnight at 4 °C.
Elisa
Interferon-γ and IL-4 concentrations were measured using ELISA kits (OptEIA™ Mouse INF-γ Set and OptEIA™ Mouse IL-4 Set (PharMingen)) on Immulon-4, 96-well microtiter plates (Nagle Nunc International, Rochester, NY, USA). Optical densities were measured on a Dynatech MR 5000 ELISA reader at a wavelength of 450 nm.
Histological analysis of the colon
The colon was divided into three portions: proximal, middle and distal. The tissue was fixed in 4% paraformaldehyde in PBS for 4 h and embedded in paraffin.
Histopathological alterations in the colonic mucosa were semi-quantified according to a modified scoring system [34]: (a) cellular infiltration in the lamina propria (score from 0 to 3); (b) mucin depletion (score from 0 to 3); (c) crypt abscesses (score from 0 to 3); (d) epithelial erosion (score from 0 to 2); (e) hyperaemia (score from 0 to 2); (f) thickness of the mucosa (score from 1 to 3). Hence, the sum of the histopathological scores for each specimen ranged from 1 (no alteration) to 16 (most severe colitis) and that of each mouse was from 3 to 48.
Western blotting
Protein was isolated from cultured spleen cells by resuspending them in lysis buffer (50 mmol/l Tris hydroxymethyl aminomethane, PH 7·5, 150 mmol/l NaCl, 100 µg/ml phenylmethylsulphonyl fluoride, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 mmol/l diethyldithiocarbamic acid, 1% Nonidet P-40, and 1% sodium deoxycholate) [35]. The cells were lysed by sonication (20 s, 4 °C). Debris was eliminated by centrifugation (10 min, 14000 r.p.m., 4 °C). Immunoblotting was performed as previously described [36]. Antibodies to 4E-BP1 and p-4E-BP1 (Thr37/Thr46) were purchased from Cell Signalling Technologies (Beverly, MA, USA) and mouse and rabbit secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence reagents were obtained from American Bioscience. The levels of protein expression were quantitatively estimated by densitometric scanning using a 1200 dpi flat bed scanner with NIH Image software, version 1·55f.
Statistical analysis
Statistical analyses were conducted using the Student's t-test on Statview® software. P-values < 0·05 were considered to be statistically significant.
Results
Everolimus resulted in a significant reduction in the severity of colitis
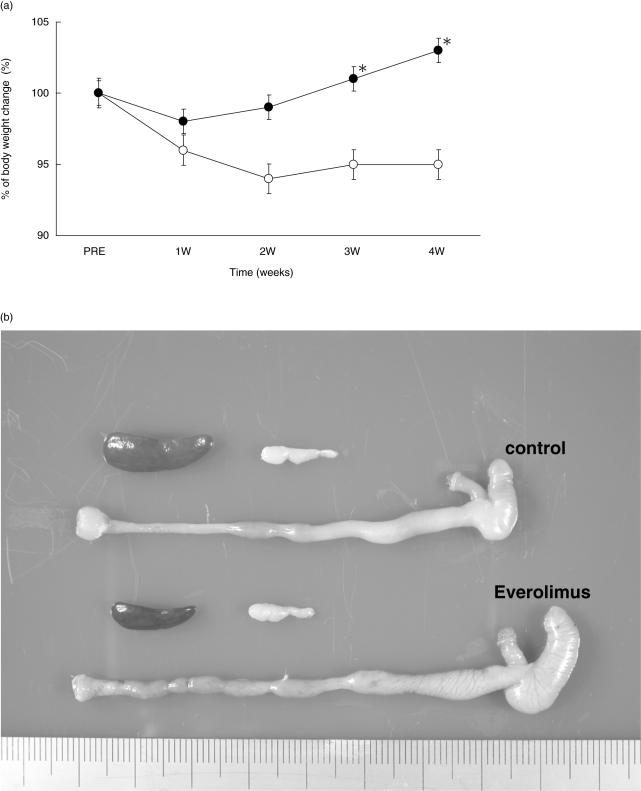
The untreated IL-10–/– mice developed the wasting syndrome: weight loss and a hunched posture. IL-10–/– mice, however, gained weight steadily and were in a good condition when treated with everolimus (Fig. 1a). Rectal prolapse did not change and diarrhoea was improved in some mice at around 2 weeks after the initiation of everolimus treatment. After 4 weeks, none of the mice treated with everolimus had any diarrhoea. Macroscopically, the colonic wall in IL-10–/– mice treated with everolimus was significantly less thickened and the length was significantly longer than that in the untreated control mice. The sizes of the spleen and MLN were also significantly smaller than those in the untreated control mice (Fig. 1b) (Table 1). Histologically, the intestinal lamina propria of IL-10–/– mice treated with everolimus had less elongation of gland crypts and less infiltration of inflammatory cells than that of the control mice (Fig. 1c). In addition, the number of goblet cells was nearly normal in the colons of IL-10–/– mice that had been treated with everolimus, but was reduced in the control mice (Fig. 1c). The mean histological score of 16- to 20-week-old IL-10–/– mice just prior to the treatment was 15·5 ± 1·7 in our animal facility. After 4 weeks, the scores of those treated with everolimus (3·0 mg/kg/day) were significantly decreased, compared with the scores of the control mice (9·8 ± 1·1 versus 17·3 ± 3·3) (Fig. 1d).
Fig. 1.
Clinical and pathologic features of everolimus-treated and untreated control mice. (a) IL-10–/– mice treated with everolimus (•) gained weight steadily throughout the study period. In contrast, untreated control mice (○) lost weight (significantly different from control weights after 3 weeks). The data represent values (mean ± s.e.m.) from three different experiments (12 mice per group). (b) Grossly, the colon of a mouse treated with everolimus is less thickened and longer than the colon of an untreated mouse. The size of the SP and MLN are smaller in treated mice. (c) Histologically, the colon of an everolimus-treated mouse has less crypt elongation, less inflammatory infiltrate in the lamina propria and more goblet cells than a control mouse. Sections were stained with haematoxylin and eosin. (d) The mean histological score in the colons of treated mice (•, 9·8 ± 1·1) is significantly lower than that in control mice (○, 17·3 ± 3·3). The data represent values (mean ± s.e.m.) (eight mice per group). *P < 0·05 versus control.
Table 1.
The weight of SP, MLN and CL in a mouse treated with everolimus is significantly less than that of control mice, and the colon of a mouse treated with everolimus is significantly longer than that of an untreated mouse.
| Control | Everolimus | |
|---|---|---|
| SP weight (mg) | 199 ± 41 | 70 ± 5* |
| MLN weight (mg) | 43 ± 4 | 25 ± 3* |
| CL weight (mg) | 574 ± 37 | 456 ± 28* |
| CL length (cm) | 8·43 ± 0·26 | 9·22 ± 0·12* |
Control (n = 12), everolimus (n = 12), mean ± s.e.m.
P < 0·05 versus control.
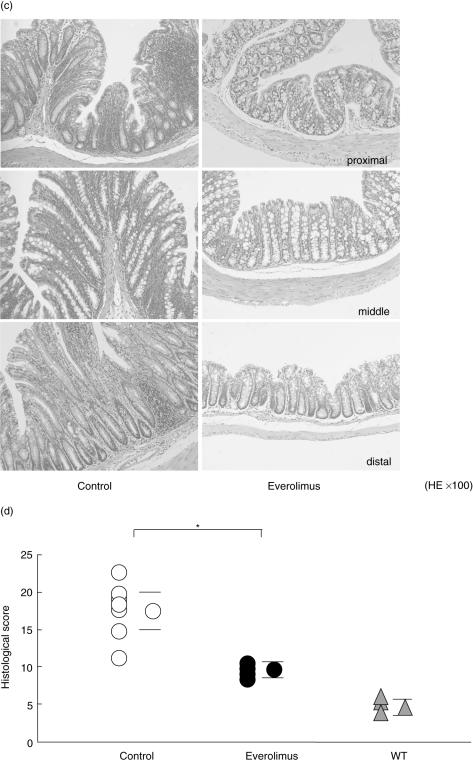
Everolimus significantly decreased the number of lymphocytes in spleen, MLN and CLP
The numbers of lymphocytes in the spleen, MLN and CLP were significantly decreased compared with those in the control mice when everolimus was administered daily for 4 weeks (Fig. 2a).
Fig. 2.
(a) Four weeks' treatment with everolimus (▪, n = 10) significantly decreased the number of SP, MLN and CLP compared with those in control (□, n = 10) mice. Mean ± s.e.m. *P < 0·05. (b) Flow cytometric analysis using lymphocytes collected after the 4-week treatment revealed that the numbers of CD3+ and CD4+ T lymphocytes from SP and CLP were significantly diminished. CD8+ lymphocytes from SP and B220+ lymphocytes from CLP were also significantly diminished. □, control (n = 8); ▪, everolimus (n = 8); mean ± s.e.m.; *P < 0·05 versus control.
Everolimus significantly decreased the number of CD4+ T cells in the spleen and CLP
Flow cytometric analysis using lymphocytes collected after the 4-week treatment of everolimus revealed that the numbers of CD3+ and CD4+ T cells from the spleen and CLP were significantly decreased. Furthermore, the numbers of CD8+ T cells from the spleen and B220+ cells from the CLP were also significantly decreased (Fig. 2b).
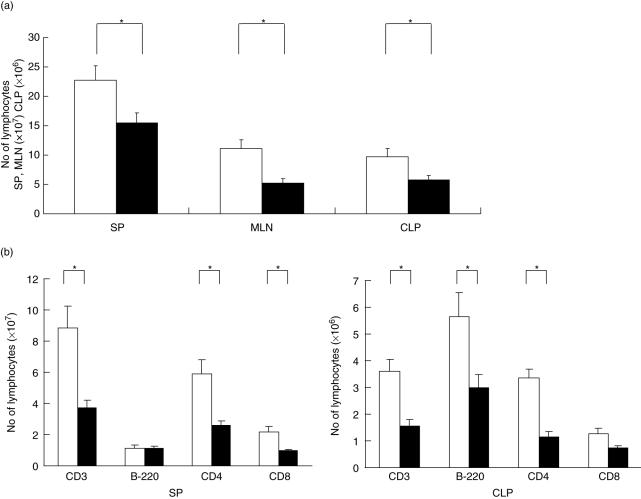
Everolimus significantly decreased the number of CD3+CD4+CD44high cells of the lamina propria
Figure 3(a) shows the results of decreasing the number of CD4+CD44high T cells, one of the activated populations, gated on CD3+ CLP lymphocytes by the administration of everolimus daily for 4 weeks. (46% to 24%)
Fig. 3.
(a) Representative results showing the decrease in the number of CD3+CD4+CD44high cells of CLP by the daily administration of everolimus for 4 weeks. This is a CD3 gated FACS analysis, CD4+CD44high double positive (RU) rate is decreased (46–24%) by treatment of everolimus. The experiment was repeated three times, and representative results are shown. (b) This figure shows that there is no significant difference between the control group and the treatment group in the number of CD4+CD25+ T cells gated on CD3+ lymphocytes of both the SP and CLP (SP, control 4·6 ± 0·9% versus everolimus 3·1 ± 0·6%; CLP, control 3·4 ± 1·1% versus everolimus 3·3 ± 1·1%; n = 5). (c) This figure shows that each of CD4+CD25+ regulatory T cells significantly suppressed the proliferation of their responder cells in the co-culture experiment. However, there was no significant difference between the untreated and the treated group in the immunoregulatory function of CD4+CD25+ T cells (each group n = 4). □, CD4+CD25–. ▪, CD4+CD25; mean ± s.e.m. *P < 0·05 versus control.
Everolimus neither expanded the number nor enhanced the function of CD4+CD25+ regulatory T cells in IL-10–/– mice
In the CD4+CD25+ T cells, however, there was no significant difference between the control group and the treatment group in the number gating on CD3+ lymphocytes of both the spleen and CLP, as shown in Fig. 3(b) (spleen, control 4·6 ± 0·9% versus everolimus 3·1 ± 0·6%; CLP, control 3·4 ± 1·1% versus everolimus 3·3 ± 1·1%; n = 5). Figure 3(c) shows that each of the CD4+CD25+ regulatory T cell populations significantly suppressed the proliferation of their responder cells in the co-culture experiments. However, there was no significant difference between the untreated and the treated group in the immunoregulatory function of CD4+CD25+ T cells (each group n = 4).
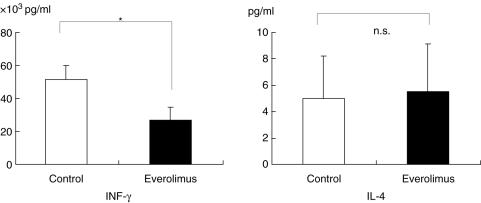
Everolimus resulted in a decreased production of IFN-γ by CLP lymphocytes
In IL-4 production by CLP lymphocytes, no significant difference between the everolimus-treated group and the untreated group was found, but INF-gamma production by the CLP was significantly decreased in the everolimus-treated group (Fig. 4).
Fig. 4.
INF-γ and IL-4 production by CLP lymphocytes after the daily administration of everolimus for 4 weeks. No difference in the production of IL-4 by CLP lymphocytes was found. However IFN-γ is significantly diminished. The data represent the values (mean ± s.e.m.) (eight mice per group). Closed (▪) and open (□) bars represent everolimus-treated group and the untreated groups, respectively. *P < 0·05 versus control.
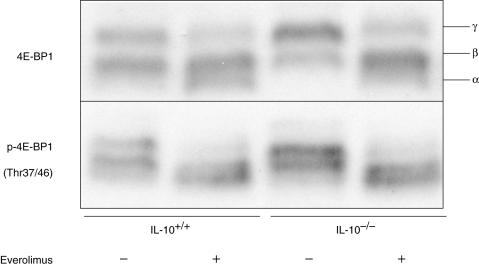
Phosphorylation of eIF4E-binding protein 1 (4E-BP1) was suppressed in CLP lymphocytes after the daily administration of everolimus for 4 weeks
We first examined the effect of everolimus on cultured splenocytes. Everolimus inhibited the phosphorylation of 4E-BP1 in vitro (data not shown). We next examined the in vivo effect of everolimus. After everolimus was administered daily for 4 weeks, the CLP lymphocytes were isolated and subjected to Western blotting analysis. When treated with everolimus, CLP lymphocytes from both wild-type and IL-10–/– mice showed lower levels of 4E-BP1 phosphorylation (Fig. 5). The suppression of 4E-BP1 phosphorylation was assessed by the mobility shift of the bands to the lowest band (α), i.e. hypo-phosphorylation status, detected by the antibodies against 4E-BP1 as well as p-4E-BP1. In the absence of everolimus treatment, the levels of expression of 4E-BP1 were slightly higher in CLP lymphocytes from IL-10–/– mice than in those from wild-type mice. The above findings indicate that everolimus suppresses the phosphorylation of 4E-BP1 in CLP lymphocytes in both wild-type and IL-10–/– mice.
Fig. 5.
Phosphorylation of eIF4E-binding protein 1 (4E-BP1), a substrate of mTOR kinase, was suppressed in CLP lymphocytes from both wild-type (IL-10+/+) and IL-10–/– mice after the daily administration of everolimus for 4 weeks. The three bands that represent the different phosphorylation status are indicated as α, β, γ. The experiment was repeated three times, and representative results are shown.
Discussion
The chronic intestinal inflammation that is characteristic of CD is thought to be due to a dysregulated immune response to luminal contents that possibly include some assumed dietary antigens. As one of the murine models of CD, IL-10–/– mice spontaneously develop enterocolitis under a specifically pathogen-free condition as the result of deviation towards a Th1-type immune response [37,38]. The chronic enterocolitis in IL-10–/– mice is mediated by Th-1 type CD4+ T cells that exclusively produce IFN-γ[39,40].
There have been some trials of inhibiting various cytokines in IL-10–/– mice in attempts to prevent colitis or to treat established colitis (anti-IFN-γ mAb, anti-IL-12 mAb and IL-10) [38–41]. But they had only a slight effect on the amelioration of the established disease in adult mice [38,39]. In contrast, there are some strategies to keep activated lymphocytes away from the effective sites, resulting in the amelioration of experimental colitis (anti-β 7 integrinAb, anti-MAdCAM-1Ab and anti-α E β 7mAb) [42,43]. Anti-α 4 integrin mAb (natalizumab) treatment also has been reported to induce clinical remission in active CD [44]. We recently reported that a novel lymphocyte homing reagent, FTY720, is effective in the treatment of established colitis in adult IL-10–/– mice [34]. FTY720 accelerated the homing of circulating lymphocytes into secondary lymphoid tissues, such as Peyer's patches (PP) and MLN and sequestrated the lymphocytes at that location, resulting in a reduction of colitis by inhibiting activated lymphocytes migrating into the CLP. Thus, it appears to be difficult to block an ongoing inflammatory process in the gut by neutralization of various inflammatory cytokines. Rather, it will be necessary under these circumstances to directly regulate the activated CD4+ T cells themselves at the site of the inflammation.
Immunosuppressive CNIs such as CsA and Tac produce no significant effect in the IL-10-deficient cell transfer model [45]. CNIs have clearly facilitated organ transplantation procedures, but they have some toxicities, such as nephrotoxicity and opportunistic infections, and continue to be a problem [46]. CNIs are also unlikely to be effective in the chronic management of autoimmune disease [47] because CNIs cannot inhibit the proliferation of antigen-primed T cells. Briefly, after signal 1 (Ca++-dependent pathway) of T cell activation and in the presence of a valid signal 2 (Ca++-independent pathway), the transcription factor NFAT (nuclear factor of activated T cell) is dephosphorylated by activated calcineurin due to Ca++/calmodulin complexes. After transition to the nucleus, NFAT promotes the binding of cytokines and growth factors (e.g. IL-2, IL-15, IFN-γ) in an autocrine or paracrine manner to specific receptors on the cell surface to deliver signal 3. The resulting kinase cascades lead to the activation of the mammalian target of rapamycin (mTOR) by autophosphorylation. The mTOR is located at a branch point of the signal 3 pathway for T cell activation [48]. Like the CNIs, sirolimus binds to a cytoplasm binding protein, FKBP (tacrolimus-binding protein). The resulting sirolimus-FKBP ligand, however, does not block calcineurin, but rather engages mTOR. As an inhibitor of mTOR, sirolimus or everolimus reduces cytokine (IL-2)-dependent cell proliferation at the G1 to the S phase of the cell division cycle.
In the current work, we first evaluated the preventive effect of everolimus on colitis in IL-10–/– mice. The daily administration of everolimus for 12 weeks in 4-week-old mice that had not yet developed colitis prevented the development of colitis (data not shown). A few mice, however, developed colitis and proctoceles within 1–2 weeks after completion of 12 weeks' treatment. We next examined the effect of the daily, 4-week administration of everolimus to IL-10–/– mice with established colitis. We found a dose dependency from 0·3 to 3·0 mg/kg/day on the effect of everolimus without any side-effects (data not shown). After the treatment, the histological score of mice treated with 3·0 mg/kg/day was significantly decreased, compared with that of control mice.
In the present study, the daily administration of everolimus significantly decreased the number of lymphocytes, especially that of CD4+ T cells in the CLP, in the population of CD3+CD4+CD44high T cells with the activation marker (control 44·2 ± 1·5% versus everolimus 35·7 ± 2·3%, n = 5). We also examined CD122 molecules as one of the other activation markers of T cells, but no significant differences were found (data not shown). The administration of everolimus resulted in a decreased production of IFN-γ by CLP, but not by spleen lymphocytes.
It has been reported that immunoregulatory T cells prevent and cure intestinal inflammation in IBD [25,26]. There are some recent reports regarding a potential role for sirolimus in the promotion of regulatory T cells. It has been reported that CNIs such as CsA may impair the development and function of immunoregulatory T cells, whereas silorimus was found to favour CD4+CD25+ T-cell-dependent immunoregulation in vitro and in vivo[27], and that silorimus selectively expands CD4+CD25+FoxP3+ regulatory T cells in vitro[28]. In our results, however, everolimus neither expanded the number nor enhanced the function of CD4+CD25+ regulatory T cells in IL-10–/– mice. It was reported that IL-10 was necessary for the effector function of the regulatory T cell population [29]. Thus, immunoregulatory T cells do not seem to be involved in the mechanism of amelioration of IL-10–/– mice colitis by everolimus.
We have considered that apoptosis in this model would be gradually and continuously induced by activation-induced cell death, but not by programmed cell death. It has been reported that by repeated activation, lamina propria T lymphocytes lead to the coexpression of two molecules, a death-inducing receptor (Fas) and its ligand (Fas-L), resulting in apoptotic cell death in situ[49]. Such activation-induced cell death might be caused also by everolimus. The most likely mechanism responsible for this effect seems to be associated with the reduction in activated CD4+ T cell numbers, which may play an important role in the pathogenesis of colitis in IL-10–/– mice, due to the inhibition of T cell proliferation by everolimus. Meanwhile, the daily everolimus administration significantly decreased not only the number of CD3+ T cells but also the number of B220+ lymphocytes from the CLP. This suggests that, unlike FTY720, everolimus has advantages in influencing the pathogenesis of colitis in IL-10–/– mice.
As described above, an everolimus/FKBP complex binds to mTOR and inhibits its kinase activity, thus suppressing mRNA translation and DNA synthesis. As a consequence, cells are arrested at the G1 stage [50]. In a signal transduction downstream of mTOR, 4E-BP1 is a key molecule. The findings show that everolimus suppresses the phosphorylation of 4E-BP1 in CLP lymphocytes in both wild-type and IL-10–/– mice. It might be clinically useful to monitor the 4E-BP1 phosphorylation status of a mucosal biopsy sample to assess the efficacy of the drug. The finding that the expression levels of 4E-BP1 were higher in the CLP lymphocytes from IL-10–/– mice supports a scenario in which mTOR signalling is enhanced under chronic inflammation in IL-10–/– mice. It provides further rationale for the use of everolimus on IBD.
Everolimus displayed a side-effect profile similar to that observed for sirolimus. The long-term safety of everolimus was reported in a 3-year randomized phase III trial [51] that compared the efficacy and safety of everolimus with those of mycophenolate mofetil (MMF). There were no significant differences in the rates of death and incidence of infections, whereas viral infections, in particular cytomegalovirus (CMV) infections, occurred more frequently in MMF-treated patients. In another report concerning the incidence of malignancies, clinical and laboratory data revealed fewer malignancies (3·6%) among renal transplant recipients treated with sirolimus [52]. Because immunosuppressive therapy in general is associated with an increased incidence of malignancies and infection, the avoidance of CMV infections and malignancies are important, and so everolimus may be suitable for maintenance therapy of patients with CD for a long period.
In summary, we demonstrate herein that a new immunosuppressive agent, everolimus, is effective in the treatment of established colitis in adult IL-10–/– mice. The most likely mechanism responsible for this effect is associated with the decreased production of IFN-γ in CLP and a reduction in activated CD4+ T cell numbers, which may play an important role in the pathogenesis of colitis in IL-10–/– mice, due to inhibiting the mTOR signalling pathway and the proliferation of activated lymphocytes on CLP in IL-10–/– mice. We conclude that the immunosuppressive activity of everolimus may be applicable for maintenance therapy after remission in cases of chronic IBD.
Acknowledgments
We express special thanks to Drs Takanori Kanai, Hiroaki Ito, Hideki Iijima and Satoshi Egawa for critical comments, Aya Kakuda and Chika Ariga for technical assistance, and Dr Milton S. Feather for editing our manuscript.
References
- 1.Shanahan F. Crohn's disease. Lancet. 2002;359(9300):62–9. doi: 10.1016/S0140-6736(02)07284-7. [DOI] [PubMed] [Google Scholar]
- 2.Scribano ML, Prantera C. Review article: medical treatment of active Crohn's disease. Aliment Pharmacol Ther. 2002;16(Suppl. 4):35–9. doi: 10.1046/j.1365-2036.16.s4.16.x. [DOI] [PubMed] [Google Scholar]
- 3.Rizzello F, Gionchetti P, Venturi A, Morselli C, Campieri M. Review article: the management of refractory Crohn's disease. Aliment Pharmacol Ther. 2002;16(Suppl. 4):40–7. doi: 10.1046/j.1365-2036.16.s4.6.x. [DOI] [PubMed] [Google Scholar]
- 4.Forbes A. Review article: Crohn's disease – the role of nutritional therapy. Aliment Pharmacol Ther. 2002;16(Suppl. 4):48–52. doi: 10.1046/j.1365-2036.16.s4.7.x. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol. 1996;91(3):423–33. [PubMed] [Google Scholar]
- 6.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150(3):823–32. [PMC free article] [PubMed] [Google Scholar]
- 7.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120(3):622–35. doi: 10.1053/gast.2001.22122. [DOI] [PubMed] [Google Scholar]
- 8.Nakase H, Okazaki K, Tabata Y, et al. Development of an oral drug delivery system targeting immune-regulating cells in experimental inflammatory bowel disease: a new therapeutic strategy. J Pharmacol Exp Ther. 2000;292(1):15–21. [PubMed] [Google Scholar]
- 9.Sandborn WJ, Hanauer SB. Infliximab in the treatment of Crohn's disease: a user's guide for clinicians. Am J Gastroenterol. 2002;97(12):2962–72. doi: 10.1111/j.1572-0241.2002.07093.x. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5(2):119–33. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Ito H. Anti-interleukin-6 therapy for Crohn's disease. Curr Pharm Des. 2003;9(4):295–305. doi: 10.2174/1381612033391900. [DOI] [PubMed] [Google Scholar]
- 12.Ding C, Jones D. Technology evaluation: MRA, Chugai. Curr Opin Mol Ther. 2003;5(1):64–9. [PubMed] [Google Scholar]
- 13.Matsui T, Nishimura T, Matake H, Ohta T, Sakurai T, Yao T. Granulocytapheresis for Crohn's disease: a report on seven refractory patients. Am J Gastroenterol. 2003;98(2):511–2. doi: 10.1111/j.1572-0241.2003.07251.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandborn WJ. Cyclosporine therapy for inflammatory bowel disease: definitive answers and remaining questions. Gastroenterology. 1995;109(3):1001–3. doi: 10.1016/0016-5085(95)90413-1. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn WJ, Tremaine WJ. Cyclosporine treatment of inflammatory bowel disease. Mayo Clin Proc. 1992;67(10):981–90. doi: 10.1016/s0025-6196(12)60930-6. [DOI] [PubMed] [Google Scholar]
- 16.Egan LJ, Sandborn WJ, Tremaine WJ. Clinical outcome following treatment of refractory inflammatory and fistulizing Crohn's disease with intravenous cyclosporine. Am J Gastroenterol. 1998;93(3):442–8. doi: 10.1111/j.1572-0241.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- 17.Bousvaros A, Leichtner AM, Book L. Treatment of pediatric autoimmune enteropathy with tacrolimus (FK506) Gastroenterology. 1996;111(1):237–43. doi: 10.1053/gast.1996.v111.pm8698205. [DOI] [PubMed] [Google Scholar]
- 18.Bousvaros A, Wang A, Leichtner AM. Tacrolimus (FK-506) treatment of fulminant colitis in a child. J Pediatr Gastroenterol Nutr. 1996;23(3):329–33. doi: 10.1097/00005176-199610000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Fellermann K, Ludwig D, Stahl M, David-Walek T, Stange EF. Steroid-unresponsive acute attacks of inflammatory bowel disease: immunomodulation by tacrolimus (FK506) Am J Gastroenterol. 1998;93(10):1860–6. doi: 10.1111/j.1572-0241.1998.539_g.x. [DOI] [PubMed] [Google Scholar]
- 20.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43(2):83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 21.Bierer BE, Hollander G, Fruman D, Burakoff SJ. Cyclosporin A and FK506: molecular mechanisms of immunosuppression and probes for transplantation biology. Curr Opin Immunol. 1993;5(5):763–73. doi: 10.1016/0952-7915(93)90135-f. [DOI] [PubMed] [Google Scholar]
- 22.Dumont FJ, Staruch MJ, Koprak SL, Melino MR, Sigal NH. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144(1):251–8. [PubMed] [Google Scholar]
- 23.Lorber MI, Basadonna GP, Friedman AL, et al. The evolving role of tor inhibitors for individualizing posttransplant immunosuppression. Transplant Proc. 2001;33(7–8):3075–7. doi: 10.1016/s0041-1345(01)02311-9. [DOI] [PubMed] [Google Scholar]
- 24.Majewski M, Korecka M, Kossev P, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: a potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci USA. 2000;97(8):4285–90. doi: 10.1073/pnas.080068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 26.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170(8):3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 27.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107(3):1018–23. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 29.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190(7):995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi I, Kiyono H, Hamada S. CD4+ T-cell population mediates development of inflammatory bowel disease in T-cell receptor alpha chain-deficient mice. Gastroenterology. 1997;112(6):1876–86. doi: 10.1053/gast.1997.v112.pm9178680. [DOI] [PubMed] [Google Scholar]
- 31.Fujihashi K, McGhee JR, Kweon MN, et al. gamma/delta T cell-deficient mice have impaired mucosal immunoglobulin A responses. J Exp Med. 1996;183(4):1929–35. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iijima H, Takahashi I, Kishi D, et al. Alteration of interleukin 4 production results in the inhibition of T helper type 2 cell-dominated inflammatory bowel disease in T cell receptor alpha chain-deficient mice. J Exp Med. 1999;190(5):607–15. doi: 10.1084/jem.190.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172(11):6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 34.Mizushima T, Ito T, Kishi D, et al. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis. 2004;10(3):182–92. doi: 10.1097/00054725-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Berg DJ, Zhang J, Weinstock JV, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123(5):1527–42. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 36.Imamura F, Shinkai K, Mukai M, et al. rho-Mediated protein tyrosine phosphorylation in lysophosphatidic-acid-induced tumor-cell invasion. Int J Cancer. 1996;65(5):627–32. doi: 10.1002/(SICI)1097-0215(19960301)65:5<627::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 38.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98(4):1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161(6):3143–9. [PubMed] [Google Scholar]
- 40.Davidson NJ, Leach MW, Fort MM, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184(1):241–51. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10-/- mice: an overview. J Leukoc Biol. 1997;61(4):389–96. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 42.Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal address in cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158(5):2099–106. [PubMed] [Google Scholar]
- 43.Ludviksson BR, Strober W, Nishikomori R, Hasan SK, Ehrhardt RO. Administration of mAb against alpha E beta 7 prevents and ameliorates immunization-induced colitis in IL-2-/- mice. J Immunol. 1999;162(8):4975–82. [PubMed] [Google Scholar]
- 44.Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348(1):24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 45.Ikenoue Y, Tagami T, Murata M. Development and validation of a novel IL-10 deficient cell transfer model for colitis. Int Immunopharmacol. 2005;5(6):993–1006. doi: 10.1016/j.intimp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Assmann T, Homey B, Ruzicka T. Applications of tacrolimus for the treatment of skin disorders. Immunopharmacology. 2000;47(2–3):203–13. doi: 10.1016/s0162-3109(00)00187-9. [DOI] [PubMed] [Google Scholar]
- 47.Allison AC. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47(2–3):63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 48.Neuhaus P, Klupp J, Langrehr JM. mTOR inhibitors: an overview. Liver Transpl. 2001;7(6):473–84. doi: 10.1053/jlts.2001.24645. [DOI] [PubMed] [Google Scholar]
- 49.MacDonald TT. Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr Top Microbiol Immunol. 1999;236:113–35. doi: 10.1007/978-3-642-59951-4_7. [DOI] [PubMed] [Google Scholar]
- 50.Nashan B. Review of the proliferation inhibitor everolimus. Expert Opin Invest Drugs. 2002;11(12):1845–57. doi: 10.1517/13543784.11.12.1845. [DOI] [PubMed] [Google Scholar]
- 51.Vitko S, Margreiter R, Weimar W, et al. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2005;5(10):2521–30. doi: 10.1111/j.1600-6143.2005.01063.x. [DOI] [PubMed] [Google Scholar]
- 52.Kahan BD, Yakupoglu YK, Schoenberg L, et al. Low incidence of malignancy among sirolimus/cyclosporine-treated renal transplant recipients. Transplantation. 2005;80(6):749–58. doi: 10.1097/01.tp.0000173770.42403.f7. [DOI] [PubMed] [Google Scholar]