Abstract
To investigate the molecular effects of the periodontopathogens Fusobacterium nucleatum (FN) and Porphyromonas gingivalis (PG) on the oral epithelium, the H400 oral epithelial cell line was cultured in the presence of non-viable bacteria. Following confirmation of the presence of transcripts for the bacterial pattern recognition receptors in H400 cells, Toll-like receptors -2, -4 and -9, and components of the NF-κB signalling pathway, immunocytochemical analyses were performed showing that NF-κB was activated within 1 h of exposure to both periodontopathogens. A significantly greater number of NF-κB nuclear translocations were apparent following H400 cell exposure to FN as compared with PG. Gene expression analyses indicated that transcripts known to be regulated by the NF-κB pathway, including cytokines/chemokines TNF-α, IL-1β, IL-8, MCP-1/CCL2 and GM-CSF, were up-regulated following 4 and 24 h of exposure to both periodontopathogens. In addition, H400 periodontopathogen exposure resulted in differential regulation of transcripts for several cytokeratin gene family members. Consistent with the immunocytochemical data, microarray results indicated that FN induced a greater number of gene expression changes than PG following 24 h of exposure, 609 and 409 genes, respectively. Ninety-one genes were commonly differentially expressed by both periodontopathogens and represented biological processes commonly associated with periodontitis. Gene expression analyses by reserve transcriptase-polymerase chain reaction (RT-PCR) of molecules identified from the microarray data sets, including Heme oxygenase-1, lysyl oxidase, SOD2, CCL20 and calprotectin components, confirmed their differential expression profiles induced by the two periodontopathogens. FN and PG have clearly different molecular effects on oral epithelial cells, potentially highlighting the importance of the composition of the plaque biofilm in periodontitis pathogenesis.
Keywords: bacteria, epithelium, NF kappaB, periodontitis
Introduction
Periodontitis is a chronic inflammatory disease affecting the supporting tissues of the teeth and is a major cause of tooth loss in the developed world. Epidemiological associations between periodontitis and more serious systemic conditions, such as coronary heart disease, atherosclerosis and type 2 diabetes, are also now emerging [1–3]. Although periodontitis is initiated by the accumulation of the dental plaque biofilm, the tissue destruction arises secondary to an abnormal host response to specific bacteria within it [4,5]. Of the 500 species of periodontal bacteria estimated to colonize the periodontium, only relatively few have been implicated as being key to periodontal disease progression, with the Gram-negative anaerobic bacteria Fusobacterium nucleatum (FN) and Porphyromonas gingivalis (PG) being widely regarded as key aetiological agents [6]. Whilst PG is strongly associated with later stages of periodontitis, FN appears to interact with other periodontal bacteria, acting as a bridge between early and late colonizers, mediating the shift from a non-pathogenic to a pathogenic microflora [7]. FN and PG are also potentially important regulators of the host response due to their ability to invade cells and tissues subsequently modifying the immune response [8,9].
Whilst exaggerated neutrophilic inflammation, in terms of reactive oxygen species (ROS) and proteolytic enzyme production, in response to periodontal bacteria is believed to underpin the tissue destruction seen in periodontitis [5], recent research has now highlighted the possibility that oral epithelium not only provides the first physical line of defence against colonizing pathogens, but also orchestrates the local immune reaction [10]. Towards a better understanding of the oral epithelium's role, work has begun on elucidating how it detects and responds to the microbial world. The major receptors involved in pathogen recognition are Toll-like receptors (TLRs) of which TLR-2, -4 and -9, whose ligands are the bacterial products lipotechoic acid (LTA), lipopolysaccharide (LPS) and bacterial DNA (bDNA), respectively, have now been shown to be present in oral epithelial cells and tissues [11–13]. Recent in vitro studies have shown that the microbial products of periodontopathogens can stimulate oral and immune cells via these receptors subsequently activating intracellular signalling cascades [11,14–16]. Genetic studies in periodontitis also indicate that sequence variations within these molecules may contribute to disease susceptibility [15,17–19].
TLRs are potent activators of the NF-κB intracellular pathway that subsequently triggers inflammatory and immune processes, such as induction of cytokines/chemokines and destructive enzymes [20], molecules known to be abundant in periodontitis tissues [21,22]. In addition, recent analysis of the localization of the p50 and p65 transcription factor components of the NF-κB complex demonstrated significantly increased activity beneath periodontal lesions [23]. Furthermore, elevated NF-κB activity has been demonstrated in the pathogenesis of several chronic inflammatory diseases associated with periodontitis, including atherosclerosis and type 2 diabetes [24,25]. Whilst different molecular and immune effects have recently been demonstrated between Gram-negative and Gram-positive periodontopathogens [26], our current understanding of bacterial–epithelium interactions in periodontitis still remains limited. In addition, previous analyses have tended to focus on specific, well-characterized pro-inflammatory mediators. Data therefore now indicate that a more thorough analysis of the TLR-NF-κB pathway, by comparing the effects of key periodontopathogens and determining the molecules they regulate in the oral epithelium, could potentially identify new opportunities for therapeutic intervention in periodontitis.
The aim of this study was therefore to extend previous knowledge by comparing how the non-viable whole periodontopathogens, PG and FN, activate the NF-κB pathway, affect selected molecules involved in the innate immune response and epithelial tissue homeostasis, and stimulate large-scale gene expression in a well-characterized oral epithelial cell line (H400) [27]. Such analyses will contribute to a better understanding of oral epithelial responses to these key periodontopathogens during periodontitis.
Materials and methods
Bacterial culture and suspensions
Fusobacterium nucleatum (ATCC 10953) and Porphyromonas gingivalis (ATCC 33277) were grown anaerobically at 37 °C in broth culture and concentrations determined as previously described [28]. Cells were pelleted by centrifugation, washed three times in sterile phosphate buffered saline (PBS), heat-inactivated (100 °C for 10 min) and diluted with sterile PBS to give a final suspension of 4 × 108 cells/ml. Heat inactivation was confirmed by plating experiments. Bacterial suspensions were stored at − 30°C prior to use.
Oral epithelial cell (OEC) culture
Experiments were performed using OEC line H400 [27]. Cells for RNA extraction were cultured in flasks (1 × 105 cells/25 cm2 flask) using Dulbecco's MEM (DMEM) (Invitrogen, Paisley, UK), containing 10% foetal calf serum (Labtech, Ringmer, UK), 4 mM glutamine (Sigma, Poole, UK), and 0·5 µg/ml hydrocortisone (Sigma), in an atmosphere of 5% CO2 at 37 °C. The media was changed on day 4 after initial seeding and cells were passaged at day 7. All experiments were performed between passages 4 and 20 using subconfluent monolayers exposed to either 1 × 109 heat-killed bacteria (approximately 100 bacteria per epithelial cell; data not shown), 10 or 20 µg E.coli LPS (Sigma) per ml of culture media, or culture media alone (control) for the times stated. The ratio of bacteria to epithelia cells used in this study was based on previous analyses [29], which have indicated the maximum number of periodontal bacteria that may associate with epithelial cells from within the periodontal pocket. This approach therefore potentially enables physiological relevance for our studies with regard to periodontitis.
For immunocytochemical analyses, Petri dishes containing multiwell slides (CA Hendley, Loughton, UK) in growth media were seeded with 4 × 105 cells. On day 4, the media was removed and the cells stimulated with medium ± PG, FN (109 cfu/ml) or E.coli LPS (10 or 20 µg/ml) (Sigma) for 0·5, 1 and 2 h. E.coli LPS served as a positive control for NF-κB activation [30]. Negative control slides were treated with media alone.
RNA isolation
Total RNA was extracted from H400 cells using the RNeasy mini kit (Qiagen, Crawley, UK). Following removal of media from culture dishes, cells were directly lysed in situ using RLT buffer (Qiagen). DNA-free RNA was subsequently isolated as recommended by the manufacturer and eluted in a final volume of 30 µl of sterile water. RNA concentrations were determined from absorbance values at 260 nm using a BioPhotometer (Eppendorf, Cambridge, UK). RNA samples used in this study had 260/280 nm ratios equal to or greater than 1·8 and RNA integrity was additionally verified by visual inspection of samples on 1% non-denaturing agarose gels stained with SYBR Gold (Molecular Probes, Paisley, UK).
Semi-quantitative reverse transcriptase-polymerase chain reaction (sq-RT-PCR)
DNase-digested total RNA (1–2 µg) was used for oligo(dT) (Ambion, Warrington, UK) reverse transcription to generate single-stranded cDNA using the Omniscript kit (Qiagen). Sq-RT-PCR assays were performed using the RedTaq PCR system (Sigma). For analyses, 50 ng of single-stranded cDNA was used to seed 25 µl PCRs containing 12·5 µl RedTaq ready reaction mix, 10·5 µl dH2O, 1 µl of 25 µM forward and reverse primer. Table 1 provides primer sequences and details used for PCR analysis. Reactions were amplified in a Thermal Cycler (Mastercycler Gradient, Eppendorf) for between 19 and 38 cycles. The initial denaturation step lasted for 5 min at 94 °C, followed by an amplification cycle consisting of 94 °C for 20 s, 60–61 °C for 20 s and 72 °C for 20 s ending with a 10-min extension at 72 °C. Following the designated number of cycles, 6 µl of the reaction was removed and the product was separated and visualized on a 1·5% agarose gel containing 0·5 µg/ml ethidium bromide (Sigma), after which gels were scanned and images captured using EDAS 120 software (Kodak, Hemel Hempstead, UK). Scanned gel images were imported into AIDA image analysis software (FUJI, Sheffield, UK) and the volume density of amplified products calculated and normalized against the GAPDH housekeeping gene control values. All sq-RT-PCR experiments were performed in duplicate.
Table 1.
Details of primer sequence and semi-quantitative RT-PCR conditions.
| Gene | Symbol | Accession Number | Sequence | Tm (°C) | Product (bp) | Cycle no. |
|---|---|---|---|---|---|---|
| Toll-like receptor-2 | TLR-2 | NM_003264.3 | F-GAT GCC TAC TGG GTG GAG AA R-CGC AGC TCT CAG ATT TAC CC | 61 | 392 | 31 |
| Toll-like receptor-4 | TLR-4 | NM_138444-2 | F-AAC CAT CCT GGT CAT TCT CG R-CGG AAA TTT TCT TCC CGT TT | 61 | 315 | 36 |
| Toll-like receptor 9 | TLR-9 | AB045180.1 | F-CTG CGT CTC CGT GAC AAT TA R-GTC CTG TGC AAA GAT GCT GA | 61 | 443 | 36 |
| NF-KappaB1 | NFKB1 | NM_003998 | F-CCT GGA TGA CTC TTG GGA AA R-CTT CGG TGT AGC CCA TTT GT | 61 | 366 | 26 |
| NF-kappaB2 | NFKB2 | NM_002502.2 | F-CGT ACC GAC AGA CAA CCT CA R-CCG TAC GCA CTG TCT TCC TT | 61 | 186 | 26 |
| NF-kappaB1 epsilon | NFKB1E | NM_004556 | F-GTG AAG CCT GTT TGC CTC TC R-AGG GTC CTC AAC AGC AAG AA | 61 | 172 | 35 |
| l-kappaB-alpha | IKB-ALPHA/NFKBIA | NM_020529 | F-AAC CTG CAG CAG ACT CCA CT R-ACA CCA GGT CAG GAT TTT GC | 61 | 375 | 23 |
| l-kappaB-beta | IKB-BETA/NFKBIB | AY736284 | F-ATG GAC CTG CAG AAT GAC CT R-CAG CTT CCA GTC CTC CTC AC | 61 | 416 | 30 |
| Tumour necrosis factor-α | TNFα | NM_000594.2 | F-AAG AAT TCA AAC TGG GGC CT R-GGC TAC ATG GGA ACA GCC TA | 60 | 402 | 38 |
| Interleukin-1beta | IL1B | NM_000576.2 | F-TCC AGG GAC AGG ATA TGG AG R-TTC TGC TTG AGA GGT GCT GA | 60 | 292 | 28 |
| Interleukin-8 | IL8 | NM_000584.2 | F-TAG CAA AAT TGA GGC CAA GG R-GGA CTT GTG GAT CCT GGC TA | 60 | 204 | 30 |
| Granulocyte macrophage colony stimulating factor | GMCSF | M11220.1 | F-ACT ACA AGC AGC ACT GCC CT R-CTT CTG CCA TGC CTG TAT CA | 60 | 303 | 31 |
| Monocyte Chemoattractant Protein-1/CCL2 | MCP-1/CCL2 | NM_002982.3 | F-GCC TCC AGC ATG AAA GTC TC R-GCT GCA GAT TCT TGG GTT GT | 60 | 339 | 35 |
| Cytokeratin-1 | CK1 | NM_0061521.2 | F-TGG CAA GAC CGA GGT CGA TT R-TGT GGG TGG TGG TCA CTG CT | 60 | 202 | 31 |
| Cytokeratin-4 | CK4 | NM_002272.2 | F-GCC ATG ATT GCC AGA CAG CAG TGT R-GGG GGT GAG CAA GCT CTG GTT G | 60 | 407 | 31 |
| Cytokeratin-5 | CK5 | NM_000424.2 | F-CCA AGC CAA TTG CAG AAC CA R-AAA TTT GGG ATT GGG GTG GG | 60 | 198 | 19 |
| Cytokeratin-6 | CK6 | NM_005554 | F-GTC CTC AGG CCC CTC TCT GG R-CCC CTG GCA ATT TTC TGC AA | 60 | 317 | 21 |
| Cytokeratin-10 | CK10 | NM_000421.2 | F-CCG TGG GCG AGT CTT CAT CT R-GGA GAC TTT GTT TTC CAT GCA TCT | 60 | 208 | 24 |
| Cytokeratin-13 | CK13 | X52426.1 | F-TCT AAT GCC TCT GGT CGC CG R-AGG GCC CAC CAT CAG GAG AG | 60 | 197 | 21 |
| Cytokeratin-16 | CK16 | AF061812.1 | F-CTT GGC ACC ATG ACC ACC TG R-CAC CGA AGC CAG CAC CAA G | 60 | 294 | 31 |
| Cytokeratin-19 | CK19 | NM_002276.3 | F-CCT ACA GCT ATC GCC AGT CG R-ATG GTT AGC TTC TCG TTG CC | 60 | 243 | 33 |
| Heme oxygenase 1 | HM0X1 | NM_0002133.1 | F-AAC CTC CAA AAG CCC TGA GT R-CAC CCC AAC CCT GCT ATA AA | 61 | 207 | 32 |
| S100 calcium binding protein A8 | MRP8/S100A8 | BC005928 | F-ATT TCC ATG CCG TCT ACA GG R-CAG CCT CTG GGC CCA GTA ACT C | 61 | 232 | 30 |
| S100 calcium binding protein A9 | MPR14/S100A9 | NM_002965.2 | F-CAG CTG GAA CGC AAC ATA GA R-TCA GCA TGA TGA ACT CCT CG | 61 | 228 | 23 |
| CCL20 | CCL20/MIP-3α | NM_004591.1 | F-GCA AGC AAC TTT GAC TGC TG R-CAA GTC CAG TGA GGC ACA AA | 60 | 341 | 27 |
| Lysyl oxidase | LOX | NM_002317 | F-ACA GGG TGC TGC TCA GAT TT R-CCA GGT AGC TGG GGT TTA CA | 61 | 381 | 24 |
| Superoxide dismutase 2 | SOD2 | S77127 | F-TGG AGG CAT CTA GTG GAA AAA R-CCC AGT CTC TCC CCA TTA CA | 61 | 305 | 34 |
Tm = Annealing temperature (°C); bp = base pairs; (F) = Forward primer; (R) = Reverse primer. All DNA sequences are shown in the 5′ to 3′ orientation.
Immunocytochemical staining
Cell monolayers were fixed in dry acetone (10 min at room temperature), air dried (10 min) and immediately stained using a monoclonal antibody to p65 (clone F-6, Santa Cruz Biotechnology, USA; 1 : 100) and a biotin–streptavidin immunoperoxidase technique (StrAviGen, Biogenex, San Ramon, CA, USA) as previously reported [31]. Bound peroxidase was visualized using 3,3′-diaminobenzidine reagent (5 min) and, if required, cells were counterstained with Meyer's haematoxylin before mounting in Xam. All reagent dilutions and washings were performed in 0·01 M PBS, pH 7·6. Immunostaining for each stimulant (PG, FN or E.coli LPS) was performed simultaneously to ensure comparability. Negative staining controls consisted of replacement of the primary antibody with PBS or a monoclonal antibody of irrelevant specificity but the same immunoglobulin isotype. A positive method control utilized a monoclonal antibody to Ki67 (clone MM1; Novacastra™, Vision Biosystems, Newcastle, UK; 1 : 100).
Cell counts
Cell counts were performed on cell monolayers after 1 h stimulation using a microscope fitted with an eyepiece graticule at a magnification of ×100. For cells to be classified as demonstrating NF-κB activation, nuclear staining for p65 was required with no staining being present within the cytoplasm. Preliminary analysis of eight slides indicated that counts from a total of 30 randomly selected microscope fields would provide a representative cell count for a given specimen. All cells within each of 30 graticule areas (1 = 0·01 mm2) were counted and recorded as positive or negative. The total and mean number of positive cells for each specimen were used to calculate the percentage of cells demonstrating NF-κB activation.
Microarray target preparation and hybridization
Gene expression in epithelial cells grown in 25 cm2 cell culture flasks (Corning, Loughborough, UK) and stimulated with (a) PG (109 cfu/ml) (b) FN (109 cfu/ml) and (c) media alone (negative control) were analysed using human Affymetrix HG_U133A oligonucleotide arrays, as described at http://www.affymetrix.com/products/arrays/specific/hgu133.affx and [32]. RNA quantity and integrity were determined by spectrophotometer (Biophotometer, Eppendorf) and agarose gel electrophoresis. All samples used for microarray analysis had a 260/280 ratio of greater than 1·8. Total RNA from each sample was used to prepare biotinylated target RNA, according to the manufacturer's instructions (http://www.affymetrix.com/support/technical/manual/expression_manual.affx). Briefly, 5 µg of DNase digested total RNA was used to generate double-stranded cDNA using SuperScript reagents (Life Technologies, Paisley, UK) and a T7-linked oligo(dT) primer. cRNAs were synthesized using the Enzo Bioarray High Yield RNA transcript labelling kit (Affymetrix, High Wycombe, UK) and resulting biotinylated labelled cRNA was subsequently fragmented into 35- to 200-bp lengths using the fragmentation buffer (Affymetrix). As recommended, RNA, cDNA and cRNA quality and size distribution were visually confirmed by agarose gel electrophoresis. Spike controls B2, bio-B, bio-C, bio-D and Cre-x were added to the hybridization cocktail before overnight hybridization at 45 °C for 16 h. Arrays were stained and washed on the Fluidics Station 400 (Affymetrix) using the EukGE-WS2 protocol (dual staining) before being scanned twice on the GeneChip Scanner 3000 at an excitation wavelength of 488 nm. cRNA was initially hybridized to Test3 GeneChips to confirm sample integrity and quality prior to hybridization to U133A microarrays and all hybridization control results were within recommended parameters (data not shown). HG_U133A microarrays were hybridized with 15 µg of each of the target cRNA samples. Analysis of control parameter data confirmed hybridization success and scaling factors were within Affymetrix recommended guidelines (data not shown). Microarray analysis complied with MIAME standards (http://www.mged.org/Workgroups/MIAME/miame.html).
Microarray data analysis
The analysis of the microarray data was performed using the DNA-Chip analyser (dChip) software package [33,34]. Array normalization to adjust overall brightness was performed by default selecting an array with median overall intensity as the baseline array against which all other arrays were normalized at probe intensity levels. The Invariant Set of Normalization method was used to identify a subset of probes with small, within subset rank, differences in the different arrays to serve as the basis for fitting a normalization curve [33,34]. Following normalization, we identified genes differentially expressed between the control and FN or PG stimulated OEC samples. Based on previous experiences, Affymetrix and dCHip recommendations and published literature [35], the criteria we used to identify differentially expressed genes were that they showed at least a two-fold change in expression level and had an absolute difference of more than 100 in the expression level between the samples on the control versus test arrays. In addition, the following criteria were used in the analysis: the P-value threshold was set at 0·05 (per default), filtering genes that differ within the group means with a two-tail P-value 0·05.
To classify genes according to standardized Gene-Ontology (GO), vocabulary for the categories of biological process information from the NetAffx software (Affymetrix) was utilized or entire data sets were imported into the Onto-Express software (http://vortex.cs.wayne.edu/projects.htm) [36]. The default binomial probability distribution and Bonferroni multiple experiment correction settings of the software were used for data analysis.
Microsoft Access (Microsoft, Seattle, US) database queries were used to compare genes in the PG and FN datasets.
Statistical analysis
Unpaired non-parametric two-tailed Mann–Whitney analyses were performed to determine the significance of differences between p65 translocation data. P-values are shown where appropriate.
Results
Activation of NF-κB intracellular signalling in OEC by periodontal pathogens
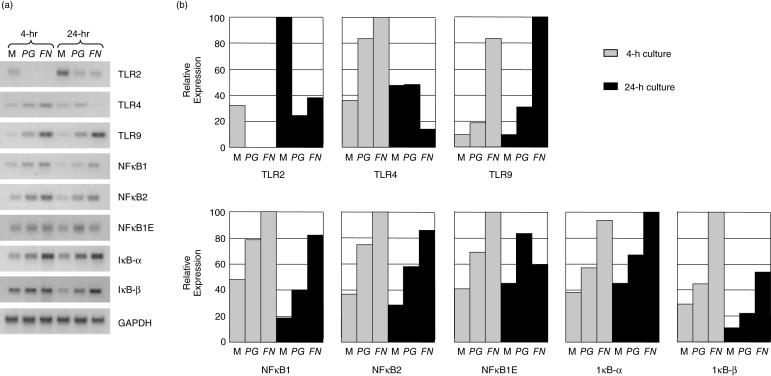
Initially, to assess whether the H400 cells were likely to respond to the heat-killed bacteria, studies were performed to analyse gene expression of relevant TLRs (TLR-2, -4, -9) and components of the NF-κB pathway (NF-κB1, NF-κB2, NF-κB1-epsilon, IκB-α and IκB-β) in unstimulated and stimulated cells. Data indicated that the H400 cells basally expressed all transcripts and that these were, in general, up-regulated at both 4- and 24-h time points in response to the periodontal pathogens, with the exceptions of TLR-2 and -4 (Fig. 1). In addition, bacterial 16 s PCR assays were performed to confirm that the ligand for TLR-9, bDNA, was present within the heat-killed bacterial samples (data not shown).
Fig. 1.
Semi-quantitative RT-PCR analysis of selected genes involved in the detection of bacterial products and downstream intracellular signalling cascade, including TLR and NF-κB molecules. H400 cells were exposed to media alone (M) or 109 cfu/ml of PG or FN for 4 and 24 h. (a) Representative gel images of duplicate analyses are shown. (b) Densitometric analysis of gel products. Expression levels are shown as percentage of the highest level detected. Amplified product values were normalized to GAPDH housekeeping gene levels.
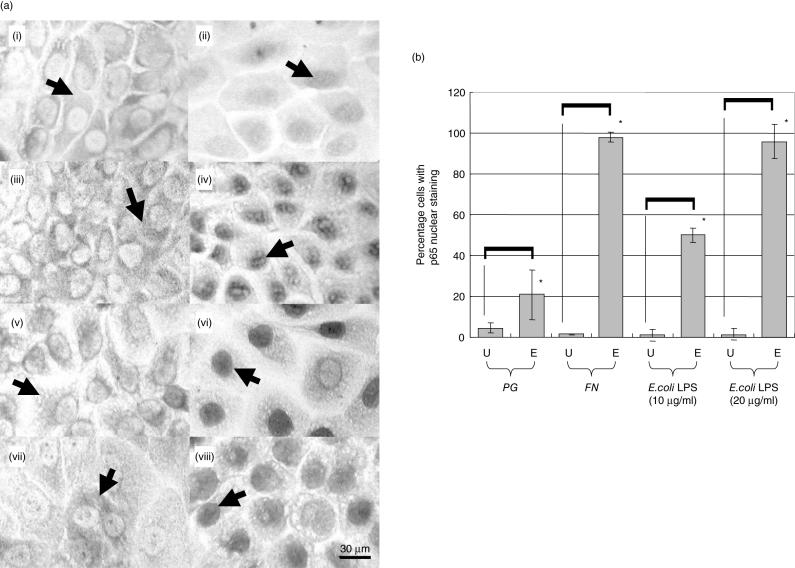
Following 1 h exposure to PG, FN or E.coli LPS, p65 nuclear staining was observed (Fig. 2a). Cell counts demonstrated that FN caused a greater percentage activation of NF-κB (98·1%) than PG (21·0%) that was comparable with that induced by 20 µg/ml E.coli LPS (95·8%) (Fig. 2b).
Fig. 2.
Immunocytochemical analysis of NF-κB activity in H400 cells following 60 min of exposure to stimuli. (a) Histological images of H400 cells in media alone (controls (i) (iii) (v) & (vii)) or exposed to the peridontopathogens PG (ii), FN (iv) and E.coli LPS controls 10 µg/ml (vi) and 20 µg/ml (viii). Arrows indicate either p65 cytoplasmic ((i) (iii) (v) & (vii)) or nuclear ((ii) (iv) (vi) & viii)) localization in H400 cells. (b) Quantitative immunocytochemical analysis of p65 nuclear staining following exposure to PG, FN and 10 or 20 µg/ml of E.coli LPS. U = unexposed H400 cells; E = exposed H400 cells. Asterisks indicate statistically significant differences (P< 0·001).
Gene expression in OECs following exposure to periodontal pathogens
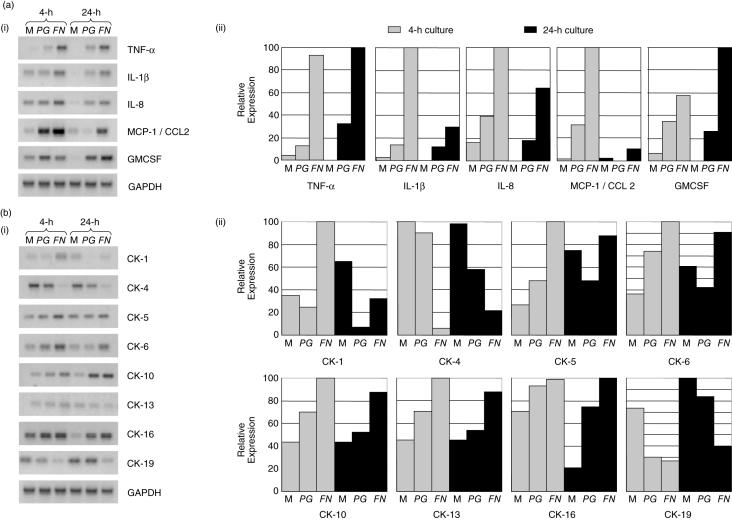
To determine whether the observed NF-κB activation by FN and PG resulted in associated gene expression changes, semi-quantitative RT-PCR analyses were performed using selected candidate genes at both 4- and 24-h post-stimulation. Genes were selected that had previously been implicated in periodontal inflammation (e.g. TNF-α, IL-1) or were important to the structural integrity of the oral epithelium (cytokeratins). Data clearly indicated that transcripts for all cytokines/chemokines investigated were up-regulated by both PG and FN at both the time points (Fig. 3a). Cytokeratin gene expression showed a more complex expression pattern with both bacteria causing down-regulation of cytokeratins 4 and 19 and up-regulation of cytokeratins 10, 13 and 16, at 4 and 24 h (Fig. 3b). Differential effects of FN and PG were observed for cytokeratins 1, 5 and 6. In general, it was notable that FN appeared to have a greater effect than PG on cytokine/chemokine and cytokeratin gene expression (Fig. 3), data consistent with the previous PCR and immunocytochemical findings (Figs 1 and 2).
Fig. 3.
Semi-quantitative RT-PCR analysis of selected cytokines/chemokines (a) and cytokeratin genes (b). H400 cells were exposed to media alone (M) or 109 cfu/ml of PG or FN for 4 and 24 h. (i) Representative gel images of duplicate analyses are shown. (ii) Densitometric analysis of gel products. Expression levels are shown as percentage of the highest level detected. Amplified product values were normalized to GAPDH housekeeping gene levels. All semi-quantitative RT-PCR analyses were performed in duplicate.
High-throughput gene expression analysis of OEC transcripts
In order to obtain a broader picture of the genes expressed in OECs following exposure to the periodontal pathogens and to identify potential genes previously associated with periodontitis, HG_U133A microarrays were screened. Analyses were performed following 24-h exposure as it was hypothesized that this time point may better represent molecular changes occurring in vivo due to interactions and feedback mechanisms between bacteria, cytokines/chemokines and other signalling molecules during chronic disease.
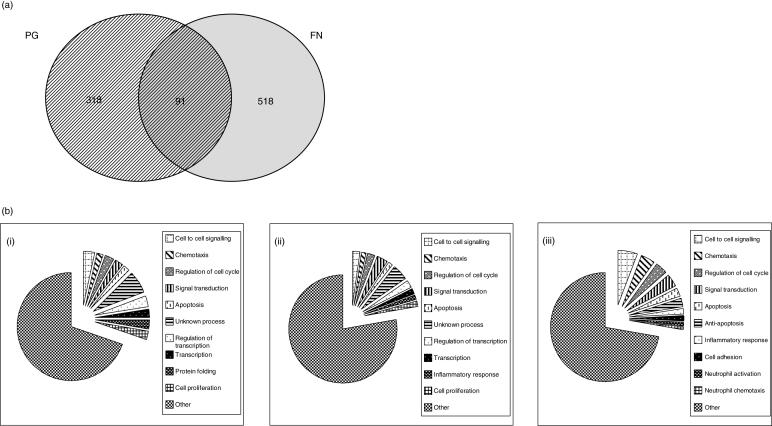
Analysis of hybridization results using dChip software indicated that PG and FN resulted in the differential expression of 409 and 609 genes, respectively. Data indicated that PG up- and down-regulated 170 and 239 transcript features, respectively, whilst FN up- and down-regulated 247 and 362 transcript features, respectively. To determine similarities between the differentially expressed gene datasets results were compared using Access database software (Microsoft) using the Affymetrix Probe ID as the primary key. Analyses indicated that of the total 1018 differentially expressed genes identified only 91 were common to both datasets and that 46 and 45 were up- and down-regulated, respectively, by both periodontal pathogens (Tables 2 and 3, Fig. 4a).
Table 2.
Genes more abundantly expressed (2-fold or greater) in H400 oral epithelial cells exposed to P. gingivalis (PG) or F. nucleatum (FN). The higher fold changes for genes induced to a greater degree by either PG or FN are shown with a grey background. GO groupings were determined from data provided by the NetAFFX web-tool for the Affymetrix probe ID number.
| Fold change | |||||
|---|---|---|---|---|---|
| GO annotation | Affymetrix ID | GENE | Gene symbol | PG | FN |
| Immune/Inflammatory/chemotaxis response | 211506_s_at | interleukin 8 | IL8 | 10.2 | 33.08 |
| 210118_s_at | interleukin 1, alpha | IL1A | 10.77 | 17.45 | |
| 202859_x_at | interleukin 8 | IL8 | 9.37 | 15.61 | |
| 220322_at | interleukin 1 family, member 9 | IL1F9 | 3.28 | 14.69 | |
| 205067_at | interleukin 1, beta | IL1B | 5.92 | 9.79 | |
| 39402_at | interleukin 1, beta | IL1B | 5.59 | 9.69 | |
| 211668_s_at | plasminogen activator, urokinase | PLAU | 3.73 | 6.95 | |
| 203828_s_at | interleukin 32 | IL32 | 3.72 | 6.9 | |
| 209774_x_at | chemokine (C-X-C motif) ligand 2 | CXCL2 | 3.25 | 6.77 | |
| 210845_s_at | plasminogen activator, urokinase receptor | PLAUR | 2.61 | 6.45 | |
| 205476_at | chemokine (C-C motif) ligand 20 | CCL20 | 6.39 | 5.7 | |
| 204420_at | FOS-like antigen 1 | FOSL1 | 5.17 | 4.09 | |
| Cell cycle/proliferation/differentiation | 210511_s_at | inhibin, beta A (activin A, activin AB alpha polypeptide) | INHBA | 6.14 | 14.87 |
| 205767_at | epiregulin | EREG | 20.59 | 14.29 | |
| 205239_at | amphiregulin (schwannoma-derived growth factor) | AREG | 8.55 | 8.51 | |
| 203414_at | monocyte to macrophage differentiation-associated | MMD | 2.95 | 4.45 | |
| 202147_s_at | interferon-related developmental regulator 1 | IFRD1 | 2.65 | 3.31 | |
| 200953_s_at | cyclin D2 | CCND2 | 2.59 | 3.15 | |
| 202146_at | interferon-related developmental regulator 1 | IFRD1 | 2.69 | 2.67 | |
| 202934_at | hexokinase 2 | HK2 | 2.63 | 2.58 | |
| 208892_s_at | dual specificity phosphatase 6 | DUSP6 | 3.08 | 2.37 | |
| Protein metabolism/processing | 205180_s_at | a disintegrin and metalloproteinase domain 8 | ADAM8 | 6.85 | 9.84 |
| 203889_at | secretory granule, neuroendocrine protein 1 (7B2 protein) | SCG5 | 4.23 | 6.26 | |
| 202843_at | DnaJ (Hsp40) homolog, subfamily B, member 9 | DNAJB9 | 6.91 | 4.94 | |
| 215009_s_at | SEC31-like 1 (S. cerevisiae) | SEC31L1 | 2.36 | 2.89 | |
| 213262_at | spastic ataxia of Charlevoix-Saguenay (sacsin) | SACS | 4.32 | 2.56 | |
| 217168_s_at | homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 | HERPUD1 | 2.92 | 2.37 | |
| Signal transduction | 202644_s_at | tumor necrosis factor, alpha-induced protein 3 | TNFAIP3 | 3.01 | 15.01 |
| 202643_s_at | tumor necrosis factor, alpha-induced protein 3 | TNFAIP3 | 3.84 | 13.61 | |
| 203821_at | heparin-binding EGF-like growth factor | HBEGF | 4.37 | 12.12 | |
| 210517_s_at | A kinase (PRKA) anchor protein (gravin) 12 | AKAP12 | 3.25 | 10.58 | |
| 221577_x_at | growth differentiation factor 15 | GDF15 | 3.58 | 2.81 | |
| Apoptosis/Anti-apoptosis | 204614_at | serine (or cysteine)proteinase inhibitor, clade B (ovalbumin), member 2 | SERPINB2 | 3.02 | 7.59 |
| 217997_at | pleckstrin homology-like domain, family A, member 1 | PHLDA1 | 3.77 | 7.11 | |
| 218000_s_at | pleckstrin homology-like domain, family A, member 1 | PHLDA1 | 2.56 | 4.49 | |
| 210538_s_at | baculoviral IAP repeat-containing 3 | BIRC3 | 2.52 | 2.43 | |
| Transcription regulation | 208025_s_at | high mobility group AT-hook 2 /// high mobility group AT-hook 2 | HMGA2 | 3.84 | 5.72 |
| 202936_s_at | SRY (sex determining region Y)-box 9 | SOX9 | 3.2 | 4.71 | |
| 36711_at | v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | MAFF | 3.23 | 3.4 | |
| Cell adhesion | 201645_at | tenascin C (hexabrachion) | TNC | 3.12 | 8.47 |
| 203562_at | fasciculation and elongation protein zeta 1 (zygin I) | FEZ1 | 3.21 | 3.68 | |
| Metabolism | 212473_s_at | microtubule associated monoxygenase, calponin and LIM domain containing 2 | MICAL2 | 2.93 | 8.09 |
| 210041_s_at | phosphoglucomutase 3 | PGM3 | 2.79 | 3.53 | |
| Nitric oxide/oxidative stress response | 203946_s_at | arginase, type II | ARG2 | 3.89 | 9.25 |
| 215223_s_at | superoxide dismutase 2, mitochondrial | SOD2 | 2.57 | 4.69 | |
| Autophagy/Cytolosis | 203827_at | WD40 repeat protein Interacting with phospholnositides of 49 kDa | WIPI1 | 3.01 | 3.24 |
Table 3.
Genes decreased in expression (2-fold or greater) in H400 oral epithelial cells exposed to P. gingivalis (PG) or F. nucleatum (FN). Fold changes for genes decreased by a greater degree by either PG or FN are shown with a grey background. GO groupings were determined from data provided by the NetAFFX web-tool for the Affymetrix probe ID number.
| Fold change | |||||
|---|---|---|---|---|---|
| Go annotation | Affymetrix ID | GENE | Gene symbol | PG | FN |
| Protein metabolism/processing/proteolysis | 209596_at | matrix-remodelling associated 5 | MXRA5 | −4.18 | −4.99 |
| 221215_s_at | receptor-interacting serine-threonine kinase 4 | RIPK4 | −3.02 | −4.88 | |
| 213640_s_at | lysyl oxidase | LOX | −6.07 | −4.14 | |
| 205959_at | matrix metalloproteinase 13 (collagenase 3) | MMP13 | −7.34 | −4.02 | |
| 204580_at | matrix metalloproteinase 12 (macrophage elastase) | MMP12 | −9.05 | −3.19 | |
| 204298_s_at | lysyl oxidase | LOX | −6.92 | −3.17 | |
| 214041_x_at | Ribosomal protein L37a | RPL37A | −3.34 | −2.68 | |
| 215446_s_at | lysyl oxidase | LOX | −4.75 | −2.48 | |
| Cell cycle/growth/proliferation | 211607_x_at | epidermal growth factor receptor | EGFR | −2.72 | −8.42 |
| 210984_x_at | epidermal growth factor receptor | EGFR | −2.85 | −7.83 | |
| 204379_s_at | fibroblast growth factor receptor 3 | FGFR3 | −4.34 | −7.48 | |
| 202718_at | insulin-like growth factor binding protein 2, 36 kDa | IGFBP2 | −2.37 | −3.99 | |
| 213413_at | stoned B-like factor | STON1 | −3.39 | −3.78 | |
| 209784_s_at | jagged 2 | JAG2 | −3.01 | −2.5 | |
| 32137_at | jagged 2 | JAG2 | −2.98 | −2.48 | |
| 201141_at | glycoprotein (transmembrane) | GPNMB | −3.29 | −2.46 | |
| Cell adhesion/motility | 209872_s_at | plakophilin 3 | PKP3 | −4.95 | −4.17 |
| 201668_x_at | myristoylated alanine-rich protein kinase C substrate | MARCKS | −4.3 | −3.74 | |
| 211905_s_at | integrin, beta 4 | ITGB4 | −3.11 | −3.33 | |
| 208153_s_at | FAT tumor suppressor homolog 2 | FAT2 | −3.42 | −3.19 | |
| 201107_s_at | thrombospondin 1 | THBS1 | −2.9 | −2.7 | |
| 201107_s_at | thrombospondin 1 | THBS1 | −2.9 | −2.7 | |
| 204989_s_at | integrin, beta 4 | ITGB4 | −3.92 | −2.37 | |
| Epidermis development | 209351_at | keratin 14 | KRT14 | −6.17 | −8.63 |
| 213240_s_at | keratin 4 | KRT4 | −2.59 | −4.89 | |
| 222242_s_at | kallikrein 5 | KLK5 | −2.51 | −3.38 | |
| 204734_at | keratin 15 | KRT15 | −3.47 | −2.67 | |
| 207935_s_at | keratin 13 | KRT13 | −2.71 | −2.49 | |
| Fatty acid/lipid metabolism | 211548_s_at | hydroxyprostaglandin dehydrogenase 15-(NAD) | HPGD | −3.26 | −3.69 |
| 211708_s_at | stearoyl-CoA desaturase (delta-9-desaturase) | SCD | −2.8 | −3.38 | |
| 203980_at | fatty acid binding protein 4, adipocyte | FABP4 | −5.72 | −2.79 | |
| 203914_x_at | hydroxyprostaglandin dehydrogenase 15-(NAD) | HPGD | −2.57 | −2.75 | |
| Nervous system signalling/development | 205413_at | chromosome 11 open reading frame 8 | MPPED2 | −4.76 | −5.19 |
| 200648_s_at | glutamate-ammonia ligase (glutamine synthase) | GLUL | −2.75 | −3.78 | |
| 217202_s_at | glutamate-ammonia ligase (glutamine synthase) | GLUL | −3.07 | −3.46 | |
| Transcription regulation | 207826_s_at | inhibitor of DNA binding 3 | ID3 | −4.01 | −3.61 |
| 210892_s_at | general transcription factor II, I | GTF21 | −2.67 | −3.31 | |
| 212016_s_at | polypyrimidine tract binding protein 1 | PTBP1 | −2.45 | −3.18 | |
| Transport | 206165_s_at | chloride channel, calcium activated, family member 2 | CLCA2 | −3.94 | −3.89 |
| Unclassified | 207761_s_at | DKFZP586A0522 protein | METTL7A | −2.9 | −5.47 |
| 221805_at | neurofilament, light polypeptide 68 kDa | NEFL | −2.43 | −3.57 | |
| 208156_x_at | epiplakin 1 III epiplakin 1 | EPPK1 | −3.27 | −3.41 | |
| 213075_at | olfactomedin-like 2A | OLFML2A | −2.83 | −2.99 | |
| 208978_at | cysteine-rich protein 2 | CRIP2 | −3.22 | −2.77 | |
| 213069_at | HEG homolog 1 (zebrafish) | HEG1 | −2.58 | −2.74 | |
| 213100_at | Unc-5 homolog B (C. elegans) | – | −3.71 | −2.51 | |
Fig. 4.
Microarray data analyses. (a) Venn diagram representing the number of gene probes differentially expressed (≥ two-fold) in H400 cells as a result of 24-h exposure to the periodontopathogens FN and PG as determined by dChip analysis of microarray hybridization experiments. (b) Pie chart representations of the biological process ontological groups identified using Onto-Express for the genes up-regulated in H400 OECs for (i) PG (170 genes), (ii) FN (247 genes) and the genes common to both data sets, (iii) PG/FN (46 genes). Groups for the 10 most represented gene ontologies are shown for each data set.
To represent the data in a more meaningful biological context and to characterize more thoroughly sets of functionally related genes, the differentially expressed data sets were subsequently organized into gene ontology (GO) groupings using the publicly available software Onto-Express (OE). The OE software automatically translates the non-redundant datasets using standardized GO terms, identifying biological processes that may be affected as a result of OEC exposure to the bacteria. Figure 4(b) provides graphical representation of the biological processes identified for genes up-regulated in the individual data sets and in the genes commonly induced by PG and FN. It was notable that the biological process GO groupings identified by the core/common dataset mapped to functions associated with our current understanding and knowledge of the molecular aspects of periodontitis pathogenesis, such as neutrophil activation and chemotaxis (Fig. 4biii).
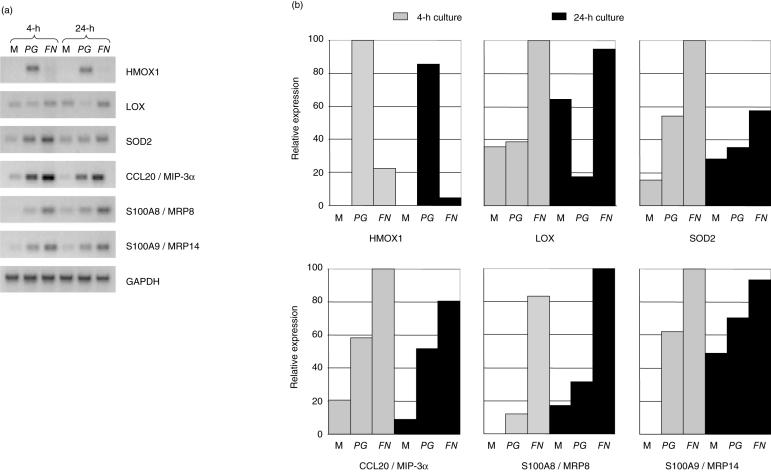
Whilst validity of the microarray gene expression results was supported by (i) our initial semi-quantitative RT-PCR analyses (Fig. 3, Tables 2 and 3), (ii) ontological analyses and (iii) published literature indicating many of the genes identified have previously been described as being up-regulated by bacterial and other pro-inflammatory stimuli [13,37], further confirmatory sq-RT-PCRs were also performed. Genes for analysis were selected based on several criteria, including (i) previously not described as being induced in oral epithelial cells by periodontal bacteria, (ii) previously not described as being involved in periodontitis pathogenesis, or (iii) genes differentially induced or repressed by FN or PG in H400 cells. Results of the analysis of LOX, HMOX1, SOD2, CCL20 and S100A8/A9 shown in Fig. 5 provide further support for the validity of the microarray data (Table 2) and indicate that FN and PG induce different molecular effects in oral epithelial cells.
Fig. 5.
Semi-quantitative RT-PCR analysis of genes identified from microarray data as being differentially expressed in H400 cells due to PG or FN exposure. H400 cells were exposed to media alone (M) or 109 cfu/ml of PG or FN for 4 and 24 h. (a) Representative gel images of amplified products. (b) Densitometric analysis of gel products. Expression levels are shown as percentage of the highest level detected. Amplified product values were normalized to GAPDH housekeeping gene levels. All semi-quantitative RT-PCR analyses were performed in duplicate.
Discussion
This study extends current published data on the up-regulation of the NF-κB pathway and certain inflammatory/immune response transcripts by OECs exposed to periodontal bacteria and provides new molecular data that significantly extend our understanding of these areas. Our results now show that FN and PG have differential effects at the molecular level on oral epithelial cells and that their differences in activating NF-κB nuclear translocation in OECs may at least in part be responsible for the change in dynamics and kinetics of downstream gene expression. Data presented here provide a more comprehensive understanding of the transcript changes established by the two bacteria identifying a variety of genes and pathways involved in signalling recruitment and activation of immune system cells, along with genes important for cellular and tissue protection from bacteria and other cellular insults. The significance of the transcripts that are regulated specifically by PG and FN requires further analysis in clinically derived samples of which biofilm composition is concurrently determined. It is conceivable that such molecules may identify different stages of the disease and provide useful diagnostic markers enabling targeted therapy.
Several studies have shown the production of anti-microbial peptides, such as β-defensins, cathelicidin LL-37, PLUNCs and bactericidal/permeability-increasing protein (BPI), by the oral epithelium [10,38]. The data presented here identified the up-regulation by both bacteria of genes coding for the anti-microbial peptides S100A9 (MRP14) and S100A8 (MRP8), which combine to form the calprotectin complex, as well as the multifunctional cytokine CCL20 (LARC/MIP-3a). Interestingly, recent studies have shown that calprotectin expression in vitro by OECs confers resistance to infection by PG, rendering the cells more resistant to detachment by PG gingipains, which contribute to the breakdown of the periodontal tissues [39]. Calprotectin may therefore augment both barrier and innate immune functions in the oral epithelium, promoting resistance to infection. Whilst increased expression of CCL20 has been demonstrated in human gingival fibroblasts in response to TNF-α, IL-1β and E.coli LPS [40], we are the first to show its up-regulation in OECs due to periodontopathogens. Data show CCL20 to have broad-spectrum anti-microbial properties similar to defensins, including activity against lung and gut pathogens and Candida albicans [41]. To our knowledge, no data currently exist in vivo on its role in periodontal health and disease, and our findings now support the need for further analysis of CCL20 in periodontitis.
In addition to the anti-microbial activity of the oral epithelium, its adaptive barrier function is an important feature of innate immunity. Genes encoding for structural components of the cytoskeleton, namely cytokeratins, were detected as being differentially expressed in OECs in response to challenge by PG and FN. These genes are involved in control of cell size and shape and cell–cell or cell–matrix interactions, all of which are key to maintenance of epithelial barrier integrity and function. Interestingly, the periodontal bacteria stimulated a cytokeratin expression profile that is similar to that described for periodontal pocket epithelium in vivo [42]. It is therefore conceivable that bacteria may stimulate alterations in cytokeratin expression to gain entry into the underlying gingival tissues. Similarly, if individuals harbour deleterious mutations or polymorphisms in cytokeratins they may be predisposed to periodontitis. Our results now provide data on how periodontal bacteria affect cytokeratin gene expression, potentially indicating the importance of these molecules in epithelial barrier function and repair.
Another novel finding was the significant up-regulation of ADAM8/CD156 (a disintegrin and metalloproteinase domain 8) after stimulation with both FN and PG (Table 2). ADAM family members are membrane-anchored glycoproteins that can act as cell-to-cell and cell-to-matrix adhesion molecules, degrade the extracellular matrix, and play a role in tissue morphogenesis. Moreover, current evidence indicates that ADAM8 regulates neutrophil infiltration and migration [43] and can stimulate osteoclast formation [44]. Interestingly, this molecule has been proposed as a promising therapeutic target for asthma and our data combined with that of the literature now indicate that ADAM8 therapeutic modulation may also be applicable in periodontitis.
Other differentially expressed genes previously not studied in relation to periodontitis, include lysyl oxidase (LOX), heme oxygenase-1 (HMOX1) and interleukin-32 (IL-32). LOX, a copper-dependent enzyme important in epithelial tissue development, function and repair, in that it catalyses both elastin and collagen cross-linking [45], was down-regulated by PG (Fig. 5). Conceivably, if PG controls expression of this molecule during periodontal infection it may contribute to tissue breakdown and frustrate wound healing. Microarray results indicated that HMOX1 was 21·1-fold up-regulated in OECs due to PG but was unchanged by FN, and subsequent PCR analyses confirmed these findings (Fig. 5a). HMOX1 enzyme activity has been strongly implicated in tissue protection against oxidative stress during the inflammatory process [46]. That FN does not elicit HMOX1 mRNA expression in OECs, may potentially result in reduced antioxidant defence capacity within the periodontal tissues and subsequent exacerbation of tissue damage, especially as FN has been shown to stimulate significant oxygen-radical production by neutrophils [47]. IL-32 was significantly up-regulated by both PG and FN, 3·72- and 6·9-fold, respectively (Table 2). This cytokine is mainly found in epithelial cells, NK cells, T cells and blood monocytes and possesses properties of a classical inflammatory mediator, including an ability to induce IL-8, TNF-α and macrophage inflammatory protein (MIP-2) by both MAP kinase and NF-κB pathways [48]. Recent work [49] has suggested that IL-32 is specifically induced by intracellular pathogens, which is of potential interest as both PG and FN are known to invade oral epithelial cells [8,9]. Whilst the periodontal pathogens employed here were heat-inactivated, it is conceivable that OECs can detect virulence factors on the bacterial outer coats, signalling the potential for intracellular invasion, hence triggering IL-32 production. Further investigations analysing other periodontal organisms' ability to induce IL-32 expression is warranted.
Interestingly, compared with PG, FN induced (i) higher NF-κB activity (Fig. 2b), (ii) a generally greater effect on differential gene expression at both 4 and 24 h (Figs 1, 3 and 5), and (iii) an increased number of gene expression changes at 24 h detected by microarray analysis (Fig. 4a). It therefore appears reasonable to conclude that PG's diminished activation of NF-κB signalling resulted in decreased transcriptional activation as compared with FN. Whilst our data cannot thoroughly explain the differential effects of PG and FN, it is possible to speculate that the mechanism by which they are detected by OECs may contribute to these molecular differences. It is notable that studies suggest that components of PG can activate cells via TLR-2 [50,51], which our data suggest becomes down-regulated relatively early following bacterial stimulation (Fig. 1). This may potentially down-modulate PG's stimulatory effect and may represent an epithelial de-sensitizing mechanism to avoid chronic stimulation. These data are, however, in contrast to TLR-4 and -9, which were initially up-regulated in our system and represent probable receptors for FN components (Fig. 1).
In addition, it was notable that expression of several molecules including TLRs, NF-κB components, cytokeratins and cytokines exhibited differential expression at the 4 and 24 h time points. Several factors may have contributed to this observation. Cellular stress due to the initial media change for synchronization of gene expression may result in the elevated or decreased gene expression observed at the 4-h time point, which subsequently may return to more homoeostatic levels by 24 h. Also, the cellular growth in the extended cultures will result in increased cellular density and confluency, which subsequently may additionally affect gene expression. It is therefore possible to speculate that the 24-h culture period may better represent in vivo events as these cell cultures provide more epithelial-like tissue conditions with cell responses being attributable solely to the bacterial stimuli.
Whilst stimulation of epithelial cells by periodontal bacteria is known to activate several signal transduction cascades, including the NF-κB, JNK and p38 MAP kinase pathways [52], the dramatic NF-κB-activating effect of FN and accompanying up-regulation of pro-inflammatory gene transcripts clearly suggests that this pathway is central to the pathogenicity of this key ‘quorum sensing’ periodontal organism. In addition, a recent study by Carayol et al. [53] indicated that the majority of genes induced by PG LPS in a monocyte cell line could be abolished or attenuated by inactivating NF-κB signalling. It is therefore interesting to speculate that neutralization of the NF-κB pathway might provide a useful therapeutic strategy in periodontitis, especially as specific NF-κB inhibitors have been found to be effective in other chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease [54]. However, another potential therapeutic approach may be the inhibition of intracellular signalling pathways at source and thereby drugs being developed to TLR signalling may have important therapeutic implications in periodontitis [55].
The data presented here confirm and extend previous studies on the effects of periodontal pathogens on OEC gene expression and highlight many previously unreported effects that may be important in initiation and progression of periodontitis. It is clear from our results, and that of previous studies, that periodontopathogens, whether alive or dead, have differential effects on OEC gene expression, although all appear to induce mediators of neutrophilic inflammation. Clinically this is potentially important as the composition of the plaque biofilm, in terms of presence of both live and dead bacterial species, may be crucial to disease progression. Our data, however, continue to support a central role for FN in orchestrating disease pathogenesis as it induces higher levels of OEC NF-κB activation and gene transcription.
Acknowledgments
We would like to thank Professor Stephen Prime, University of Bristol, for the gift of the oral epithelial cell line and advice on its growth and maintenance. The technical assistance of Dr Gillian Mason and the help with microarray data analysis by Dr John Arrand are also gratefully acknowledged.
References
- 1.Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996;1:1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Garcia RI, Janket SJ, et al. The association between cumulative periodontal disease and stroke history in older adults. J Periodontol. 2006;77:1744–54. doi: 10.1902/jop.2006.050339. [DOI] [PubMed] [Google Scholar]
- 3.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;19:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 4.Page RC, Kornman K. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapple ILC, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 6.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 7.Kolenbrander PE, Parrish KD, Andersen RN, Greenberg EP. Intergeneric coaggregation of oral Treponema spp. with Fusobacterium spp. & intrageneric coaggregation among Fusobacterium spp. Infection Immunity. 1995;63:4584–8. doi: 10.1128/iai.63.12.4584-4588.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YW, Shi W, Huang GT, et al. Interactions between periodontal bacteria and human oral epithelial cells. Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000;68:3140–6. doi: 10.1128/iai.68.6.3140-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eick S, Reissmann A, Rodel J, Schmidt KH, Pfister W. Porphyromonas gingivalis survives within KB cells and modulates inflammatory response. Oral Microbiol Immunol. 2006;21:231–7. doi: 10.1111/j.1399-302X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 10.Dale BA. Periodontal epithelium: a newly recognized role in health and disease. Periodontol 2000. 2002;30:70–8. doi: 10.1034/j.1600-0757.2002.03007.x. [DOI] [PubMed] [Google Scholar]
- 11.Mori Y, Yoshimura A, Ukai T, Lien E, Espevik T, Hara Y. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol Immunol. 2003;18:54–8. doi: 10.1034/j.1399-302x.2003.180109.x. [DOI] [PubMed] [Google Scholar]
- 12.Ren L, Leung WK, Darveau RP, Jin L. The expression profile of lipopolysaccharide-binding protein, membrane-bound CD14, and toll-like receptors 2 and 4 in chronic periodontitis. J Periodontol. 2005;76:1950–9. doi: 10.1902/jop.2005.76.11.1950. [DOI] [PubMed] [Google Scholar]
- 13.Kusumoto Y, Hirano H, Saitoh K, et al. Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via toll-like receptor 2. J Periodontol. 2004;75:370–9. doi: 10.1902/jop.2004.75.3.370. [DOI] [PubMed] [Google Scholar]
- 14.Asai Y, Ohyama Y, General K, Ogawa T. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect Immun. 2001;69:7387–95. doi: 10.1128/IAI.69.12.7387-7395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinane DF, Shiba H, Stathopoulou PG, et al. Gingival epithelial cells heterozygous for Toll-like receptor 4 polymorphisms Asp299Gly and Thr399ile are hypo-responsive to Porphyromonas gingivalis. Genes Immun. 2006;7:190–200. doi: 10.1038/sj.gene.6364282. [DOI] [PubMed] [Google Scholar]
- 16.Nonnenmacher C, Dalpke A, Zimmermann S, Flores-De-Jacoby L, Mutters R, Heeg K. DNA from periodontopathogenic bacteria is immunostimulatory for mouse and human immune cells. Infect Immun. 2003;71:850–6. doi: 10.1128/IAI.71.2.850-856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroder NW, Meister D, Wolff V, et al. Chronic periodontal disease is associated with single-nucleotide polymorphisms of the human TLR-4 gene. Genes Immun. 2005;6:448–51. doi: 10.1038/sj.gene.6364221. [DOI] [PubMed] [Google Scholar]
- 18.Folwaczny M, Glas J, Torok HP, Limbersky O, Folwaczny C. Toll-like receptor (TLR) 2 and 4 mutations in periodontal disease. Clin Exp Immunol. 2004;135:330–5. doi: 10.1111/j.1365-2249.2004.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laine ML, Morre SA, Murillo LS, van Winkelhoff AJ, Pena AS. CD14 and TLR4 gene polymorphisms in adult periodontitis. J Dent Res. 2005;84:1042–6. doi: 10.1177/154405910508401114. [DOI] [PubMed] [Google Scholar]
- 20.Andreakos E, Sacre S, Foxwell BM, Feldmann M. The toll-like receptor-nuclear factor kappaB pathway in rheumatoid arthritis. Front Biosci. 2005;10:2478–88. doi: 10.2741/1712. [DOI] [PubMed] [Google Scholar]
- 21.Bascones A, Noronha S, Gomez M, Mota P, Gonzalez Moles MA, Dorrego MV. Tissue destruction in periodontitis: bacteria or cytokines fault? Quintessence Int. 2005;36:299–306. [PubMed] [Google Scholar]
- 22.Gemmell E, Carter CL, Seymour GJ. Chemokines in human periodontal disease tissues. Clin Exp Immunol. 2001;125:134–41. doi: 10.1046/j.1365-2249.2001.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambili R, Santhi WS, Janam P, Nandakumar K, Pillai MR. Expression of activated transcription factor nuclear factor-kappaB in periodontally diseased tissues. J Periodontol. 2005;76:1148–53. doi: 10.1902/jop.2005.76.7.1148. [DOI] [PubMed] [Google Scholar]
- 24.Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc. 2006;137:14S–20S. doi: 10.14219/jada.archive.2006.0402. [DOI] [PubMed] [Google Scholar]
- 25.Taylor GW, Manz MC, Borgnakke WS. Diabetes, periodontal diseases, dental caries, and tooth loss: a review of the literature. Compend Contin Educ Dent. 2004;25:179–84. 186–8, 190. [PubMed] [Google Scholar]
- 26.Tietze K, Dalpke A, Morath S, Mutters R, Heeg K, Nonnenmacher C. Differences in innate immune responses upon stimulation with gram-positive and gram-negative bacteria. J Periodontal Res. 2006;41:447–54. doi: 10.1111/j.1600-0765.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 27.Prime SS, Nixon SVR, Crane LJ, et al. The behaviour of human oral squamous cell carcinoma in cell culture. J Pathol. 1990;160:259–69. doi: 10.1002/path.1711600313. [DOI] [PubMed] [Google Scholar]
- 28.Roberts A, Matthews JB, Socransky SS, Freestone PP, Williams PH, Chapple IL. Stress and the periodontal diseases: effects of catecholamines on the growth of periodontal bacteria in vitro. Oral Microbiol Immunol. 2002;17:296–303. doi: 10.1034/j.1399-302x.2002.170506.x. [DOI] [PubMed] [Google Scholar]
- 29.Dierickx K, Pauwels M, Van Eldere J, Cassiman JJ, Van Steenberghe D, Quirynen M. Viability of cultured periodontal pocket epithelium cells and Porphyromonas gingivalis association. J Clin Periodontol. 2002;29:987–96. doi: 10.1034/j.1600-051x.2002.291103.x. [DOI] [PubMed] [Google Scholar]
- 30.Harada K, Ohira S, Isse K, et al. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Laboratory Invest. 2003;83:1657–67. doi: 10.1097/01.lab.0000097190.56734.fe. [DOI] [PubMed] [Google Scholar]
- 31.Huntley SP, Davies M, Matthews JB, et al. Attenuated type 2 TGF-B receptor signalling in human malignant oral keratinocytes induces a less differentiated and more aggressive phenotype that is associated with metastatic dissemination. Int J Cancer. 2004;110:170–6. doi: 10.1002/ijc.20111. [DOI] [PubMed] [Google Scholar]
- 32.McLachlan JL, Smith AJ, Bujalska IJ, Cooper PR. Gene expression profiling of pulpal tissue reveals the molecular complexity of dental caries. Biochim Biophys Acta. 2005;25:271–81. doi: 10.1016/j.bbadis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Hung-Wong W. Model-based analysis of oligonucleotide arrays. Expression index computation and outlier detection. Proc Natl Acad Sci. 2001;98:31–6. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Hung-Wong W. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;8:0032.1–0032.11. doi: 10.1186/gb-2001-2-8-research0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wodicka L, Dong H, Mittmann M, Ho MH, Lockhart DJ. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:1359–67. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- 36.Draghici S, Khatri P, Martins RP, Ostermeier GC, Krawetz SA. Global functional profiling of gene expression. Genomics. 2003;81:98–104. doi: 10.1016/s0888-7543(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 37.Baqui AA, Meiller TF, Chon JJ, Turng BF, Falkler WA., Jr Granulocyte-macrophage colony-stimulating factor amplification of interleukin-1beta and tumor necrosis factor alpha production in THP-1 human monocytic cells stimulated with lipopolysaccharide of oral microorganisms. Clin Diagn Laboratory Immunol. 1998;5:341–7. doi: 10.1128/cdli.5.3.341-347.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bingle CD, Gorr SU. Host defense in oral and airway epithelia: chromosome 20 contributes a new protein family. Int J Biochem Cell Biol. 2004;36:2144–52. doi: 10.1016/j.biocel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect Immun. 2001;69:4242–7. doi: 10.1128/IAI.69.7.4242-4247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Increase of CCL20 expression by human gingival fibroblasts upon stimulation with cytokines and bacterial endotoxin. Clin Exp Immunol. 2005;142:285–91. doi: 10.1111/j.1365-2249.2005.02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D, Chen Q, Hoover DM, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–55. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 42.Papaioannou W, Cassiman JJ, Van den Oord J, De Vos R, van Steenberghe D, Quirynen M. Multi-layered periodontal pocket epithelium reconstituted in vitro: histology and cytokeratin profiles. J Periodontol. 1999;70:668–78. doi: 10.1902/jop.1999.70.6.668. [DOI] [PubMed] [Google Scholar]
- 43.Higuchi Y, Yasui A, Matsuura K, Yamamoto S. CD156 transgenic mice. Different responses between inflammatory types. Pathobiology. 2002;70:47–54. doi: 10.1159/000066003. [DOI] [PubMed] [Google Scholar]
- 44.Choi SJ, Han JH, Roodman GD. ADAM8: a novel osteoclast stimulating factor. J Bone Miner Res. 2001;16:814–22. doi: 10.1359/jbmr.2001.16.5.814. [DOI] [PubMed] [Google Scholar]
- 45.Maki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–36. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willis D, Moore AR, Frederick R, Willoughby DA. Heme Oxygenase: a novel target for modulation of inflammatory respose. Nature Med. 1996;2:87–93. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 47.Matthews JB, Wright HJ, Roberts A, Cooper PR, Chapple ILC. Hyper-active and reactive peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol. 2007;147:255–64. doi: 10.1111/j.1365-2249.2006.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65:iii61–iii64. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Netea MG, Azam T, Lewis EC, et al. Mycobacterium tuberculosis induces Interleukin-32 production through a Caspase- 1/IL-18/Interferon-γ-dependent mechanism. PLOS Med. 2006;3:1310–9. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bainbridge BW, Coats SR, Darveau RP. Porphyromonas gingivalis lipopolysaccharide displays functionally diverse interactions with the innate host defense system. Ann Periodontol. 2002;7:29–37. doi: 10.1902/annals.2002.7.1.29. [DOI] [PubMed] [Google Scholar]
- 51.Nemoto E, Darveau RP, Foster BL, Nogueira-Filho GR, Somerman MJ. Regulation of cementoblast function by P. gingivalis lipopolysaccharide via TLR2. J Dent Res. 2006;85:733–8. doi: 10.1177/154405910608500809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang GT, Zhang HB, Dang HN, Haake SK. Differential regulation of cytokine genes in gingival epithelial cells challenged by Fusobacterium nucleatum and Porphyromonas gingivalis. Microb Pathog. 2004;37:303–12. doi: 10.1016/j.micpath.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Carayol N, Chen J, Yang F, et al. A dominant function of IKK/NF-kB signaling in global LPS-induced gene expression. J Biol Chem. 2006;16:1–26. doi: 10.1074/jbc.M603417200. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rezaei N. Therapeutic targeting of pattern-recognition receptors. Int Immunopharmacol. 2006;6:863–9. doi: 10.1016/j.intimp.2006.02.005. [DOI] [PubMed] [Google Scholar]