Abstract
Delayed-type hypersensitivity (DTH) is an immune reaction induced by antigen. In the mice footpads at which DTH is elicited, transient swellings which usually peaks at 24–48 h after the antigen challenge are observed. We found that the footpad swellings of mice are sustained for at least 7 days after the antigen challenge if the mice were injected with anti-type II collagen monoclonal antibody (anti-CII MoAb) before the antigen challenge. A histological section of the swelled hindpaw revealed that severe joint inflammation and bone destruction was induced. These features were not observed in the footpads of the DTH-induced mice. Analysis of the inflammatory reaction induced by both the DTH and the anti-CII MoAb injection, here named as DTH arthritis, revealed the following: (1) DTH arthritis is elicited in an antigen-specific manner; and (2) the development of DTH arthritis is mediated by antigen-specific T cells, especially CD4+ T cells.
Keywords: anti-type II collagen antibody, arthritis, delayed-type hypersensitivity, T cells
Introduction
Rheumatoid arthritis (RA) is a progressive, destructive systemic autoimmune disease [1]. Autoantibodies against type II collagen are reported to be detected in some RA patients [2]. It is not understood precisely how antibodies against type II collagen are involved in the progression of RA. On the other hand, antibodies against type II collagen are utilized for the induction of arthritis in animal model in anti-type II collagen antibody-induced arthritis (CAIA) [3], which is the model induced by an injection of mixtures of anti-type II collagen monoclonal antibody (anti-CII MoAb) and subsequent lipopolysaccharide (LPS) injection. In our previous study, we observed that arthritis could be induced by injecting a mixture of recombinant proteins of tumour necrosis factor-α and interleukin-1β instead of LPS into mice footpads after an anti-CII MoAb injection [4]. The injection of these recombinant cytokines alone caused only transient footpad swelling but not arthritis [4]. We also observed anti-CII MoAb localized on the surface of the cartilage in the joint tissue, and revealed that its Fc portion is indispensable for the development of CAIA [4]. These observations led to the hypothesis that the anti-CII MoAb forces a transient inflammatory stimulation to develop a chronic inflammation at the joint tissue, where anti-CII MoAb has been observed to be localized [4]. To test this hypothesis, we induced delayed-type hypersensitivity (DTH) in mice footpads as a transient inflammation in this study.
It is well known that DTH is the immune response mediated by T cells [5]. T cells activated by antigen-presenting cells release cytokines and chemokines which stimulate vascular endothelial cells to enhance permeability and induce phagocytes infiltration and body fluid accumulation at the site of the DTH reaction [6]. The DTH reaction is usually tranquillized after a few days. Even if a DTH reaction has occurred in the footpad, this reaction might not lead to arthritis characterized by chronic synovial joint inflammation, including synovial hyperplasia, infiltration of inflammatory cells, fibrin deposition and erosion of cartilage and bone [1].
In the present study, we found that the administration of anti-CII MoAb sustained the footpad swelling of mice caused by the DTH reaction and induced severe arthritis. We named this sustained inflammation DTH arthritis, and showed that antigen-specific T cells are required for the induction of arthritis. Considering these results, it is speculated that anti-CII MoAb has an important role in the sustenance of inflammatory reactions induced by cell-mediated immune response.
Materials and methods
Animals
Male BALB/cAnNCrj (BALB/c) mice, C.B-17/lcr-scid (scid/scid) mice and the corresponding control, C.B-17/lcr-+/+(+/+control) were purchased from Charles River (Tokyo, Japan). All mice were purchased at the age of 5–6 weeks, housed at Sankyo Laboratories (Tokyo, Japan), and given a standard rodent chow diet and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Sankyo Co. Ltd (Tokyo, Japan). We used BALB/c mice in this study because BALB/c mice develop DTH easily in the footpad and because it is convenient for comparison with our previous studies about CAIA, in which BALB/c mice were used [4,7,8].
Reagents
Arthritogenic MoAb mixture was purchased from Immuno-Biological Laboratories (Gunma, Japan). Methylated bovine serum albumin (mBSA) was purchased from Sigma (St Louis, MO, USA). Key-hole limpet haemocyanin (KLH) was purchased from Calbiochem (Darmstadt, Germany). Complete Freund's adjuvant (CFA) containing Mycobacterium tuberculosis H37Ra was purchased from Difco Laboratories (Detroit, MI, USA). Magnetic cell sorting (MACS) beads coupled to anti-CD4, anti-CD8 and anti-B220 MoAbs were purchased from Miltenyi Biotech (Sunnyvale, CA, USA).
Induction of arthritis in mice
The mice were immunized intradermally with emulsified mBSA in an equal amount of CFA at two sites on the abdomen (0·25 mg/body). For DTH arthritis induction, the mice were injected intravenously with anti-CII MoAb solution (0·5 mg/0·5 ml/body) 4 days after the immunization. DTH or DTH arthritis was elicited in the mice by challenging mBSA solution (0·05 mg/0·05 ml/footpad) subcutaneously in saline into the right footpad 7 days after immunization. As a control, the left footpad was challenged with a comparable volume of saline. For the induction of DTH arthritis in SCID mice, anti-CII MoAb solution (1 mg/0·5 ml/body) was injected. In this study, the day of the antigen challenge was designated as day 0, and anti-CII MoAb was given as a single injection.
Clinical assessment of arthritis and measurement of hindpaw thickness
The mice were observed carefully for swelling of the hindpaws as a sign of arthritis each day after arthritis induction by antigen challenge or LPS injection. The severity of the arthritis was graded on a 0–3 scale as follows: 0, normal; 1, swelling of one digit; 2, swelling of two digits or more or swelling of the ankle or wrist; 3, severe swelling of the entire paw. The degree of hindpaw swelling was evaluated as the net increase in hindpaw thickness (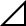 mm) attributable to the challenge calculated by subtracting an increase in the thickness of the right hindpaw from an increase in the thickness of the left hindpaw. The hindpaw thickness (mm) was measured using a dial thickness gauge (Ozaki seisakusho). Statistical significance was determined by a non-parametric Dunnett's test for the clinical score and hindpaw thickness.
mm) attributable to the challenge calculated by subtracting an increase in the thickness of the right hindpaw from an increase in the thickness of the left hindpaw. The hindpaw thickness (mm) was measured using a dial thickness gauge (Ozaki seisakusho). Statistical significance was determined by a non-parametric Dunnett's test for the clinical score and hindpaw thickness.
Histopathology
For the histopathology examinations, DTH arthritis was induced in the mice as described above. The DTH arthritis-induced mice were killed on days 1 and 7 and the hindpaws were removed by cutting them between the knee and ankle. The cut hindpaws were fixed in phosphate-buffered saline (PBS) containing 10% formaldehyde and decalcified in 10% ethylenediamine tetraacetic acid (EDTA) and embedded in paraffin. The hindpaws were sliced horizontally to the footpad to make sections, and the sections were stained with haematoxylin and eosin. Evaluations were carried out on the synovial tissues, bone and the cartilage tissues of the tarsal joints. With regard to the synovial tissues, scoring was performed for the following events: oedema, congestion and/or haemorrhage, presence of debris in the cavity, deposition of fibrin, infiltration of neutrophils, infiltration of macrophages, infiltration of lymphocytes, infiltration of blood plasma cells, proliferation of fibroblasts, proliferation of papilla (villi) and proliferation of synovial cells. With regard to the bone and cartilage tissues, scoring was performed for detachment of chondrocytes, destruction of bone tissues, increase of osteoclasts and ostitis and/or periostitis. The scoring was conducted as follows: –, normal; +, slight change; + +, mild change; + + +, severe change. Scoring was performed as a blind test.
Adoptive transfer of DTH arthritis into SCID mice
B220+, CD8+ or CD4+ cells were depleted from BALB/c splenocytes using immunomagnetic beads conjugated with MoAbs to B220, CD8 or CD4, according to the manufacturer's instructions. Briefly, splenocytes were washed in MACS buffer (PBS without Mg2+ and Ca2+ supplemented with 2 mM EDTA and 0·5% BSA) and incubated for 20 min at 4°C with immunomagnetic beads conjugated with MoAbs against B220, CD8 or CD4. The cells were passed through MACS columns (Miltenyi Biotech), and any magnetically negative cells which passed through the columns were collected. The percentage of B220+, CD8+ or CD4+ cells remaining in each of the collected cells was less than approximately 3%. The collected cells were injected intravenously into SCID mice (1 × 107 cells/0·5 ml/body). The next day the SCID mice were immunized with mBSA, and DTH arthritis was induced as described above.
Results
Administration of anti-CII MoAb sustains footpad swelling of mice caused by a DTH reaction and induces severe arthritis
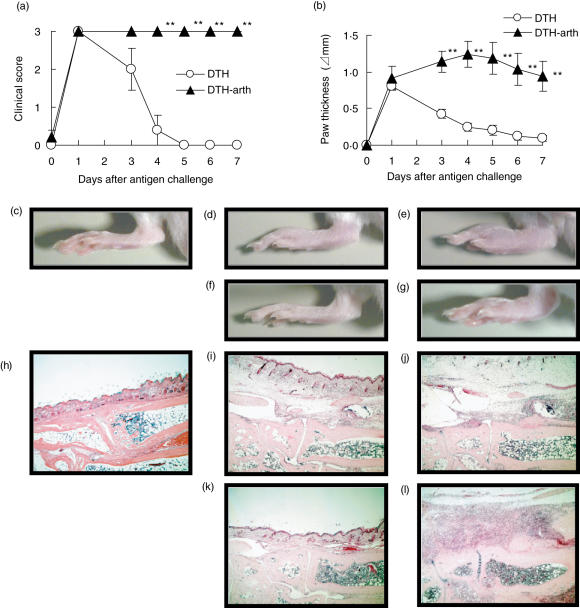
In the group of DTH-induced mice, the clinical score reached maximum on day 1 and decreased gradually on days 3–5 (Fig. 1a); the paw thickness reached maximum on day 1 and gradually decreased on days 3–7 (Fig. 1b). In contrast, in the group of mice injected with anti-CII MoAb 3 days before mBSA injection, the clinical score reached maximum on day 1 and was maintained until day 7 (Fig. 1a) and the paw thickness was sustained from days 1–7 (Fig. 1b). A representative photograph of the hindpaw shows that similar swelling was observed in both groups on day 1 (Fig. 1d,e). However, on day 7, the paw swelling was still observed in the group of mice injected with anti-CII MoAb (Fig. 1g), while the swelling subsided to almost the same extent as the normal mice in the group of DTH-induced mice (Fig. 1c,f). We did not inject the isotype control antibody into the DTH-induced mice. However, injection of the control antibody would not influence critically the swelling induced by the DTH reaction, judging from our previous study [7]. These results have shown clearly that injection of anti-CII MoAb before the antigen challenge prolonged significantly the paw swelling of DTH-induced mice.
Fig. 1.
Administration of anti-CII monoclonal antibody (MoAb) sustains footpad swelling of mice caused by a delayed-type hypersensitivity (DTH) reaction and induces severe arthritis. Clinical score (a) and paw thickness (b) of mice induced with DTH or DTH arthritis. The mice were immunized intradermally with methylated bovine serum albumin (mBSA) (0·25 mg/body). Four days after immunization, the mice were injected intravenously with anti-CII MoAb (0·5 mg/0·5 ml/body) for DTH arthritis induction. Seven days after the immunization, DTH (○) or DTH arthritis (DTH-arth) (▴) was elicited in the mice by challenging mBSA into their right footpads (0·05 mg/0·05 ml/footpad). As a control, their left footpads were challenged by saline. As described in Materials and methods, the severity is represented by the clinical score and paw thickness. The data were expressed as the mean ± s. e.m. of the five mice in each group. The clinical score and paw thickness of the DTH arthritis-induced mice was significantly different from those of the DTH-induced mice (**P < 0·01). Representative photographs of the footpad (c–g) and the histological sections of the tarsal joints stained with haematoxylin and eosin (h–l) in mice are shown. Normal mice (c and h), the DTH-elicited mice 1 day (d and i) or 7 days (f and k) after the mBSA challenge, the DTH arthritis-elicited mice 1 day (e and j) or 7 days (g and l) after the mBSA-challenge. The photographs and the sections, except c and h, were from the mBSA-challenged footpads.
Next, we investigated histological sections of the tarsal joints of the mice in both groups on days 1 and 7. On day 1, inflammatory events such as oedema and infiltration of neutrophils were observed in the synovial tissue in both groups (Fig. 1I,j, Table 1). The severities were similar in both groups. On day 7, in the group of DTH-induced mice all the severities observed were only mild, and amelioration was observed in many of these inflammatory events (Fig. 1k, Table 1). It appears that the inflammation status would begin to subside toward that of normal mice (Fig. 1h). However, in the group of mice injected with anti-CII MoAb, inflammatory events were still observed on day 7 and the severities became more severe or sustained compared with those on day 1 (Fig. 1l, Table 1). In particular, proliferation of fibroblasts and neutrophil infiltration were prominent in all three mice (Fig. 1l, Table 1). Furthermore, it is noteworthy that inflammation such as ostitis, periostitis and increase of osteoclasts were observed in the bone and cartilage tissues in only the group of mice injected with anti-CII MoAb.
Table 1.
Histological examination of tarsal joints of delayed-type hypersensitivity (DTH) or DTH arthritis-induced mice.a
| DTH | DTH arthritis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | |||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Synovial tissues | ||||||||||||
| Oedema | ++ | + | ++ | – | – | – | ++ | ++ | +++ | ++ | ++ | ++ |
| Congestion and/or haemorrhage | + | – | + | – | – | – | + | – | + | + | + | + |
| Presence of debris in the cavity | + | ++ | ++ | + | + | + | +++ | ++ | +++ | +++ | ++ | ++ |
| Deposition of fibrin | – | + | ++ | – | – | – | – | + | + | +++ | +++ | +++ |
| Infiltration of neutrophils | + | +++ | ++ | + | – | + | ++ | + | ++ | +++ | +++ | +++ |
| Infiltration of macrophage cells | – | + | + | – | – | + | + | + | + | + | + | + |
| Infiltration of lymphocytes | – | – | – | – | – | – | – | – | – | – | + | – |
| Infiltration of blood plasma cells | + | + | + | + | + | + | + | + | + | + | + | + |
| Proliferation of fibroblasts | + | + | + | – | – | – | – | + | + | +++ | +++ | +++ |
| Proliferation of papilla (villi) | – | + | – | + | + | + | + | – | + | +++ | + | + |
| Proliferation of synovial cells | + | + | – | + | + | − | – | – | + | + | + | + |
| Bone and cartilage tissues | ||||||||||||
| Detatchment of chondrocytes | – | – | – | – | – | – | – | – | – | – | + | + |
| Destruction of bone tissues | – | – | – | – | – | – | – | – | – | + | + | – |
| Increase in osteoclasts | – | – | – | – | – | – | – | – | – | + | + | + |
| Ostitis and/or periostitis | – | – | – | – | – | – | – | – | – | ++ | ++ | +++ |
DTH or DTH arthritis was induced using the same procedure as in Fig. 1. Three mice were killed on days 1 and 7, and histological sections were made. The severity of each inflammatory event in the synovial tissues, bone and cartilage tissues was graded as follows: –, normal; +, slight change; + +, mild change; + + +, severe change.
As a result of these observations, it was shown that the transient inflammation induced by a DTH reaction in mice footpads developed into arthritis by an anti-CII MoAb injection. It appeared that this arthritis development is due to the synergistic effects of both the DTH reaction and the anti-CII MoAb injection. The histological features seem to have similarities to those of human RA in that inflammation such as proliferation of fibroblasts and destruction of the bone and cartilage tissues were observed [1]. We designated this arthritis as DTH arthritis and investigated this arthritis model as described below.
Antigen specificity in the development of DTH arthritis
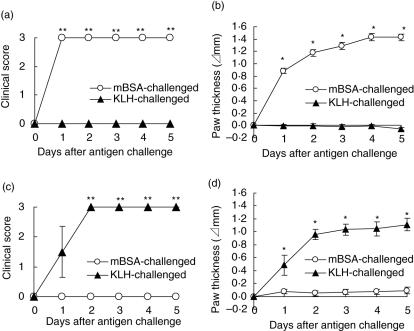
To confirm antigen specificity in DTH arthritis the mice were immunized with mBSA or KLH, and then challenged with mBSA or KLH into their footpads. The mBSA-immunized mice developed DTH arthritis as a result of the mBSA challenge but not the KLH challenge, as judged by both the clinical scores (Fig. 2a) and paw thickness (Fig. 2b). In contrast, KLH-immunized mice developed DTH arthritis as a result of the KLH challenge but not the mBSA challenge, as judged by both clinical score (Fig. 2c) and paw thickness (Fig. 2d). These results indicate that the induction of DTH arthritis could be induced in an antigen-specific manner.
Fig. 2.
Antigen-specific induction of delayed-type hypersensitivity (DTH) arthritis. The mice were immunized intradermally with methylated bovine serum albumin (mBSA) (a, b) or KLH (c, d) (0·5 mg/body). Four days after the immunization, the mice were injected intravenously with anti-CII monoclonal antibody (MoAb) (0·5 mg/0·5 ml/body), and 7 days after the immunization the mice were challenged with mBSA (○) or key-hole limpet haemocyanin (KLH) (▴) into their right footpad (0·05 mg/0·05 ml/footpad). As a control, the other footpad was challenged by saline. As described in Materials and methods, the severity is represented by the clinical score and paw thickness. The data were expressed as the mean ± s.e.m. of the four mice in each group. The clinical score and the paw thickness of the mBSA- or KLH-challenged mice was significantly different from those of the KLH- or mBSA-challenged mice, respectively (*P < 0·05; **P < 0·01).
Involvement of CD4+ T cells in the development of DTH arthritis
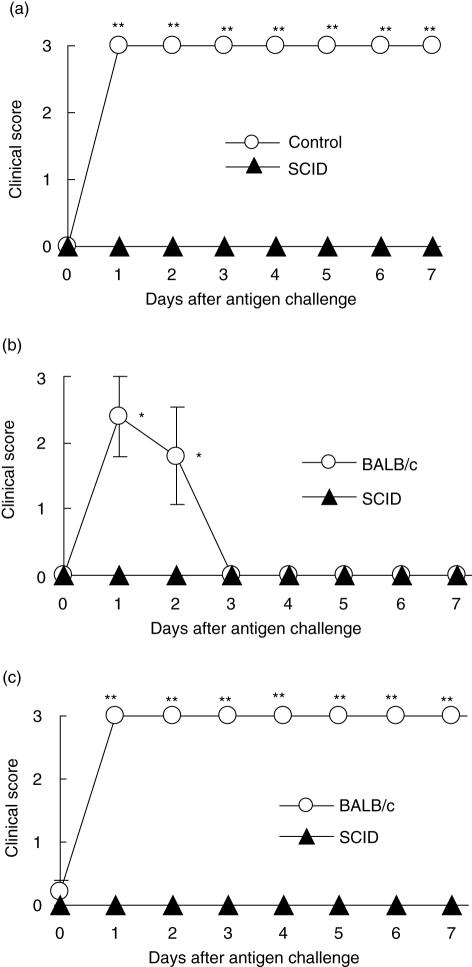
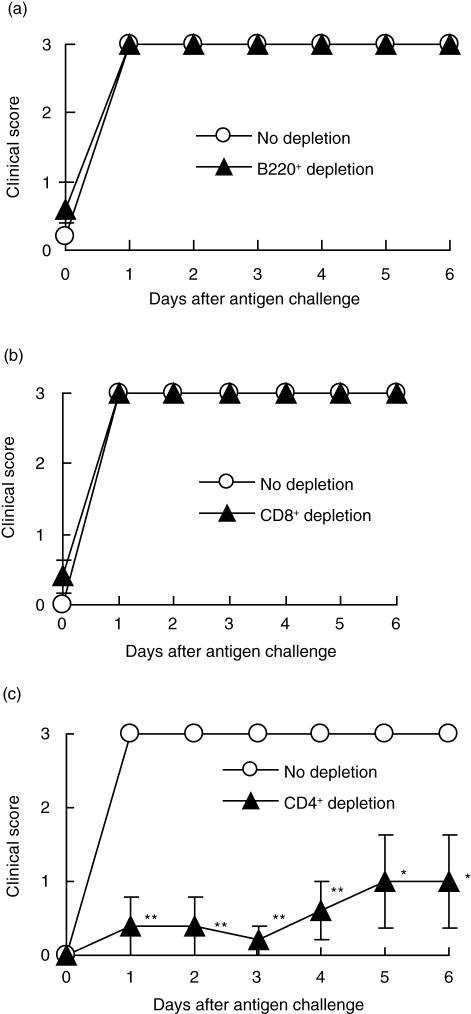
To investigate what kinds of cells are required for the development of DTH arthritis, we induced DTH arthritis in SCID mice that lack both T and B cells. As shown in Fig. 3a, SCID mice were completely resistant to DTH arthritis. To confirm the requirement of T or B cells or both in the development of DTH arthritis, we used reconstituted SCID mice. As a result, the SCID mice reconstituted with splenocytes of BALB/c mice developed both DTH and DTH arthritis (Fig. 3b.c), but SCID mice reconstituted with the splenocytes of SCID mice did not (Fig. 3b,c). These results indicate that T or B cells are probably involved in the development of DTH arthritis. Furthermore, to identify the cell populations that contribute to the development of DTH arthritis, we reconstituted SCID mice with splenocytes of BALB/c mice from which B220+, CD8+ or CD4+ cells had been depleted, and induced DTH arthritis in the SCID mice. Mice reconstituted with splenocytes depleted of CD8+ or B220+ cells developed DTH arthritis to the same extent as did the mice reconstituted with undeleted splenocytes (Fig. 4a,b). However, in the group of mice reconstituted with splenocytes depleted of CD4+ cells, DTH arthritis development was suppressed significantly compared with the group of mice reconstituted with undeleted splenocytes (Fig. 4c). These results indicated that CD4+ but not CD8+ or B220+ cells might be involved in the development of DTH arthritis.
Fig. 3.
Requirement of T or B cells in delayed-type hypersensitivity (DTH) arthritis. DTH arthritis was induced in C,B-17/lcr-scid (SCID) mice (▴) and the corresponding control C.B-17/lcr-+/+ mice (○) (a), as described in Materials and methods. One day before immunization, the SCID mice were reconstituted with the splenocytes (1 × 107 cells/body) derived from BALB/ c (○) or SCID mice (▴), and then DTH (b) or DTH arthritis (c) was induced, as described in Materials and methods. The severity is represented by the clinical score as described in Materials and methods. The data were expressed as the mean ± s.e.m. of the five mice in each group. The clinical score of control C.B-17/lcr-+/+ or BALB/c mice was significantly different from those of SCID mice (*P < 0·05; **P < 0·01).
Fig. 4.
Requirement of CD4+ cells but not CD8+ cells and B220+ in the development of delayed-type hypersensitivity (DTH) arthritis. B220+ (a), CD8+ (b) or CD4+ (c) cells were depleted from splenocytes of BALB/c mice using immunomagnetic beads conjugated with monoclonal antibodies (MoAb) to B220, CD8 and CD4, as described in Materials and methods. Then these depleted splenocytes (▴) or undepleted splenocytes (○) were transferred adoptively (1 × 107 cells/0·5 ml/body) into SCID mice. The next day, the SCID mice were immunized with methylated bovine serum albumin (mBSA) (0·5 mg/body). Four days after the immunization, the mice were injected intravenously with anti-CII MoAb (1 mg/0·5 ml/body). Seven days after the immunization, DTH arthritis was elicited in the mice by challenging mBSA into the footpad (0·05 mg/0·05 ml/footpad). The clinical scores were graded, as described in Materials and methods. The data were expressed as the mean ± s.e.m. of the five mice in each group. The clinical scores of the SCID mice reconstituted with the CD4+ cell-depleted splenocytes were significantly different from those of the SCID mice reconstituted with the undepleted splenocytes (*P < 0·05; **P < 0·01).
Discussion
In the present study, we have shown that an injection of anti-CII MoAb before a DTH induction in the footpads of mice prolonged significantly the inflammation of DTH, and arthritis was developed (DTH arthritis) (Fig. 1). We have also shown that the development of DTH arthritis is mediated by CD4+ T cells (Fig. 4c).
According to histopathological examination, the inflammation of the synovial tissues was sustained in DTH arthritis, while it seemed to be transient in DTH (Fig. 1, Table 1). In DTH arthritis the proliferation of fibroblasts and neutrophil infiltration became more severe, and the destruction of bone and cartilage tissues was observed 7 days after the antigen challenge (Fig. 1, Table 1). These histological features are also observed in human RA [1]. However, there seem to be some differences in the histological features between DTH arthritis and human RA. For example, the formation of pannus and lymphoid follicles, which are characteristics of human RA [1], were not observed in DTH arthritis (data not shown). Even though there might be some differences, the pathogenesis of DTH arthritis seems to have many similarities to that of human RA.
We have shown that inflammation of DTH is significantly prolonged by the injection of anti-CII MoAb. However, we did not investigate how the presence of anti-CII MoAb induces prolonged inflammation in DTH arthritis. We have examined previously the effect of anti-CII MoAb injection alone and observed only slight infiltration of neutrophils and macrophages in the joint tissue 3 days after the anti-CII MoAb injection (2 mg/body) [8]. The amount of anti-CII MoAb used in the present study was less than 2 mg/body, and therefore the anti-CII MoAb injection alone might have induced very slight inflammatory changes in the joint tissue without swelling. Accordingly, DTH arthritis might be induced by the synergistic effect of both the anti-CII MoAb and DTH reactions. Among many arthritis models, CAIA is known to be a mouse arthritis model induced with anti-CII MoAb [3]. Because the anti-CII MoAb used in this report is the same antibody used in our previous report about CAIA [4,7,8], it is considered that there might be some similarities in pathogenesis between DTH arthritis and CAIA. In CAIA, it was shown that Fcγ receptors are indispensable for the development of arthritis [4]. Fcγ receptors are expressed on neutrophils, macrophages, mast cells, dendritic cells, B cells, and so on [9]. Among these cells, neutrophils are shown to be involved in the development of CAIA [8]. In DTH arthritis, neutrophils were observed to be infiltrated into the synovial tissues (Table 1). It is possible that neutrophils are also involved in DTH arthritis, as reported in CAIA. Activation of signalling from Fcγ receptors on neutrophils might contribute to the development of DTH arthritis by promoting their lifespan [10] or enhancing the production of inflammatory mediators [11].
It has been reported that SCID mice develop CAIA although they lack both T and B cells. As the kinds of cell population that participate in DTH arthritis are unknown, we examined whether DTH arthritis is induced in SCID mice. We showed that SCID mice did not develop DTH arthritis (Fig. 3a); however, after the transfer of the splenocytes of BALB/c mice into SCID mice they did develop DTH arthritis (Fig. 3c). Among the cell populations of splenocytes, we showed that B cells might not be involved (Fig. 4a). This is in contrast to previous reports that B cells are involved in the development of a collagen-induced arthritis model (CIA) [12]. In CIA, B cells contribute to the induction of CIA by producing anti-collagen antibody [12]. In DTH arthritis, it is considered that B cells might be dispensable as anti-CII MoAb is supplied by injection. Next we addressed the involvement of T cells, as it has been reported that T cells are involved both in the pathogenesis of human RA and in animal arthritis models [13]. First, we showed that CD8+ cells might not be involved (Fig. 4b). Although CD8+ cells are reported to contribute to the pathogenesis of autoimmune diseases such as those found in non-obese diabetic mice [14], a different cell population seemed to be participating in DTH arthritis induction. We then showed that CD4+ cells are important for the development of DTH arthritis (Fig. 4c). However, the development of DTH arthritis was not suppressed completely by reconstitution with CD4+ cell-depleted splenocytes (Fig. 4c). This is because other cell populations in CD4+ cell-depleted splenocytes might contribute to the DTH arthritis development.
In this report, we have shown an animal arthritis model induced by both anti-CII MoAb injection and DTH elicitation. The requirement of both anti-CII MoAb and CD4+ cells is characteristic of this arthritis model. According to histological observation, the pathogenesis of DTH arthritis might have similarities to that of human RA. We did not elucidate the detailed mechanism of the development of DTH arthritis in this study. Further studies on DTH arthritis might provide an insight into the pathogenesis of RA.
Acknowledgments
We greatly thank Dr Azusa Seki for his technical support on histological analyses. We also acknowledge Dr Wataru Tomisato and Mr Philip Snider for proofreading this manuscript.
References
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim WU, Yoo WH, Park W, et al. IgG antibodies to type II collagen reflect inflammatory activity in patients with rheumatoid arthritis. J Rheumatol. 2000;27:575–81. [PubMed] [Google Scholar]
- 3.Terato K, Harper DS, Griffiths MM, et al. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22:137–47. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- 4.Kagari T, Tanaka D, Doi H, Shimozato T. Essential role of Fcγ receptors in anti-type II collagen antibody-induced arthritis. J Immunol. 2003;170:4318–24. doi: 10.4049/jimmunol.170.8.4318. [DOI] [PubMed] [Google Scholar]
- 5.Marchal G, Seman M, Milon G, Truffa-Bachi P, Zilberfarb V. Local adoptive transfer of skin delayed-type hypersensitivity initiated by a single T lymphocyte. J Immunol. 1982;129:954–8. [PubMed] [Google Scholar]
- 6.Kraneveld AD, Buckley TL, van Heuven-Nolsen D, van Schaik Y, Koster AS, Nijkamp FP. Delayed-type hypersensitivity-induced increase in vascular permeability in the mouse small intestine: inhibition by depletion of sensory neuropeptides and NK1 receptor blockade. Br J Pharmacol. 1995;114:1483–9. doi: 10.1111/j.1476-5381.1995.tb13374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagari T, Doi H, Shimozato T. The importance of IL-1β and TNF-α, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169:1459–66. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119:195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Durand V, Renaudineau Y, Pers JO, Youinou P, Jamin C. Cross-linking of human FcγRIIIb induces the production of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor by polymorphonuclear neutrophils. J Immunol. 2001;167:3996–4007. doi: 10.4049/jimmunol.167.7.3996. [DOI] [PubMed] [Google Scholar]
- 11.Chouchakova N, Skokowa J, Baumann U, et al. FcγRIII-mediated production of TNF-α induces immune complex alveolitis independently of CXC chemokine generation. J Immunol. 2001;166:5193–200. doi: 10.4049/jimmunol.166.8.5193. [DOI] [PubMed] [Google Scholar]
- 12.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–6. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7:S4–14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vizler C, Bercovici N, Cornet A, Cambouris C, Liblau RS. Role of autoreactive CD8+ T cells in organ-specific autoimmune diseases: insight from transgenic mouse models. Immunol Rev. 1999;169:81–92. doi: 10.1111/j.1600-065x.1999.tb01308.x. [DOI] [PubMed] [Google Scholar]