Abstract
Evidence is emerging that exposure to mercury (Hg) may elicit many pathological manifestations, including immunomodulation. We tested whether changing cellular activation pathways may affect the immunomodulation by Hg. Human cell cultures were set up where isolated peripheral blood mononuclear cells, activated by monoclonal antibodies (MoAb: anti-CD3/-CD28/-CD40) or heat-killed Salmonella enterica serovar Enteritidis (hk-SE), exposed to mercuric chloride (HgCl2) for 24 h. Cell vitality was assessed by MTT assay, and modulation of cytokine profiles were monitored by enzyme-linked immunosorbent assay (ELISA), intracellular cytokine staining and reverse transcription–polymerase chain reaction (RT–PCR). Results show that Hg doses above 15 ng/ml significantly reduced cell vitality (P < 0·05). Lower doses elicited distinct effects on T helper 1 (Th1) and Th2 cytokine expression depending on cellular activation pathways. In MoAb-stimulated cells, interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interleukin (IL)-6 production was reduced. Doses up to 0·150 and 0·5 µg/ml increased IL-10 and IL-4 production, respectively, resulting in significantly reduced Th1/Th2 ratios. Stimulation by hk-SE, however, elevated Th1/Th2 ratios due to induction of IFN-γversus IL-10 production. Taken together, we conclude that low-level exposure to Hg, in the absence of inflammation, polarizes the immune response toward Th2, but not in the case of Th1-polarized responses elicited by Salmonella antigens that can be promoted instead. This demonstrates differential in vitro effects of Hg on the Th1/Th2 balance produced by different stimuli, which may have important experimental and scientific implications.
Keywords: cellular activation, helper T cells, immunotoxicity, inflammation, mercuric chloride
Introduction
Anthropogenic use has led to global dispersion of heavy metals such as mercury (Hg) into the environment. Mercury exists in three forms: elemental Hg, inorganic and organic forms, each of which has unique characteristics for the target organ specificity [1]. Potential sources of human exposure to Hg include inhalation of Hg vapours, ingestion of contaminated water and foodstuffs, chloro-alkali plants, gold mining, the production of lamps and batteries and through medical treatments and dental amalgam fillings that contain about 50% Hg by weight [2] and is assumed to be the important source of inorganic Hg exposure in the general population [3]. Dietary intake of fish and other seafood products is the sole source of non-occupational exposure to the highly absorbable methylmercury form [2,4].
Many pathological manifestations caused by exposure to heavy metals have been reported. In general, substantial evidence exists to support the hypothesis that mammalian immunocompetent cellular components are sensitive to modulation by metal contaminants, including different forms of Hg that show diverse effects [5,6]. Exposure to Hg vapours and to organic Hg compounds affects specifically the central nervous system (CNS), while the kidney is the target organ for inorganic Hg. Implication of Hg in induction or exacerbation of diseases included allergic manifestations, neurodegenerative and autoimmune disorders in genetically susceptible animals as well as humans [2,4,6–10]. Together with other findings, results regarding low-level doses of Hg emerged from two large studies [4] have led many regulatory agencies to revise their tolerable limits. Thus, in 1997, the US Environmental Protection Agency (EPA) has lowered the safety level for Hg exposure from 0·5 µg Hg/kg body weight/day, the threshold established by the World Health Organization (WHO) in 1978 [11], to 0·1 µg Hg/kg body weight/day, which is equivalent to blood Hg levels of 4·0–5·0 µg/l [2,12,13].
Mercury has been studied extensively in animal models for its immunotoxic properties, which include both immunosuppression and immunostimulation [14]. Except for those evaluating perinatal exposure [15] or in vivo effects of human relevant concentrations [16–19], most animal experiments have only dealt with exposure at higher Hg concentrations. In addition, many reports have addressed the health consequences resulting from low-level exposures such as those occur among dental personnel handling Hg-containing amalgam or among those who have dental amalgam fillings [3,20,21].
The present study was conducted to test the hypothesis that immunomodulation by HgCl2in vitro may be affected by changes in cell stimulation pathways. To this end, we adopted two agents of immune cell stimulation, either through monoclonal antibodies (MoAb) or bacterial antigens, to test their effects on the course of immune modulation induced by Hg. We investigated the in vitro changes in cell vitality and cytokine secretion profiles of isolated human peripheral blood mononuclear cells (PBMC). In order to avoid potential confounding by non-immunological toxicity of Hg, we intentionally used very low doses of Hg compared with the range used commonly in studies of Hg immunotoxicity.
Materials and methods
Preparation of cells
Blood samples used in this study were obtained as buffy coats of healthy donors from the Blood Bank of Leipzig University Clinic, Germany. The experiments were approved by the local authorities and the informed consent of all participating subjects was obtained. Density gradient centrifugation technique (Ficoll®; Amersham Biosciences, Freiburg, Germany) was applied to separate PBMC that were then collected and washed with phosphate-buffered saline (PBS).
Distribution of PBMC subpopulations
Analysis of the isolated PBMC subpopulations was performed using surface marker staining and flow cytometry. For immunofluorescence staining of cells, the manufacturer's instructions were followed (BD Biosciences, Heidelberg, Germany). A standard set of MoAb against surface antigens was used: Simultest™ CD3/CD8, CD3/CD4, CD3/CD19, CD3/CD16CD56, Simultest™ Leucogate™ (CD45/CD14) and Simultest™ control γ1/γ2a [IgG1 fluorescein isothiocyanate (FITC)/IgG2a phycoerythrin (PE)]. The results are given as percentage of positively stained cells in the sample. A gate was set on the lymphocytes in the forward–side scatter plot, which were then analysed to estimate the percentages of T helper (CD3+, CD4+), T suppressor (CD3+, CD8+), B (CD3–, CD19+) and natural killer (NK) cells (CD3–, CD16+/CD56+).
Cell cultures
Isolated cells were suspended in HybridoMed DIF 1000 (Biochrom, Berlin, Germany) containing 10 μg/ml gentamycin, 100 μg/ml streptomycin, 100 U/ml penicillin and 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT, USA), incubated at 37°C with humidified 5% CO2 and finally cultured (1 ml/well) in 48-well flat-bottomed microtitre plates (Greiner Bio-one GmbH, Nürtingen, Germany). Cells were activated either by MoAb (anti-CD3: OKT3, mouse IgG1; Ortho Biotech, Bridgewater, NJ, USA; anti-CD28: clone CD28·2, mouse IgG1; Beckman-Coulter, Krefeld, Germany; and anti-CD40: clone B-B20; Trinova Biochem, Gießen, Germany; 100 ng/ml each) or heat-killed Salmonella enterica serovar Enteritidis (hk-SE: ade–, his–; Salmovac SE®, Impfstoffwerk Dessau-Tornau, Rosslau, Germany; 1·25 × 105 colony-forming units (CFU)/ml). First, MoAb mixture or hk-SE was diluted in culture medium and 450 µl were added to the wells and the plates were preincubated for 1 h for homologous distribution and binding to the plate surface. Finally, cells were added (2 × 106/500 µl medium) and followed by the application of 50 µl serial doses of the heavy metal.
Application of mercuric chloride
Mercuric chloride (Sigma, Steinheim, Germany) was dissolved in deionized water immediately before application. Final concentrations from 15 pg to 50 µg/ml were prepared using the same culture medium. Control samples were established where cells received either MoAb or hk-SE.
Cell vitality response
Vitality response of Hg-exposed cells to MoAb or hk-SE was assessed by MTT assay as described previously [22]. Following harvesting culture supernatant for enzyme-linked immunosorbent assay (ELISA) after 24-h incubation with HgCl2, the remaining cells were suspended in 200 µl medium, then 25 µl/well MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 5 g/l PBS; Sigma] reagent were added and the plates were incubated in the dark for 4 h at 37°C. Then 300 µl/well stop reagent (10% w/v sodium dodecyl sulphate in 50% v/v N,N′-dimethylformamide; SERVA, Heidelberg, Germany) was added and the platesincubated overnight. The optical density (OD) was evaluated by an automatic plate spectrophotometer (Spectra Image; Tecan, Crailsheim, Germany) at a wavelength of 570 nm and 450 nm reference.
Detection of cytokine release by ELISA
Following a 24-h incubation period, the release of interleukin (IL)-4, IL-6, IL-10, interferon (IFN)-γ and tumour necrosis factor (TNF)-α was determined in cell culture supernatants by commercially available ELISA kits, with lower detection limits of 4 pg/ml (OptEIA™ Kits; BD Biosciences) according to the manufacturer's instructions. Briefly, 384-well microtitre ELISA plates (MaxiSorp, Nunc, Wiesbaden, Germany) were sterile-coated with 50 µl relevant capture antibodies (1 µg/ml) using an automated pipetter (Biomek 2000; Beckmann, Unterschleißheim, Germany) and incubated overnight in a moist chamber at − 4°C. The plates were then washed three times with wash buffer using a microtitre-plate wash automate (Tecan, Crailsheim, Germany) and were then blocked with 100 µl/well assay buffer and incubated at room temperature for 1 h. Following three washing steps, samples and standards were applied and the plates were incubated in a moist chamber for 2 h at room temperature and then washed five times. Detection antibodies (0·5 µg/ml) were applied (50 µl/well) and incubated for 1 h. Following a further seven washes, 3,3′,5,5′-tetramethylbenzidine (TMB)-substrate (50 µl/well; BD Biosciences) was added and the plates were kept in the dark for 20 min before stopping the reaction using 2 M H2SO4 (50 µl/well; Merck, Darmstadt, Germany). The OD was measured by automatic plate spectrophotometer (Spectra Image; Tecan) at a wavelength of 450 nm and 620-nm reference.
Intracellular cytokine staining
To evaluate the functional differentiation of T helper (Th) cells following exposure to Hg, flow-cytometric detection of intracellular cytokines was performed as described previously [22]. Briefly, MoAb-activated human PBMC, isolated as described above, were cultured (2 × 106/ml) in cell-culture tubes (TPP, Trasadingen, Switzerland) at 37°C/5% CO2 for 24 h in the absence or presence of serial doses of HgCl2. Cells were then washed and received 1·0 ml fresh medium (HybridoMed DIF 1000; Biochrom) containing 1·0 µM ionomycin (Calbiochem, Bad Soden, Germany), 2·5 nM phorbol myristate acetate (PMA) and 2·5 µM monensin (Sigma). After a 4-h incubation period, cells were fixed with 4% cold paraformaldehyde, permeabilized with 0·1% saponin and stained with FITC-labelled MoAb against CD3 (UCHT1) and PE-labelled MoAb against the cytokines IFN-γ (45·15) or IL-4 (4D9; Beckman-Coulter). Mouse IgG1 PE-labelled isotype control was used. Cells were analysed by flow cytometry using Cellquest Software [fluorecence activated cell sorter (FACS)Calibur; BD Biosciences]. For analysis, cytokine-PE labelled cells were then plotted against total T cells. Results were expressed as percentages of the control cells.
Quantitative real-time polymerase chain reaction (PCR)
A similar set of cell cultures were set up as described above to determine changes in cytokine mRNA expression following 24-h exposure to Hg. Total RNA was isolated using the E.Z.N.A.® total RNA Kit (PeqLab, Erlangen, Germany). Transcription into cDNA at 42°C for 1 h was followed using AMV Reverse Transcriptase (Promega, Mannheim, Germany), primer deoxyribonucleoside triphosphate (dNTP) set and oligo-dT [5′-pd(T)12−18-3′; Amersham Biosciences]. The LightCycler PCR and real-time detection system (FastStart DNA Master SYBR Green I reagent Kit; Roche, Mannheim, Germany) were used for detecting IFN-γ and IL-4 mRNA. The sequences of the forward and reverse primers used were: (i) the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH):5′-G TC AGT GGT GGA CCT GAC CT and 5′-AGG GGA GAT TCA GTG TGG TG; (ii) IL-4: 5′-AGA AGA CTC TGT GCA CCG AGT TGA and 5′-CTC TCA TGA TCG TCT TTA GCC TTT; and (iii) IFN-γ: 5′-TTC AGC TCT GCA TCG TTT TG and 5′-TCA GCC ATC ACT TGG ATG AG. The level of GAPDH mRNA was used to normalize the amounts of mRNA in each sample. Cytokine standards (GenExpress, Berlin, Germany) were used for quantification. Results were expressed as percentages of the controls.
Statistical analysis
Experiments were carried out in triplicate. Data analysis was performed using statistica version 5·1 software (Statsoft, Hamburg, Germany). Variations among cytokine profiles of different donors were tested using a non-parametric analysis of variance (anova), the Kruskal–Wallis test and, in the case of an indication of significant variation, a post-test was performed (Dunn's test). Wilcoxon's rank test for paired samples was used to analyse the differences between controls and Hg-treated cells. The correlation between the different combinations of cytokine release, intracellular cytokine levels and cytokine mRNA expression was analysed using Spearman's rank correlation test. In all tests, significance was determined at P < 0·05.
Results
Phenotypes of PBMC
For evaluating the immunomodulatory effects of exposure to HgCl2, human Ficoll®-isolated PBMC were used. The percentage of lymphocytes varies within a narrow range of 73·11 ± 6·56 of the total PBMC; monocytes were 15·37 ± 5·95% and granulocytes were 4·98 ± 3·04%. Of the total lymphocytes, the percentage distribution of the main lymphocyte subpopulations was 70·36 ± 6·88 for total CD3+ T cells, 41·72 ± 6·42 CD4+ T cells, 27·25 ±8·82 for CD8+ T cells, 9·35 ± 3·15 for B cells (CD3–,CD19+) and 18·04 ± 7·18 for NK (CD3–, CD16+, CD56+) cells.
Cell vitality response
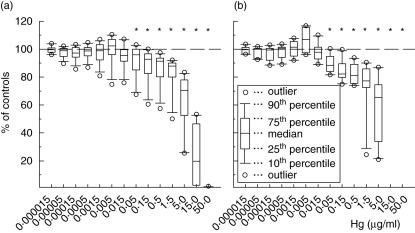
Cell vitality responses of human PBMC to MoAb or hk -SE were significantly decreased at 24 h in cells exposed to HgCl2 (Fig. 1). Doses above 15·0 ng/ml exerted significant inhibitory effects on cell vitality, and those above 15·0 µg/ml showed strong cytotoxic effects, where cell vitality decreased by 80–100% incomparison to controls. Mercury doses below 50 ng/ml caused insignificant decrease in cell vitality, and some samples even showed a slight insignificant increase in vitality response at doses up to 15·0 ng/ml.
Fig. 1.
The vitality response of human peripheral blood mononuclear cells to monoclonal antibodies (MoAb: anti-CD3/-CD28/-CD40) or heat-killed Salmonella enterica serovar Enteritidis (hk-SE) followingexposure to HgCl2. Box-plots show the results of MoAb-activated cells obtained from 12 donors (a) and of hk-SE-stimulated cells from 10 donors (b) following exposure to the heavy metal. Results are shown as percentages of control cells not exposed to Hg. The asterisks (*) indicate significant inhibition (P < 0·05 in Wilcoxon's rank test).
Cytokine release
Table 1 shows the cytokine ranges of control samples, where cells were activated either by MoAb or hk-SE in the absence of HgCl2. Figures 2 and 3 demonstrate changes of the cytokines produced by MoAb- or hk-SE-stimulated human PBMC following 24-h exposure to HgCl2in vitro.
Table 1.
Ranges of the different cytokines (pg/ml) produced by monoclonal antibodies [monoclonal antibodies (MoAb): anti-CD3/-CD28/CD40] or heat-killed Salmonella enterica serovar Enteritidis (hk-SE)-activated human peripheral blood mononuclear cells (PBMC) cultured for 24 h. These data were used as controls to assess the effects of exposure to HgCl2 on cytokine production in vitro.
| Cytokine | Human MoAb-activated PBMC median (10%-90%)a | Human hk-SE-activated PBMC median (10%-90%) |
|---|---|---|
| IL-4 | 61·5 (34·0–306·7) | – |
| IL-6 | 2507·8 (1010·2–8216·2) | 2301·3 (1079·5–5298·1) |
| IL-10 | 265·9(76·7–505·6) | 219·3(140·2–948·2) |
| IFN-γ | 1476·3 (640·0–6957·1) | 114·5 (77·1–415·2) |
| TNF-α | 6793·9 (3047·7–9329·9) | 8466·1 (2685·4–15271·0) |
The median, the 10th and the 90th percentiles of each cytokine are represented as measured by enzyme-linked immunosorbent assay. IL: interleukin; IFN: interferon; TNF: tumour necrosis factor.
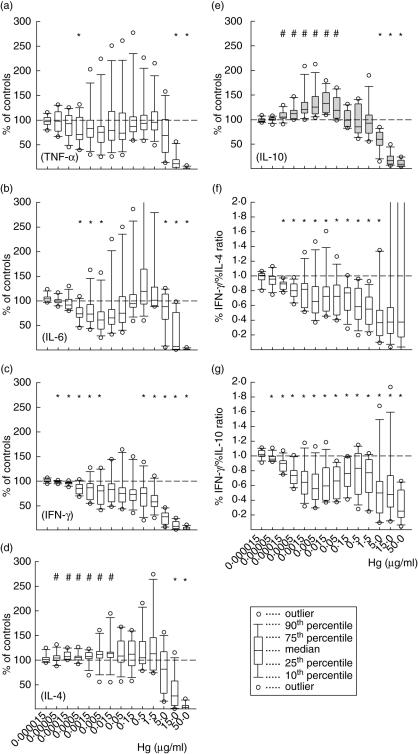
Fig. 2.
The effect of HgCl2 on the in vitro monoclonal antibody (MoAb) -activated release of tumour necrosis factor (TNF)-α (a), interleukin (IL)-6 (b), interferon (IFN)-γ (c), IL-4 (d) and IL-10 (e) by human peripheral blood mononuclear cells (PBMC). Box-plots show data of 12 samples, each with three replicates. T helper 1 (Th1)/Th2 ratios were evaluated from %IFN-γ/%IL-4 (f) and %IFN-γ/%IL-10 (g). Symbols above each plots show whether, at each dose, cytokine release was significantly stimulated (#) or suppressed (*) compared with the controls (P < 0·05 in Wilcoxon's rank test).
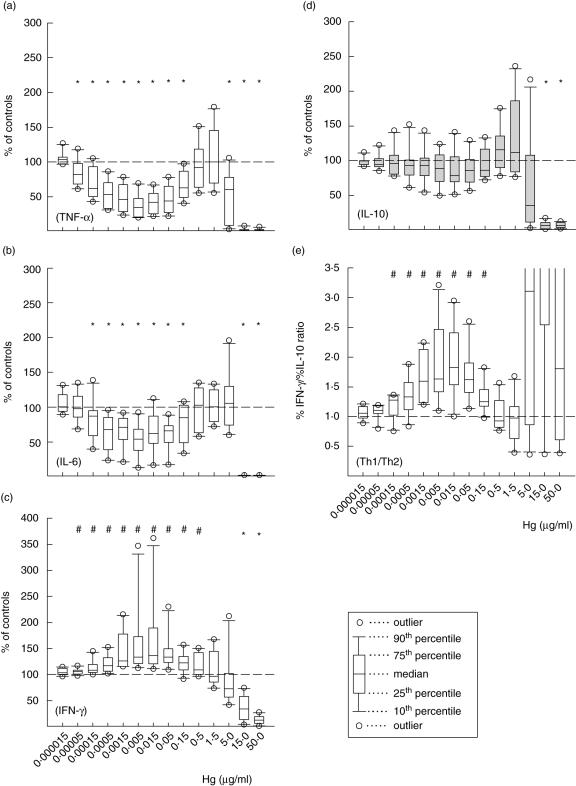
Fig. 3.
The effect of HgCl2 on the in vitro heat-killed Salmonella enterica serovar Enteritidis (hk-SE)-stimulated release of tumour necrosis factor (TNF)-α (a), interleukin (IL)-6 (b), interferon (IFN)-γ (c) and IL-10 (d) by human peripheral blood mononuclear cells. Data of 10 samples, each with three replicates, are demonstrated. T helper 1 (Th1)/Th2 ratios were evaluated from %IFN-γ/%IL-10 ratio (e). Symbols above each plot show whether, at each dose, cytokine release was significantly stimulated (#) or suppressed (*) compared with the controls (P < 0·05 in Wilcoxon's rank test).
Following exposure of MoAb-activated PBMC to HgCl2, the release of the proinflammatory cytokines TNF-α (Fig. 2a), IL-6 (Fig. 2b) and the Th1 cytokine IFN-γ (Fig. 2c) was reduced. Of 12 samples tested, exceptions were the stimulation of TNF-α and IL-6 release at doses between 1·5 ng/ml and 5·0 µg/ml of three donors, which was remarkable in the case of IL-6 release at doses from 0·5 to 1·5 µg/ml; variations between samples were insignificant (P > 0·05, Kruskal–Wallis test). In the case of IFN-γ, only two samples showed an increase of IFN-γ at doses from 15 pg/ml to 1·5 µg/ml and 5–500 ng/ml; data not shown separately. The levels of IL-4 and IL-10 increased significantly (P < 0·05 at Wilcoxon's rank test) at lower doses from 50 pg/ml to 15 and from 150 pg/ml to 50 ng/ml, respectively (Fig. 2d–e). Because a balance between both T helper cell types Th1 and Th2 is hypothesized to exist, the ratios of %IFN-γ/%IL-4 and %IFN-γ/%IL-10 were estimated (Fig. 2f–g) to assess any modulation of this balance. At all Hg doses from 50 pg/ml to 5 µg/ml significantly reduced ratios (P < 0·05 at Wilcoxon's rank test) were found, indicating Th2-biased immune responses.
Figure 3 represents changes in cytokine profiles of hk-SE-stimulated cells exposed to HgCl2 for 24 h. Compared to the case of MoAb-activated cells, the inhibition of TNF-α and IL-6 were more pronounced at Hg doses from 50 pg to 150 ng/ml and from 150 pg to 150 ng/ml for both cytokines, respectively. The release of IFN-γ (Fig. 3c), however, was stimulated significantly at doses from 50 pg up to 0·5 µg/ml, and then decreased steadily when vitality drops below 80% (Fig. 1b). The release of IL-4 was undetectable, and IL-10 release showed an insignificant variation in the 10 samples tested (Fig. 3d). The ratios of %IFN-γ/%IL-10 indicated deviation of the immune response toward Th1 type (Fig. 3e) due to the relative increase of IFN-γversus IL-10 production.
Intracellular cytokines
Intracellular cytokine staining of MoAb-activated cells exposed to HgCl2 for 24 h revealed changes in the proportion of IFN-γ- as well as IL-4-producing cells of the total viable T cells in comparison to control cells (Fig. 4). With few exceptions, a decrease in the number of IFN-γ-producing cells was found as a result of exposure to HgCl2 (Fig. 4a).
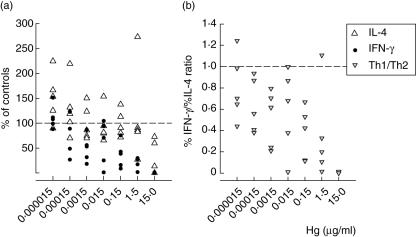
Fig. 4.
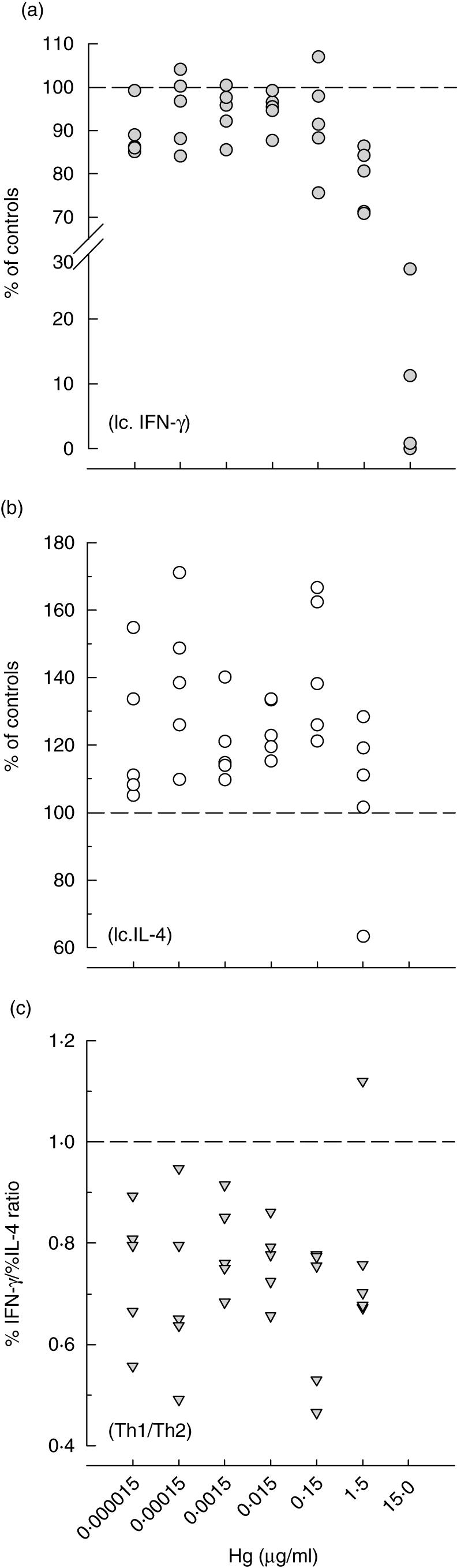
The effect of HgCl2 on the in vitro MoAb-induced production of interferon (IFN)-γ (a) and interleukin (IL)-4 (b) by human peripheral blood mononuclear cells as evaluated by intracellular cytokine staining. Results of five donors are shown as percentages of intracellular cytokine-phycoerythrin labelled to the total fluorescein isothiocyanate-labelled CD3+T cells. T helper 1 (Th1)/Th2 ratios (c) were estimated from %IFN-γ/%IL-4.
On the other hand, the number of IL-4-producing T cells increased in all tested samples at doses from 15 pg to 1·5µg/ml, and then decreased with the increase in cytotoxicity (Fig. 4b). This led to a decrease in the %IFN-γ/%IL-4 ratio at all tested doses up to 1·5 µg/ml, which indicates favouring IL-4-producing cells, namely Th2 cells, in comparison to IFN-γ-producing Th1 cells (Fig. 4c).
Cytokine mRNA expression
Figure 5 represents modulation of the mRNA expression of IL-4 and IFN-γ by MoAb-activated human PBMC following 24-h exposure to HgCl2. Although both show a declining tendency with the increase of Hg dose, it is clear that expression of the IL-4 mRNA was stimulated comparable to IFN-γ mRNA (Fig. 5a). This is represented clearly by estimating the Th1/Th2 ratios (Fig. 5b). With the exception of only one of five tested samples at 15 pg/ml and 1·5 µg/ml, these ratios indicate that all tested doses deviate the immune response toward Th2 type, and refer therefore to the modulation of the immune response by Hg at the early stages of cytokine synthesis.
Fig. 5.
Cytokine mRNA expression, measured by reverse transcription–polymerase chain reaction, of monoclonal antibody (MoAb)-stimulated human peripheral blood mononuclear cells (PBMC) exposed for 24 h to HgCl2. Results of five donors are shown as percentages of controls for both IL-4 and IFN-γ (a). The ratios of %IFN-γ/%IL-4 were estimated to assess modulation of the Th1/Th2 balance(b).
Discussion
Heavy metal pollution has attained high visibility in the public arena and increasingly become a major scientific concern. Most of the early studies addressed the effects of relatively higher doses that are not relevant to most populations. In addition, only recently has the threshold of Hg toxicity been considered with regard to the immune system as a direct target of mercurial exposure [5]. Nowadays, exposure of humans to low levels of Hg through its use in industry and as a component of amalgam fillings warrants further studies targeted at investigating the possible effects of these levels and their relevance to diseases. Because of the great sensitivity of the immune system, determining the optimal exposure doses for immunological testing and attempting to extrapolate between different endpoints in risk assessment is of crucial importance [5]. Among others, disturbances in cytokine production, an important agent in the aetiology of many diseases [23], may be the most relevant indicator to address the effect of xenobiotic agents [24]. Therefore, through applying two model systems of immune cell stimulation and three different methods to detect changes in cytokine profiles, the present study was undertaken to evaluate modulation of cytokine production following exposure to HgCl2. We used a wide range of exposure doses, from toxic levels as indicated by cell cytotoxicity and downwards to very low levels, thought to be ineffective.
Based on their cytokine secretion patterns and effector functions, naive CD4+ T helper cells (Th0) have been shown to differentiate into at least two subsets, Th1 and Th2, both in mice [25–27] and in humans [28,29]. These cells secrete different but overlapping sets of cytokines. Th0 cells secrete cytokines including IL-2, IL-4 and IFN-γ. Whereas Th1 cells produce IL-2, IFN-γ, TNF-β and low levels of IL-10, Th2 cells produce IL-4, IL-5, IL-9, IL-10, IL-13 [25,26,30] and IL-6 [31]. Differentiation of Th0 towards Th1 or Th2 is believed to be a consequence of several cellular influences, such as the cytokine milieu. Th0 cells differentiate into Th1 cells when stimulated in the presence of IL-12 and into Th2 cells when stimulated in the presence of IL-4 [32]. Moreover, there is cross-regulation between Th1 and Th2 in a variety of ways. For example, secretion of IL-4 by Th2 cells blocks differentiation of Th0 into The cells. Production of IL-4, IL-13 and IL-10 by Th2 cells may suppress The cells and many IFN-γ-induced macrophage functions. Conversely, Th1 cells secrete IFN-γ, which inhibits the proliferation of Th2 cells[25,32,33]. The dominance of one of these subsets results in either a predominantly cellular(Th1-mediated) or antibody (Th2-mediated) response [4]. For instance, altering the Th1/Th2 balance may lead to the onset and/or exacerbation of autoimmune disorders[34–36], and may even help find therapeutic possibilities [37]. We assessed the modulation of the Th1/Th2 balance due to exposure to HgCl2 using the %IFN-γ/%IL-4 ratios. In accordance with previous reports, IFN-γ levels in all samples were higher than IL-4 levels. Therefore, according to Lawrence et al. [36], estimating %IFN-γ/%IL-4 ratios may be more relevant than the actual number of IL-4- or IFN-γ-producing cells or the amount of the producedcytokines.
Our results provide evidence that applying different pathways of immune stimulation determines the immunomodulatory effects elicited by exposure to HgCl2in vitro. While high Hg doses are highly toxic, as indicated by significant decrease in cell vitality, exposure to lower and moderate doses exerts immunomodulatory effects resulting in inclination of the immune response towards type 2 in MoAb-activated cells due to induction of IL-4 and IL-10 release. Production of the Th1 cytokine IFN-γ as well as the proinflammatory cytokines TNF-α and IL-6 was reduced. These immunomodulatory effects could be confirmed by measuring the intracellular cytokines of T cells and also at the mRNA level, indicating the preference of The response in MoAb-activated PBMC. However, some samples showed insignificantly different effects following exposure to Hg in vitro. Unfortunately, our figures are limited by the lack of background of the subjects used, which could help understanding of these differences in individual susceptibility that could be linked to genetic factors [38].
As mentioned above, we evaluated modulation of cytokine profiles following exposure to HgCl2in vitro using ELISA to detect cytokine release, flow cytometric analysis to detect intracellular cytokine levels and reverse transcription (RT)–PCR to assess cytokine mRNA expression. We carried out a correlation analysis to test the relevance of these methods (Table 2). A positive correlation was found in all cases of IFN-γ, indicating the relevance of these methods to assess the cytokine profile. In the case of IL-4, on the other hand, with the exception of two cases that showed strong positive correlation, there was no correlation between these different methods. This variation may be attributed to the instant uptake of IL-4 by cells following its release. Use of anti-IL-4 receptor antibodies would have been more relevant to assess the actual levels of IL-4 release. However, the suppression of IFN-γ production which was accompanied by induction of IL-4 release indicates that the polarization of cytokine production begins early at the transcriptional stages, which corresponds with previous studies[39–41], indicating a Th2-polarized immune response in Hg-exposed cells in the case of MoAb stimulation.
Table 2.
The correlation between cytokine release, intracellular cytokine and cytokinemRNA levels for both of interferon (IFN)-γ and interleukin (IL)-4 measured following 24-h in vitro exposure to seven doses of HgCl2 ranged from 15·0 pg to 15·0 µg/ml. Spearman's rank correlation coefficient values(rs) for five samples are listed for both cytokines. The figures in bold type represent the rs for median cytokine values.
| Intracellular cytokine | Cytokine mRNA | |||||
|---|---|---|---|---|---|---|
| IFN-γ | ||||||
| Cytokine release | 0·21 | 0·61 | 0·76 | 0·75 | 0·82** | 0·89** |
| 0·82** | 0·75** | 0·61** | 0·54 | 0·86** | 1·0*** | |
| Intracellular cytokine | – | 0·61 0·11 | 0·52 0·71 | 0·71 0·61 | ||
| IL-4 | ||||||
| Cytokine release | 0·36 | 0·38 | 0·64 | 0·39 | −0·43 | 0·32 |
| 0·54 | 0·58 | 0·34 | −0·14 | 0·83** | 0·04 | |
| Intracellular cytokine | – | 0·39 0·21 | −0·05 0·77** | 0·68 0·38 | ||
P<0·001
P <0·05.
Results of hk-SE-activated cells show that exposure to Hg promoted the type 1-polarized responses induced by bacterial antigens, as indicated by an increase in IFN-γ production. However, at identical concentrations, both TNF-α and IL-6 production were reduced significantly, implying a selective suppression of macrophage/monocyte system by Hg, which is consistent with previous studies [42]. Because of the induction of some cytokines at intermediate Hg doses, the overall behaviour may be the result of an overlap of toxic effects starting at low doses, and cytokine-induced cell proliferation or induction of cytokine production at moderate doses. Another reason for this biphasic pattern of cytokine production may be due to differences in cell sensitivity [43,44] and/or individual susceptibility. The increase of the Th1/Th2 ratio in the presence of Salmonella antigens, which is comparable to lipopolysaccharide (LPS), has recently shown its important clinical implications [45]. Moreover, we extend the earlier studies on Hg immunotoxicity by demonstrating that even low doses of Hg, that cannot affect cell vitality of human immune cells in vitro, can disturb cytokine production as reported previously [24].
Although many studies have been performed on cytotoxic effects of metals on human immunocompetent cells, some inconsistencies have evolved, especially regarding the effects of low-dose exposure in humans. Despite the well-documented effects elicited by high and moderate doses, some studies demonstrated only negligible, if any, influences of low-level exposure to Hg [46–52]. With regard to cytotoxic effects of Hg, our results are consistent with previous studies [53–56]. The immunotoxicity of Hg is complex involving, on one hand, immunosuppression, but on the other hand immunopotentiation. For a long time, the effect of mercurials on the immune system was synonymous with immunosuppression [6]. Exposure to Hg increased animal susceptibility to infectious agents possibly by inhibiting cellular [57] and/or humoral [58,59] responses. Human studies endorsed this view where a decrease in T cell proliferation [54] or B cell counts resulted following exposure to Hg [60]. On the other hand, in vitro exposure to inorganic Hg was found to induce human lymphocyte activity [61] and humoral immunity in Hg-exposed workers [62], and to increase splenic T and B cells in genetically Hg-susceptible mice strains [63]. There are many explanations for these discrepancies [3,5,44], including genetic and cellular differences as well as an array of experimental factors that may profoundly influence the outcome of the in vitro studies.
The immunomodulatory effects of Hg found in the present study are consistent with previous reports. The trend of inclination towards a type 2 response is similar to that described in several in vitro and in vivo studies [37,40,64–70], as well as in workers occupationally exposed to Hg [71]. For instance, in vitro IL-4 production by a Th2 clone was increased significantly by the addition of 27·2–1360 ng/ml HgCl2, whereas IFN-γ production by a Th1 clone was decreased [68]. Similarly, in human basophils [72], whereas 0·272–272 µg/ml HgCl2 promoted Th2 cytokine profiles as indicated by high levels of IL-4 and IL-13, higher doses of 2·72 µg/ml−272 ng/ml strikingly reduced cell viability; however,toxicity varied depending on cell density and incubation time. Using two murine T cell hybridomas (SM1·27·9 and 1H11·3), Badou et al. [64] found that exposure to HgCl2 induced both IL-4 mRNA expression as well as cytokine release. The latter authors proposed a mechanistic pathway that HgCl2 induces a protein kinase C-dependent Ca2+ influx through l-type channels leading to IL-4 gene induction in cells that are engaged in IL-4 production, thereby initiating differentiation of Th2 cells. The predominance of Th2-associated IL-4 is also a well-known feature of HgCl2-treated genetically susceptible rats [73,74] and mouse [67,68,75]. When BALB/c mice were exposed subcutaneously to HgCl2ex vivo IL-4 production by anti-CD3-stimulated splenic T cells was enhanced, but IFN-γ production was inhibited [68]. Additionally, the plasma IL-4 and IgE levels of Hg-exposed mice were increased and IFN-γ levels were lowered significantly in the absence of exogenous antigen.
It is important to consider the combined weight of evidence of Hg accumulation, bioavailability, metabolism and mode of action with regard to threshold of toxicity and potential immunomodulation [5]. This will help to develop risk estimations by comparing Hg concentrations in specific tissues and endpoints that are likely to result in adverse effects. In this study, we applied a wide range of Hg doses. In terms of Hg levels in human blood, we used biologically relevant doses lower than those found in people who live in contaminated areas (0·02 µg/ml [76]), obtained through occupational exposure (2·0–98·0 ng/ml [77]) or in people with amalgam restorations (0·1–2·55 ng/ml [78,79]) and up to toxic levels. Despite the existence of constraints in the extrapolation of in vitro to in vivo situation, for example due to many Hg neutralizing factors in the tissues, our results reveal immunomodulatory effects at doses as low as 50 pg/ml which are lower than the current acceptable threshold blood Hg level of 4·0–5·0 µg/l [2,12,13]. These results are consistent with previous reports that indicated cytotoxic effects of comparable doses [80] and should be taken into account in the preliminary assessment of the risks to human.
Due to its effects on cytokine and immunoglobulin production, Hg has been implicated in the induction of allergic [72,81] and autoimmune [10,34,82] diseases. In allergies, it is known that reactions of type 1 response, evoked normally by IL-2, TNF-α and IFN-γ, are deficient and a type 2 response, driven by IL-4, IL-13, IL-5 and IL-10 [36,83], predominates and is responsible for immunoglobulin class-switching from IgM to IgE [36]. Thus, in susceptible mouse strains, serum IgE increased dose-dependently and reached a maximum after 1–2·5 weeks following exposure to Hg [84]. The immunomodulative potency of Hg, as indicated here and by previous studies, confirms the implication of the metal in the induction of allergic and autoimmune diseases. Moreover, our results imply that the pathways of activating immune cells should be considered in such in vitro systems aiming at evaluation of immunomodulation by xenobiotic agents such as Hg, especially at low exposure levels.
In conclusion, short-term exposure to low and moderate doses of Hg suppresses the MoAb-stimulated production of IFN-γ, TNF-α and IL-6, and alternatively induces type 2 responses, as evidenced by increases in IL-4 and IL-10 levels. The two later cytokines induce and maintain a type 2 immune response, which raises the possibility of Hg-induced allergic diseases, and the development of Th2-dominant autoimmunity. Contrarily, Hg at concentrations from 50 pg/ml to 500 ng/ml can promote the type 1 biased immune response induced by bacterial antigens of S. enterica. At these doses the macrophage activity, however, is suppressed, implying that Hg exerts distinct effects on different immune cell types. Further studies may be conducted to identify specific target cells of Hg immunomodulatory effects. In order to extrapolate between different end-points of risk assessment, current in vivo studies are being undertaken to delineate the biological relevance of these findings and to determine whether low-level exposure to Hg might be problematic in terms of risk for immunological disorders and susceptibility to infectious agents. Overall, this study reflects the importance of considering immune stimulation regimes while investigating the immune modulation by xenobiotic agents such as heavy metals in vitro.
Acknowledgments
We acknowledge the valuable technical assistance of Ms Sylke Drubig (Department of Environmental Immunology, UFZ) and Ms Heike Knaack (IKIT, University of Leipzig). We are also grateful to Dr Sonya Faber (Fraunhofer Institute for Cell Therapy and Immunology) for reviewing the manuscript. This work was funded by a grant from the Interdisziplinäres Zentrum für Klinische Forschung Leipzig (IZKF) of the Medical Faculty, University of Leipzig, Germany (project Z10).
References
- 1.Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110(Suppl. 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury −current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–7. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 3.Enestrom S, Hultman P. Does amalgam affect the immune system? A controversial issue. Int Arch Allergy Immunol. 1995;106:180–203. doi: 10.1159/000236843. [DOI] [PubMed] [Google Scholar]
- 4.Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol. 2003;18:149–75. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 5.Sweet LI, Zelikoff JT. Toxicology and immunotoxicology of mercury: a comparative review in fish and humans. J Toxicol Environ Health B Crit Rev. 2001;4:161–205. doi: 10.1080/109374001300339809. [DOI] [PubMed] [Google Scholar]
- 6.Havarinasab S, Hultman P. Organic mercury compounds and autoimmunity. Autoimmun Rev. 2005;4:270–5. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ Health Perspect. 2003;111:1273–7. doi: 10.1289/ehp.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silbergeld EK, Silva IA, Nyland JF. Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol. 2005;207:282–92. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Monnet-Tschudi F, Zurich MG, Boschat C, Corbaz A, Honegger P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev Environ Health. 2006;21:105–17. doi: 10.1515/reveh.2006.21.2.105. [DOI] [PubMed] [Google Scholar]
- 10.Hultman P, Taylor A, Yang JM, Pollard KM. The effect of xenobiotic exposure on spontaneous autoimmunity in (SWR × SJL) F1 hybrid mice. J Toxicol Environ Health A. 2006;69:505–23. doi: 10.1080/15287390500354904. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) Evaluation of Certain Food Additives and Contaminants. Geneva: WHO; 1978. pp. 1–39. Twenty-second report of the joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series. [Google Scholar]
- 12.US Environmental Protection Agency (EPA) Mercury Study Report to Congress, Office of Air Quality Planning & Standards and Office of Research and Development, Volume IV: an Assessment of Exposure to Mercury in the United States. EPA-452/R-97-006, 1997. http://www.epa.gov/ttn/oarpg/t3/reports/volume4.pdf.
- 13.Ip P, Wong V, Ho M, Lee J, Wong W. Environmental mercury exposure in children: South China's experience. Pediatr Int. 2004;46:715–21. doi: 10.1111/j.1442-200x.2004.01972.x. [DOI] [PubMed] [Google Scholar]
- 14.Clarkson TW. The toxicology of mercury. Crit Rev Clin Lab Sci. 1997;34:369–403. doi: 10.3109/10408369708998098. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RJ, Osborne PB, Haubenreich JE. Dental amalgam restorations: daily mercury dose and biocompatibility. J Long Term Effect Med. 2005;15:709–21. doi: 10.1615/jlongtermeffmedimplants.v15.i6.120. [DOI] [PubMed] [Google Scholar]
- 16.Hultman P, Enestrom S. Dose–response studies in murine mercury-induced autoimmunity and immune-complex disease. Toxicol Appl Pharmacol. 1992;113:199–208. doi: 10.1016/0041-008x(92)90115-9. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, Watanabe C, Satoh M, et al. Susceptibility of metallothionein-null mice to the behavioral alterations caused by exposure to mercury vapor at human-relevant concentration. Toxicol Sci. 2004;80:69–73. doi: 10.1093/toxsci/kfh138. [DOI] [PubMed] [Google Scholar]
- 18.Warfvinge K, Hansson H, Hultman P. Systemic autoimmunity due to mercury vapor exposure in genetically susceptible mice: dose–response studies. Toxicol Appl Pharmacol. 1995;132:299–309. doi: 10.1006/taap.1995.1111. [DOI] [PubMed] [Google Scholar]
- 19.Havarinasab S, Lambertsson L, Qvarnstrom J, Hultman P. Dose–response study of thimerosal-induced murine systemic autoimmunity. Toxicol Appl Pharmacol. 2004;194:169–79. doi: 10.1016/j.taap.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Echeverria D, Woods JS, Heyer NJ, et al. The association between a genetic polymorphism of coproporphyrinogen oxidase, dental mercury exposure and neurobehavioral response in humans. Neurotoxicol Teratol. 2006;28:39–48. doi: 10.1016/j.ntt.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Woods JS, Echeverria D, Heyer NJ, Simmonds PL, Wilkerson J, Farin FM. The association between genetic polymorphisms of coproporphyrinogen oxidase and an atypical porphyrinogenic response to mercury exposure in humans. Toxicol Appl Pharmacol. 2005;206:113–20. doi: 10.1016/j.taap.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Hemdan NYA, Emmrich F, Adham K, et al. Dose-dependent modulation of the in vitro cytokine production of human immune competent cells by lead salts. Toxicol Sci. 2005;86:75–83. doi: 10.1093/toxsci/kfi177. [DOI] [PubMed] [Google Scholar]
- 23.Haggqvist B, Hultman P. Murine metal-induced systemic autoimmunity: baseline and stimulated cytokine mRNA expression in genetically susceptible and resistant strains. Clin Exp Immunol. 2001;126:157–64. doi: 10.1046/j.1365-2249.2001.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen X, Lee K, Konig R. Effects of heavy metal ions on resting and antigen-activated CD4(+) T cells. Toxicology. 2001;169:67–80. doi: 10.1016/s0300-483x(01)00483-8. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991;12:256–7. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- 29.Umetsu DT, Jabara HH, DeKruyff RH, Abbas AK, Abrams JS, Geha RS. Functional heterogeneity among human inducer T cell clones. J Immunol. 1988;140:4211–6. [PubMed] [Google Scholar]
- 30.Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–44. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 32.Seder RA, Paul WE, Davis MM, Fazekas DS. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–8. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 34.Hultman P, Johansson U, Dagnaes-Hansen F. Murine mercury-induced autoimmunity: the role of T-helper cells. J Autoimmun. 1995;8:809–23. doi: 10.1016/s0896-8411(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 35.Lafaille JJ. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998;9:139–51. doi: 10.1016/s1359-6101(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence DA, McCabe MJ., Jr Immunomodulation by metals. Int Immunopharmacol. 2002;2:293–302. doi: 10.1016/s1567-5769(01)00180-1. [DOI] [PubMed] [Google Scholar]
- 37.Haggqvist B, Hultman P. Effects of deviating the Th2-response in murine mercury-induced autoimmunity towards a Th1-response. Clin Exp Immunol. 2003;134:202–9. doi: 10.1046/j.1365-2249.2003.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gleichmann E, Kimber I, Purchase IF. Immunotoxicology: suppressive and stimulatory effects of drugs and environmental chemicals on the immune system. A discussion. Arch Toxicol. 1989;63:257–73. doi: 10.1007/BF00278639. [DOI] [PubMed] [Google Scholar]
- 39.Haggqvist B, Hultman P. Interleukin-10 in murine metal-induced systemic autoimmunity. Clin Exp Immunol. 2005;141:422–31. doi: 10.1111/j.1365-2249.2005.02878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansson M, Djerbi M, Rabbani H, et al. Exposure to mercuric chloride during the induction phase and after the onset of collagen-induced arthritis enhances immune/autoimmune responses and exacerbates the disease in DBA/1 mice. Immunology. 2005;114:428–37. doi: 10.1111/j.1365-2567.2005.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havarinasab S, Haggqvist B, Bjorn E, Pollard KM, Hultman P. Immunosuppressive and autoimmune effects of thimerosal in mice. Toxicol Appl Pharmacol. 2005;204:109–21. doi: 10.1016/j.taap.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Vimercati L, Santarelli L, Pesola G, et al. Monocyte–macrophage system and polymorphonuclear leukocytes in workers exposed to low levels of metallic mercury. Sci Total Environ. 2001;270:157–63. doi: 10.1016/s0048-9697(00)00780-4. [DOI] [PubMed] [Google Scholar]
- 43.Shenker BJ, Berthold P, Decker S, et al. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. II. Alterations in cell viability. Immunopharmacol Immunotoxicol. 1992;14:555–77. doi: 10.3109/08923979209005411. [DOI] [PubMed] [Google Scholar]
- 44.Waalkes MP, Fox DA, States JC, Patierno SR, McCabe MJ., Jr Metals and disorders of cell accumulation: modulation of apoptosis and cell proliferation. Toxicol Sci. 2000;56:255–61. doi: 10.1093/toxsci/56.2.255. [DOI] [PubMed] [Google Scholar]
- 45.Abedi-Valugerdi M, Nilsson C, Zargari A, Gharibdoost F, Depierre JW, Hassan M. Bacterial lipopolysaccharide both renders resistant mice susceptible to mercury-induced autoimmunity and exacerbates such autoimmunity in susceptible mice. Clin Exp Immunol. 2005;141:238–47. doi: 10.1111/j.1365-2249.2005.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansteen IL, Ellingsen DG, Clausen KO, Kjuus H. Chromosome aberrations in chloralkali workers previously exposed to mercury vapor. Scand J Work Environ Health. 1993;19:375–81. doi: 10.5271/sjweh.1459. [DOI] [PubMed] [Google Scholar]
- 47.Mabille V, Roels H, Jacquet P, Leonard A, Lauwerys R. Cytogenetic examination of leucocytes of workers exposed to mercury vapour. Int Arch Occup Environ Health. 1984;53:257–60. doi: 10.1007/BF00398818. [DOI] [PubMed] [Google Scholar]
- 48.Loftenius A, Sandborgh-Englund G, Ekstrand J. Acute exposure to mercury from amalgam: no short-time effect on the peripheral blood lymphocytes in healthy individuals. J Toxicol Environ Health A. 1998;54:547–60. doi: 10.1080/009841098158692. [DOI] [PubMed] [Google Scholar]
- 49.Sandborgh-Englund G, af Geijersstam E, Loftenius A. Rapid communication: acute exposure to mercury from dental amalgam does not affect the levels of C-reactive protein or interleukin-6 in peripheral blood. J Toxicol Environ Health A. 2003;66:495–9. doi: 10.1080/15287390306352. [DOI] [PubMed] [Google Scholar]
- 50.Issa Y, Watts DC, Duxbury AJ, Brunton PA, Watson MB, Waters CM. Mercuric chloride: toxicity and apoptosis in a human oligodendroglial cell line MO3.13. Biomaterials. 2003;24:981–7. doi: 10.1016/s0142-9612(02)00436-2. [DOI] [PubMed] [Google Scholar]
- 51.Luglie PF, Campus G, Chessa G, et al. Effect of amalgam fillings on the mercury concentration in human amniotic fluid. Arch Gynecol Obstet. 2005;271:138–42. doi: 10.1007/s00404-003-0578-6. [DOI] [PubMed] [Google Scholar]
- 52.Kingman A, Albers JW, Arezzo JC, Garabrant DH, Michalek JE. Amalgam exposure and neurological function. Neurotoxicology. 2005;26:241–55. doi: 10.1016/j.neuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Yamada H, Koizumi S. Metallothionein induction in human peripheral blood lymphocytes by heavy metals. Chem Biol Interact. 1991;78:347–54. doi: 10.1016/0009-2797(91)90064-e. [DOI] [PubMed] [Google Scholar]
- 54.Shenker BJ, Rooney C, Vitale L, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. I. Suppression of T-cell activation. Immunopharmacol Immunotoxicol. 1992;14:539–53. doi: 10.3109/08923979209005410. [DOI] [PubMed] [Google Scholar]
- 55.Shenker BJ, Berthold P, Rooney C, Vitale L, DeBolt K, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. III. Alterations in B-cell function and viability. Immunopharmacol Immunotoxicol. 1993;15:87–112. doi: 10.3109/08923979309066936. [DOI] [PubMed] [Google Scholar]
- 56.Steffensen IL, Mesna OJ, Andruchow E, Namork E, Hylland K, Andersen RA. Cytotoxicity and accumulation of Hg, Ag, Cd, Cu, Pb and Zn in human peripheral T and B lymphocytes and monocytes in vitro. Gen Pharmacol. 1994;25:1621–33. doi: 10.1016/0306-3623(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 57.Lawrence DA. In vivo and in vitro effects of lead on humoral and cell-mediated immunity. Infect Immun. 1981;31:136–43. doi: 10.1128/iai.31.1.136-143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koller LD, Exon JH, Brauner JA. Methylmercury: decreased antibody formation in mice. Proc Soc Exp Biol Med. 1977;155:602–4. doi: 10.3181/00379727-155-39859. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence DA. Heavy metal modulation of lymphocyte activities. 1. In vitro effects of heavy metals on primary humoral immune responses. Toxicol Appl Pharmacol. 1981;57:439–51. doi: 10.1016/0041-008x(81)90241-6. [DOI] [PubMed] [Google Scholar]
- 60.Lawrence DAS, Mudzinski U, Rudofsky G. Mechanisms of metal-induced immunotoxicity. In: Berlin A, Dean J, Draper MH, Smith EMB, Spreafico F, Warner, editors. Immunotoxicology. Dordrecht: Martinus-Nigoff; 1987. pp. 293–307. [Google Scholar]
- 61.Loftenius A, Ekstrand J, Moller E. HgCl(2)-induced human lymphocyte activation in vitro: a superantigenic mechanism? Int Arch Allergy Immunol. 1999;120:63–70. doi: 10.1159/000024221. [DOI] [PubMed] [Google Scholar]
- 62.Bencko V, Wagner V, Wagnerova M, Ondrejcak V. Immunological profiles in workers occupationally exposed to inorganic mercury. J Hyg Epidemiol Microbiol Immunol. 1990;34:9–15. [PubMed] [Google Scholar]
- 63.Johansson U, Hansson-Georgiadis H, Hultman P. The genotype determines the B cell response in mercury-treated mice. Int Arch Allergy Immunol. 1998;116:295–305. doi: 10.1159/000023959. [DOI] [PubMed] [Google Scholar]
- 64.Badou A, Savignac M, Moreau M, et al. HgCl2-induced interleukin-4 gene expression in T cells involves a protein kinase C-dependent calcium influx through 1-type calcium channels. J Biol Chem. 1997;272:32411–8. doi: 10.1074/jbc.272.51.32411. [DOI] [PubMed] [Google Scholar]
- 65.Bagenstose LM, Salgame P, Monestier M. Murine mercury-induced autoimmunity: a model of chemically related autoimmunity in humans. Immunologic Res. 1999;20:67–78. doi: 10.1007/BF02786508. [DOI] [PubMed] [Google Scholar]
- 66.Goldman M, Druet P, Gleichmann E. TH2 cells in systemic autoimmunity. insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 67.Ochel M, Vohr HW, Pfeiffer C, Gleichmann E. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J Immunol. 1991;146:3006–11. [PubMed] [Google Scholar]
- 68.Heo Y, Parsons PJ, Lawrence DA. Lead differentially modifies cytokine production in vitro and in vivo. Toxicol Appl Pharmacol. 1996;138:149–57. doi: 10.1006/taap.1996.0108. [DOI] [PubMed] [Google Scholar]
- 69.Heo Y, Lee WT, Lawrence DA. In vivo the environmental pollutants lead and mercury induce oligoclonal T cell responses skewed toward type-2 reactivities. Cell Immunol. 1997;179:185–95. doi: 10.1006/cimm.1997.1160. [DOI] [PubMed] [Google Scholar]
- 70.Fournie GJ, Saoudi A, Druet P, Pelletier L. Th2-type immunopathological manifestations induced by mercury chloride or gold salts in the rat: signal transduction pathways, cellular mechanisms and genetic control. Autoimmun Rev. 2002;1:205–12. doi: 10.1016/s1568-9972(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 71.Soleo L, Vacca A, Vimercati L, et al. Minimal immunological effects on workers with prolonged low exposure to inorganic mercury. Occup Environ Med. 1997;54:437–42. doi: 10.1136/oem.54.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strenzke N, Grabbe J, Plath KE, Rohwer J, Wolff HH, Gibbs BF. Mercuric chloride enhances immunoglobulin E-dependent mediator release from human basophils. Toxicol Appl Pharmacol. 2001;174:257–63. doi: 10.1006/taap.2001.9223. [DOI] [PubMed] [Google Scholar]
- 73.Gillespie KM, Qasim FJ, Tibbatts LM, Thiru S, Oliveira DB, Mathieson PW. Interleukin-4 gene expression in mercury-induced autoimmunity. Scand J Immunol. 1995;41:268–72. doi: 10.1111/j.1365-3083.1995.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 74.Prigent P, Saoudi A, Pannetier C, et al. Mercuric chloride, a chemical responsible for T helper cell (Th) 2-mediated autoimmunity in brown Norway rats, directly triggers T cells to produce interleukin-4. J Clin Invest. 1995;96:1484–9. doi: 10.1172/JCI118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansson U, Sander B, Hultman P. Effects of the murine genotype on T cell activation and cytokine production in murine mercury-induced autoimmunity. J Autoimmun. 1997;10:347–55. doi: 10.1006/jaut.1997.0149. [DOI] [PubMed] [Google Scholar]
- 76.Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf. 2003;56:174–9. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 77.Counter SA, Buchanan LH, Ortega F, Laurell G. Elevated blood mercury and neuro-otological observations in children of the Ecuadorian gold mines. J Toxicol Environ Health A. 2002;65:149–63. doi: 10.1080/152873902753396785. [DOI] [PubMed] [Google Scholar]
- 78.Kingman A, Albertini T, Brown LJ. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. J Dent Res. 1998;77:461–71. doi: 10.1177/00220345980770030501. [DOI] [PubMed] [Google Scholar]
- 79.Ganss C, Gottwald B, Traenckner I, et al. Relation between mercury concentrations in saliva, blood, and urine in subjects with amalgam restorations. Clin Oral Invest. 2000;4:206–11. doi: 10.1007/s007840000089. [DOI] [PubMed] [Google Scholar]
- 80.Silva-Pereira LC, Cardoso PC, Leite DS, et al. Cytotoxicity and genotoxicity of low doses of mercury chloride and methylmercury chloride on human lymphocytes in vitro. Braz J Med Res. 2005;38:901–7. doi: 10.1590/s0100-879x2005000600012. [DOI] [PubMed] [Google Scholar]
- 81.Lerch M, Bircher AJ. Systemically induced allergic exanthem from mercury. Contact Dermatitis. 2004;50:349–53. doi: 10.1111/j.0105-1873.2004.00366.x. [DOI] [PubMed] [Google Scholar]
- 82.Röger J, Zillikens D, Hartmann A, Burg G. Systemic autoimmune disease in a patient with long-standing exposure to mercury. Eur J Dermatol. 1992;2:168–70. [Google Scholar]
- 83.Dubey C, Bellon B, Druet P. TH1 and TH2 dependent cytokines in experimental autoimmunity and immune reactions induced by chemicals. Eur Cytokine Netw. 1991;2:147–52. [PubMed] [Google Scholar]
- 84.Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ Health Perspect. 2002;110(Suppl. 5):877–81. doi: 10.1289/ehp.02110s5877. [DOI] [PMC free article] [PubMed] [Google Scholar]