Abstract
Periodontal disease involves multi-bacterial infections accompanied by inflammatory bone resorption lesions. The abundant T and B lymphocyte infiltrates are the major sources of the osteoclast differentiation factor, receptor activator for nuclear factor-kB ligand (RANKL) which, in turn, contributes to the development of bone resorption in periodontal disease. In the present study, we found that the concentrations of RANKL and regulatory T cell (Treg)-associated cytokine, interleukin (IL)-10, in the periodontal tissue homogenates were correlated negatively, whereas RANKL and proinflammatory cytokine, IL-1β, showed positive correlation. Also, according to the fluorescent-immunohistochemistry, the frequency of forkhead box P3 (FoxP3)/CD25 double-positive cells was diminished strikingly in the bone resorption lesion of periodontal disease compared to healthy gingival tissue, while CD25 or FoxP3 single positive cells were still observed in lesions where abundant RANKL+ lymphocytes were present. Very importantly, few or no expressions of FoxP3 by the RANKL+ lymphocytes were observed in the diseased periodontal tissues. Finally, IL-10 suppressed both soluble RANKL (sRANKL) and membrane RANKL (mRANKL) expression by peripheral blood mononuclear cells (PBMC) activated in vitro in a bacterial antigen-specific manner. Taken together, these results suggested that FoxP3/CD25 double-positive Treg cells may play a role in the down-regulation of RANKL expression by activated lymphocytes in periodontal diseased tissues. This leads to the conclusion that the phenomenon of diminished CD25+FoxP3+ Treg cells appears to be associated with the increased RANKL+ T cells in the bone resorption lesion of periodontal disease.
Keywords: FoxP3, periodontal/oral immunology, RANKL, T cells, Treg cells
Introduction
Many research reports have demonstrated conclusively that regulatory T cells (Treg cells) suppress pathogenic adaptive immune responses, including those involved in autoimmune diseases, infectious diseases and organ transplantation [1]. Although the presence of Treg cells in periodontal diseased tissues has been demonstrated by the reverse transcription–polymerase chain reaction (RT–PCR) for forkhead box P3 (FoxP3), interleukin (IL-10) and transforming growth factor (TGF)-β [2], the role of Treg cells in the context of bone resorption lesion of periodontal disease remains unclear. For example, involvement of lymphocytes in bone loss processes was demonstrated only recently in both in vitro [3] and in vivo models for rheumatoid arthritis [4] and periodontal disease [5,6]. This finding resulted from the discovery of osteoclast differentiation factor, receptor activator for nuclear factor-kB ligand (RANKL) [7], which is expressed not only in bone marrow stromal cells and osteoblasts but also in activated lymphocytes [8]. However, the linkage between Treg cells and RANKL production by lymphocytes in the context of bone resorption lesion of periodontal disease remains unknown.
An active periodontal lesion is characterized by the prominent infiltration of B cells [9,10] and T cells [11,12]. Specifically, the occupancy of 50–60% of such cellular infiltrates by plasma cells makes periodontal disease very distinct from other chronic infectious diseases [13,14]. This is demonstrated by our recent discovery that the osteoclast differentiation factor, RANKL, is expressed distinctively by activated T cells and B cells in gingival tissues with periodontal disease, but not by these lymphocytes in healthy gingival tissues [15]. These RANKL+ lymphocytes isolated from patients' gingival tissues were functionally sufficiently potent to induce in vitro osteoclastogenesis in an RANKL-dependent manner [15]. Supporting this finding, we also demonstrated that Actinobacillus actinomycetemcomitans (Aa) Omp29-specific T helper 1 (Th1)-type T cells or Aa-reactive B cells can trigger periodontal bone resorption in rat models [5,6,16]. Because it has never been reported that any bacteria per se invade and resorb bone independently of osteoclast activation, RANKL expression by T cells and B cells is considered to be a major stimulus of osteoclast precursor cells.
To gain an insight into inflammatory bone resorption mechanisms involving RANKL-expressing activated lymphocytes, a question is raised as to whether FoxP3+ Treg cells are associated with suppression of such lymphocyte expression of RANKL in the context of periodontal bone resorption lesions. It is reported that CD4+ T cells with regulatory phenotypes, CD25 or CTLA4, along with the FoxP3, TGF-β and IL-10 mRNA, are present in diseased gingival tissues [2]. Another study has also demonstrated that a majority of CD4+ T clone cells (almost 100%), which are those established from T cells infiltrating periodontal diseased tissues, are positive for FoxP3 and IL-10 mRNA [17]. The latter results support the presence of FoxP3+CD25+ T cells in gingival tissue. It is conceivable that the protein expression level of FoxP3 in the T cells infiltrating in periodontal tissues may not be proportional to the FoxP3 mRNA. This result, however, contradicts studies of rheumatoid arthritis in which the prevalence of CD25+CD4+ T cells in the total CD4+ T cells of patients' peripheral blood and sites of inflammation was shown to be less than 10%, respectively [18]. Therefore, in the present study, we focus on investigating the expression pattern of FoxP3 protein by the T cells infiltrating in periodontal diseased tissue. Our step-by-step experimental methodology is described below.
Materials and methods
Human gingival tissue samples
For the in situ analysis of FoxP3 expression, healthy and diseased gingival tissue samples were collected in laboratories of the Department of Periodontology at the Harvard School of Dental Medicine (HSDM, Boston, MA). Healthy gingival tissue was characterized by the lack of bleeding on probing (gingival pocket depth ≤ 3 mm; n = 4, 1 male and 3 females, ages 25–51 y). Inflamed gingival tissues were collected from patients with periodontal disease at surgery for non-responsive sites after basic periodontal therapy. These samples were characterized by the presence of radiological bone resorption features and bleeding on probing (gingival pocket depth > 5 mm; n = 4, 2 males and 2 females, ages 36–55 y).
Tissue samples were also collected in the Department of Periodontology at HSDM in order to examine the cytokine expression in the gingival tissue homogenates. In this case, healthy gingival tissues were sampled, after cosmetic or preventive surgery, for non-responsive sites (gingival pocket depth ≤ 3 mm, age 27–41 y old, n = 3, one female and 2 males). Inflamed gingival tissues from patients with periodontal disease were sampled at surgery for periodontal therapy as described above (gingival pocket depth > 3 mm, average 5.7 ± 1.3 mm SD, age 32–58 y old, n = 11, 8 females and 3 males). The Institutional Review Board of HSDM approved the collection of gingival tissues after periodontal surgery, and informed consent was obtained from each subject prior to inclusion in this study.
Double-color confocal microscopy
The presence of FoxP3 expression in the human gingival tissues was determined using double-color confocal microscopy (DMRXE/TCS/SP-2 laser scan confocal microscope, Leica, Wetzler, Germany), following the previously published protocol [15]. FITC-conjugated anti-human FoxP3 antibody (clone 259D, BioLegend, San Diego, CA) and Alexa Fluor 647-conjugated anti-human CD25 antibody (clone BC96, BioLegend), with appropriate control antibodies, were reacted with frozen sections of healthy (n = 4) and diseased (n = 4) human gingival tissues. Staining pattern of FoxP3 in the healthy gingival tissues, as well as periodontal diseased lesions with RANKL+ lymphocytes, was evaluated by staining with FITC-conjugated anti-human FoxP3 antibody and with osteoprotegerin-Fc-biotin (OPG-Fc-biotin), followed by TexasRed-avidin [15]. OPG-Fc-biotin was prepared by reacting sulfo-NHS-biotin (Pierce, Rockford, IL) to OPG-Fc (gift from Dr. Dunston, Amgen), following the previously published procedure [15]. The number of FoxP3, CD25 and RANKL positive cells in the microscopic field (×1000) were counted, following the method published previously [15].
Measurement of sRANKL, IL-10 and IL-1β in gingival tissue homogenates using ELISA
Gingival tissue homogenates were prepared following the previously published method [15]. The concentrations of IL-1β and IL-10 in the homogenates were measured using DuoSet ELISA kit (R&D Systems, Minneapolis, MN). Detection of soluble RANKL (sRANKL) was carried out using ELISA kit (PeproTech, Rocky Hill, NJ).
In vitro stimulation of peripheral blood mononuclear cells (PBMC)
Peripheral blood mononuclear cells (PBMC) were isolated from healthy subjects (n = 2), and one patient with periodontal disease, by a gradient centrifugation using Histopaque™ (Sigma, St. Louis, MO) [15]. The resulting single-cell suspension of PBMC was stimulated in the RPMI 1640 medium supplemented with 10% FBS and antibiotics in a 96-well culture plate (105/well) for 4 days. The PBMC were stimulated with Aa Y4 (107/ml) in the presence or absence of anti-HLA-DR MAb (clone G46–6, BD PharMingen, San Diego, CA), control isotype matched MAb (PF18 [19]), or recombinant IL-10 (50 ng/ml). On Day 3, culture supernatant was harvested and subjected to human sRANKL ELISA (PeproTech). 3H thymidine (0.5 uCi) was added to the culture during the last 16 hours of a total incubation of 4 days.
For the confocal microscopy analysis, PBMC harvested on Day 3 were fixed on a glass slide using Cytospin (Shandon Elliott, Surrey, England) and stained with FITC-conjugated-anti-human FoxP3 antibody, Alexa Fluor 647-conjugated-anti-human CD25 antibody, Alexa Fluor 647-conjugated-anti-human CD4 and OPG-Fc-biotin/TexasRed-conjugated-avidin.
Results
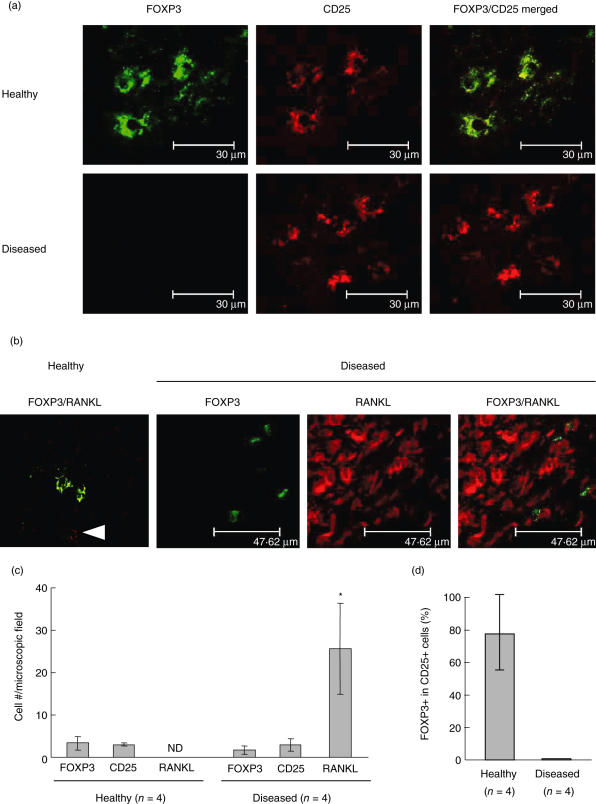
First, the expression pattern of FoxP3 protein in the gingival tissues with periodontal disease, as well as healthy gingival tissues, was determined by fluorescein isothiocyanate (FITC)-conjugated anti-FoxP3 antibody using double-colour confocal microscopy (Fig. 1a). In the healthy gingival tissues, FoxP3/CD25 double-positive lymphocytes were found in the connective tissues beneath the sulcular epithelium (Fig. 1a, healthy). Very strikingly, however, while the diseased tissue showed the presence of either CD25 or FoxP3 single-positive cells (Fig. 1a,b), the CD25+ lymphocytes did not show a positive staining pattern for FoxP3. In the gingival tissues with periodontal disease, a few FoxP3+ cells were detected within the dense infiltration of RANKL+ lymphocytes (Fig. 1b). Nevertheless, the FoxP3+ cells in the diseased tissues displayed very little or no staining pattern for membrane RANKL (mRNAKL), considerably lessening the likelihood that FoxP3+ cells contribute to local bone resorption. The relative number of cells counted for FoxP3, CD25 and mRANKL in a microscopic field is shown in Fig. 1c (× 1000). Although the number of mRANKL+ lymphocytes was increased significantly in the diseased gingival tissues compared to healthy tissues, there was no significant difference in the number of CD25+ cells or FoxP3+ cells between diseased and healthy tissues. The percentage of FoxP3+ cells, based on the number of mRANKL+ lymphocytes in the diseased tissues, was 4·7 ± 5·6% (average ± s.d., n = 4), indicating that a very low rate of FoxP3+ lymphocytes is present in the diseased tissues. Very importantly, the percentage of CD25/FoxP3 double-positive cells in the total CD25+ cells was high (about 77%) in healthy gingival tissue, while no CD25/FoxP3 double-positive cells were detected in diseased gingival tissues (Fig. 1d).
Fig. 1.
Forkhead box P3 (FoxP3) expression in CD25-positive cells diminished in periodontal diseased gingival tissue where abundant nuclear factor-kB ligand (RANKL+) lymphocytes infiltrate. Healthy gingival tissues (gingival pocket depth < 3 mm; n = 4, one male and three females, ages 25–51 years) and inflamed gingival tissues (gingival pocket depth > 5 mm; n = 4, two males and two females, ages 36–55 years) were analyzed for the expression of FoxP3, CD25 and RANKL on the infiltrating lymphocytes in the tissues. (a) The presence of FoxP3 expression in the human gingival tissues was determined using double-colour confocal microscopy. Fluorescein isothiocyanate (FITC)-conjugated anti-human FoxP3 antibody (green) and Alexa Fluor 647-conjugated anti-human CD25 antibody (red) were reacted with frozen sections of human gingival tissues of healthy (n = 4) and diseased (n = 4) gingival tissues. Representative staining patterns of healthy and diseased gingival tissues are shown. (b) Staining pattern of FoxP3 in the healthy gingival tissues and periodontal diseased lesions with RANKL+ lymphocytes was evaluated by staining with FITC-conjugated anti-human FoxP3 antibody (green) and with osteoprotegerin (OPG)-Fc-biotin, followed by TexasRed–avidin (red). The stained tissues were also analysed by the confocal microscope. In the healthy gingival tissue sample, an arrow indicates RANKL-positive cells. (c) The number of FoxP3, CD25 and RANKL-positive cells in the microscopic field (× 1000) were counted. Data from healthy and diseased gingival tissues (n = 4 and n = 4, respectively) are expressed as average cell number ± s.d. per microscopic field (n.d., not detectable). *Significantly higher than the number of FoxP3 or CD25-positive cells in either healthy or diseased tissues by Student's t-test (P < 0·05). (d) The number of FoxP3/CD25 double-positive cells and total CD25 single-positive cells were counted in each microscopic field. The percentage of FoxP3-positive cells in CD25+ cells is calculated and shown as an average percentage ± s.d.
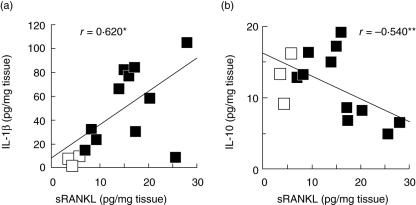
Secondly, we employed enzyme-linked immunosorbent assay (ELISA) (Fig. 2) to examine cytokines present in the gingival homogenates, including sRANKL, IL-1β and IL-10, to determine whether the Treg-associated cytokine, IL-10, is expressed in periodontal diseased tissue in relation to sRANKL. The concentrations of IL-10 and sRANKL in the gingival tissues showed negative correlation (Fig. 2b), whereas a positive correlation was detected between IL-1β and sRANKL (Fig. 2a). Interestingly, there were no significant differences in IL-10 concentrations between healthy and diseased tissues (healthy, 13·1 ± 3·6* pg/mg tissue; diseased, 11·2 ± 4·9* pg/ml tissue, *average ± s.d.). Therefore, based on reports that CD4+ T cells with regulatory phenotype CD25 produce IL-10 mRNA in diseased gingival tissues [2], the FoxP3+ cells found in healthy and diseased gingival tissues (Fig. 1) appeared to be at least one of the cellular sources of IL-10 in gingival tissues.
Fig. 2.
Measurement of soluble nuclear factor-kB ligand (sRANKL), interleukin (IL-10) and IL-1β in gingival tissue homogenates. Gingival tissue homogenates were prepared from inflamed gingival tissues from patients with periodontal disease (gingival pocket depth > 3 mm, average 5·7 ± 1·3 s.d. mm, age 32–58 years, n = 11, eight females and three males) and healthy gingival tissues (gingival pocket depth ≤ 3 mm, age 27–41 years, n = 3, one female and 2 males), following the procedure described in the Materials and Methods. Concentrations of IL-1β, IL-10 and sRANKL in the gingival tissue homogenates were measured using the DuoSet enzyme-linked immunosorbent assay (ELISA) kit. Open or closed symbols indicate the data from healthy or diseased gingival tissue homogenates, respectively. (a) Concentrations of IL-1β and sRANKL and (b) concentrations of IL-10 and sRANKL are shown in scatter diagrams. *Positive correlation between two parameters (n = 14, P < 0·05); **negative correlation between two parameters (n = 14, P < 0·05).
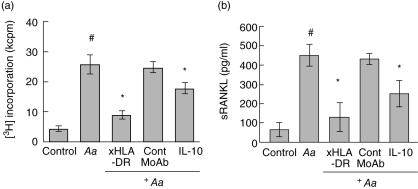
Next, in order to interpret the phenomenon of negative correlation found between IL-10 and sRANKL in the above-described human gingival tissue homogenates (Fig. 2), healthy human peripheral blood mononuclear cells (PBMC) were stimulated with the periodontal bacterium Aa in the presence or absence of IL-10. Our mouse and rat models of periodontal disease demonstrate the relevance of antigen-specific T cell activation in the context of RANKL-mediated periodontal bone loss. Therefore, an antigen-specific T cell activation assay was employed. Proliferative response of PBMC upon Aa stimulation was determined using a [3H]-thymidine incorporation assay (Fig. 3a). Aa-mediated PBMC activation was inhibited by anti-HLA-DR antibody, indicating that Aa-mediated PBMC proliferation is derived from antigen-dependent T cell proliferation via Aa-antigen presentation from human leucocyte antigen D-related (HLA-DR+) professional antigen-presenting cells (APC). The healthy subject also possessed positive serum IgG reaction to Aa as determined by ELISA (not shown), which provides evidence that this healthy subject possesses both T and B cell-mediated adaptive immune responses to Aa in an antigen-dependent manner. The stimulation of PBMC with Aa also up-regulated sRANKL expression by PBMC (Fig. 3b). Very importantly, the addition of IL-10 suppressed both proliferation and production of sRANKL by Aa-activated PBMC, indicating that a negative correlation between IL-10 and sRANKL in gingival tissue homogenates (Fig. 2a) may result from the suppression function of IL-10, as it acts to reduce sRANKL production by activated T cells. It is noteworthy that PBMC stimulation with immobilized anti-CD3 monoclonal antibody (MoAb) (UCTH-1) and anti-CD28 MoAb (clone CD28·2) also showed results similar to those of Aa stimulation (not shown) [15]. Furthermore, PBMC isolated from a patient with periodontal disease, who possessed elevated serum IgG response to Porphyromonas intermedia (Pi), also showed a soluble RANKL expression pattern similar to that of the healthy subject, as this patient's PBMC was stimulated with Pi in the presence or absence of IL-10 (not shown). To summarize, these results all demonstrated that IL-10 can suppress RANKL expression induced in T cells in an antigen-dependent manner, indicating that IL-10 present in the gingival tissues may be engaged in the suppression of T cell activation and production of RANKL by the activated T cells.
Fig. 3.
IL-10 suppressed in vitro soluble nuclear factor – KB ligand (sRANKL) expression by activated peripheral blood mononuclear cells (PBMC). In vitro stimulation of peripheral blood mononuclear cells (PBMC) induced predominant nuclear factor-kB ligand (RANKL) expression in forkhead box P3 (FoxP3)-negative cells, but little or no expression in FoxP3-positive cells. PBMC isolated from a healthy subject were stimulated with Aa Y4 (107/ml) in the presence or absence of anti-human leucocyte antigen D-related (HLA-DR) monoclonal antibody (MoAb) (clone G46-6), control isotype-matched MoAb (PF18), or recombinant interleukin (IL)-10 (50 ng/ml). On day 3, culture supernatant was harvested and subjected to human sRANKL enzyme-linked immunosorbent assay (ELISA). [3H]-thymidine (0·5 µCi) was added to the culture during the last 16 h of a total incubation of 4 days. The data from a healthy subject were expressed as average ± s.d. of (a) [3H]-thymidine incorporation (kcpm = 1000 c.p.m) or (b) secreted sRANKL concentration (pg/ml), respectively. PBMC isolated from another healthy subject and a patient with periodontal disease showed similar expression patterns of sRANKL in response to bacterial stimulation in the presence of IL-10 (not shown). *Significantly lower than Aa stimulation alone (#) by Student's t-test (P < 0·05).
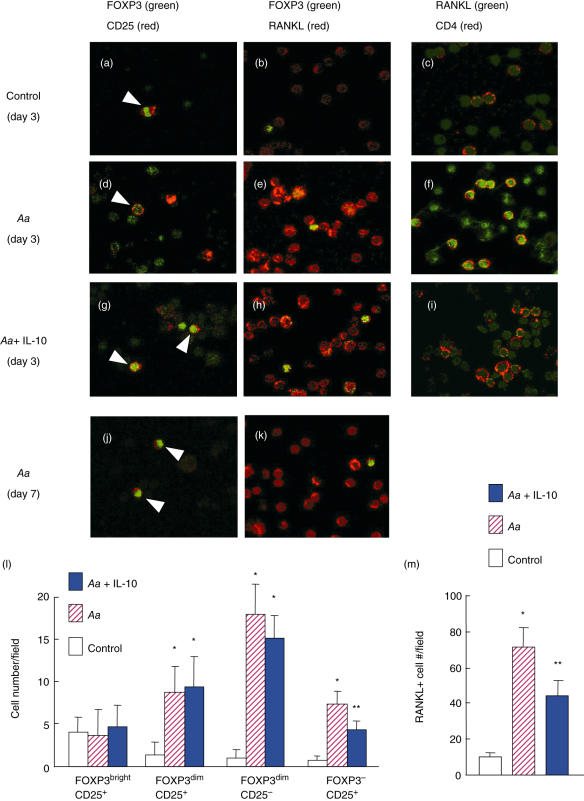
Finally, the influence of IL-10 on RANKL expression by PBMC in relation to FoxP3 expression was examined using PBMC isolated from the same healthy subject shown in Fig. 3. After 3 days of in vitro stimulation with Aa, the PBMC were fixed onto a glass slide and expressions of CD25, FoxP3 and mRANKL were determined using double-colour confocal microscopy. The stimulation with Aa, irrespective of the addition of IL-10, increased the number of CD25+ cells and FoxP3+ cells, respectively, whereas the number of CD25/FoxP3 double-positive cells remained similar to control non-stimulated PBMC (Fig. 4a,d,g, indicated by arrows). It is noteworthy that two levels of FoxP3 expression, which we term ‘bright’ and ‘dim’, were observed and then differentiated as follows: the CD25/FoxP3 double-positive cells appeared to be bright in FoxP3 expression level (FoxP3bright), while the CD25–/FoxP3+ cells appeared to be dim in FoxP3 expression level (FoxP3dim).
Fig. 4.
In vitro stimulation of peripheral blood mononuclear cells (PBMC)-induced predominant nuclear factor-kB ligand (RANKL) expression in forkhead box P3 (FoxP3)-negative cells compared to FoxP3 positive cells. PBMC isolated from healthy subjects were stimulated with formalin-fixed Actinobacillus actinomycetemcomitans (Aa) Y4 (107/ml) in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics in the presence or absence of human recombinant interleukin (IL)-10. Three days after incubation, the cells were fixed and stained with fluorescein isothiocyanate (FITC)-conjugated anti-human FoxP3 antibody, Alexa Fluor 647-conjugated anti-human CD25 antibody, Alexa Fluor 647-conjugated anti-human CD4 and osteoprotegerin (OPG)-Fc-biotin/TexasRed-conjugated avidin. The staining pattern for each molecule was analysed using the double-colour confocal microscope. Representative staining patterns of merged images between green and red colours are shown at ×640 magnification. The arrows in (a), (d) and (g) indicate the FoxP3/CD25 double-positive cells. All three samples, including two healthy subjects and one patient with periodontal disease, showed similar expression patterns of CD25, FoxP3 and RANKL after stimulation with Aa in the presence or absence of IL-10. (a–i), PBMC of a healthy subject stimulated for 3 days; (j, k) PBMC of a healthy subject stimulated with Aa for 7 days. The numbers of cells positive for respective molecules were counted in a microscopic field containing a total of 100 PBMC cells (l, FoxP3 and/or CD25; mM, RANKL). *Significantly higher than non-stimulation control by Student's t-test (P < 0·05); **significantly lower than Aa stimulation alone by Student's t-test (P < 0·05).
Aa stimulation also up-regulated mRANKL expression by PBMC (Fig. 4e) compared to control (Fig. 4b). The data enumerated for CD25, FoxP3 and mRANKL expression by in vitro-stimulated PBMC are shown in Fig. 4l,m. In contrast to the stable number of FoxP3bright/CD25+ cells, regardless of stimulations, the increase in cell numbers for the other three types of cells, FoxP3dim/CD25+, FoxP3dim/CD25– and FoxP3–/CD25+, was significant upon stimulation with Aa (Fig. 4m). Of particular importance, the increased number of FoxP3dim/CD25+ and FoxP3–/CD25+ cells seemed to be due to the transient inflammatory stress caused by mitogenic components present in Aa. This finding is confirmed by the observation that these same expression patterns were no longer found in the Aa-activated PBMC on day 7 (Fig. 4j,k). This phenomenon is supported by the study of Gorska et al., who reported that the frequency of CD25+ cells in the PBMC decreases significantly after proper periodontal surgery [20], which eliminates the generic inflammation in periodontal tissues.
The FoxP3bright cells demonstrated little or no mRANKL expression, whereas FoxP3dim cells expressed mRANKL to a relatively strong degree (Fig. 4b,e,h). Although FoxP3dim cells disappeared on day 7 in Aa-stimulated PBMC, FoxP3bright cells, as well as mRANKL+ cells, remained at the same levels of frequency and intensity as determined on day 3 (Fig. 4j,k). However, the addition of IL-10 suppressed such Aa-mediated mRANKL expression by PBMC on day 3 (Fig. 4h,i,m) and on day 7 (not shown). The increased expression of mRANKL was observed in the CD4+ T cells (Fig. 4f). These results indicate that, upon activation of PBMC with Aa, FoxP3dim expression could be induced transiently in T cells. mRANKL expression was expressed prominently in FoxP3 negative cells and in FoxP3dim cells, but not in FoxP3bright cells, which were most probably represented by the presence of CD25/FoxP3 double-positive cells. It must be emphasized that CD25/FoxP3 double-positive cells are considered to function as Treg cells [1]. As such, the production of Treg-associated cytokine IL-10 seems to suppress RANKL expression by T cells, which indicates that Treg cells can down-regulate RANKL expression by activated T cells, as long as Treg cells produce IL-10 in the periodontal tissues, as reported previously [17].
Discussion
Overall, the present study demonstrated the following novel findings about Treg cells in the context of periodontal disease:
FoxP3/CD25 double-positive cells are present in healthy gingival tissues, whereas FoxP3 or CD25 single-positive cells, but not FoxP3/CD25 double-positive cells, are found in diseased gingival tissues.
The percentage of FoxP3+ cells is as low as 5% within the otherwise massive infiltration of RANKL+ lymphocytes found in the diseased gingival tissues.
A negative correlation between concentrations of sRANKL and IL-10 in the gingival tissue homogenates has been demonstrated.
In the peripheral blood lymphocytes stimulated with bacteria (Aa) in an antigen-dependent fashion, mRANKL expression is expressed prominently in FoxP3 negative cells and in FoxP3dim cells, but not in FoxP3bright cells, which most probably represents the presence of CD25/FoxP3 double-positive cells.
Treg-associated cytokine IL-10 can suppress RANKL expression by PBMC stimulated in an antigen-specific manner.
Several examples of these finding can be found in the literature. Recurrent aphthous ulcerations (RAU) is a chronic inflammatory disease with evidence of inappropriate immune response towards microorganisms found in the oral cavity of patients with RAU [21–23]. Peripheral blood of these patients is reported to show a decreased number of CD4+CD25+high Treg cells along with the predominance of type 1 cytokine production by cultured PBMC [24]. Another example is found in systemic lupus erythematosus, where a diminished number of Treg cells is also implicated [25]. These lines of evidence suggest that a diminished Treg cell count may be attributed to ‘out-of-control’ immune responses in certain diseases.
The current study found that an amount equal to only 5% of FoxP3+ cells is present in the RANKL+ lymphocytes infiltrating diseased gingival tissues. This finding, however, contradicts the report by Ito et al. [17], where they demonstrate that a majority of CD4 T clone cells (nearly 100%) established from T cells infiltrating periodontal diseased tissues is positive for FoxP3 and IL-10 mRNA. None the less, it is reported that almost all human CD25–CD4+ T cells express FoxP3 upon activation, peaking at 72 h, whereas in mice only CD25+CD4+ Treg cells, but not CD25–CD4+ T cells, express FoxP3, irrespective of activation [26,27]. Furthermore, other studies have demonstrated that most human CD4+ clone cells are FoxP3-positive [28]. The FoxP3dim cells found in Aa-stimulated PBMC, which were also RANKL+ on day 3 (Fig. 4e,h), may represent those FoxP3+ cells that can be induced in an activation-dependent manner. It is significant that all FoxP3dim expression in Aa-stimulated PBMC diminished dramatically, whereas RANKL expression remained positive in those cells (Fig. 4k). Therefore it is conceivable that, in the study by Ito et al., T cell stimulation with CD3 and IL-2 may have induced FoxP3 mRNA in an activation-dependent manner, which then resulted in an extraordinarily high percentage of FoxP3+ T cell clones. In terms of the fluorescent-immunohistochemistry results for the gingival tissue sections shown in Fig. 1, the FoxP3-positive cells found in the diseased tissue appeared to be FoxP3dim cells, which are activated in a transitory manner under inflammatory conditions, whereas the FoxP3/CD25 double-positive cells found in healthy gingival tissues appeared to be FoxP3bright cells (Fig. 1b).
It is also reported that systemic administration of IL-10 can suppress periodontal bone resorption induced by adoptive transfer of RANKL expressing human T cells into non-obese diabetic (NOD)/SCID-mice [29]. In this SCID mouse model, although it is unclear if IL-10 reduces the amount of RANKL expression from the adoptively transferred human T cells, systemic IL-10 administration clearly suppressed the frequency of Th1-type human T cells [interferon (IFN)-γ producers] in the NOD/SCID-mice. We also previously demonstrated in the mouse periodontal disease model with P. gingivalis infection that periodontal bone loss is increased in the IL-10 gene knock out mice compared to the wild-type mice [30]. Findings in the present study have demonstrated (1) the negative correlation between IL-10 and sRANKL concentrations in the human gingival tissues and (2) the suppression function of IL-10 on RANKL expression and proliferation by activated PBMC cells. These findings are therefore consistent with the results of the two mouse models noted above, which show the suppression role of IL-10 in periodontal bone loss induced by RANKL production by activated T cells.
In addition to the suppression role of IL-10, it is noteworthy that TGF-β also plays an important role in the immune suppression function of Treg cells. As TGF-β mRNA appears to be expressed in the Treg cells present in the gingival tissue [2], the possible engagement of TGF-β in RANKL-mediated periodontal disease is yet to be elucidated. In particular, TGF-β is reported to increase osteoprotegerin (OPG) expression by bone marrow stromal cells [31]. Therefore, it is very plausible that Treg cells play a suppressive regulatory role in the inflammatory bone resorption lesion of periodontal disease, while elaboration of such a hypothesis in both in vitro and in vivo studies is still required to elucidate the relevance of Treg cells in the context of periodontal bone resorption.
Kopitar et al. [32] found that commensal oral bacteria antigens prepared from Bacteroides fragilis, Streptococcus mitis and Propionibacterium acnes prime human dendritic APC to induce Th1, Th2 and Treg differentiation, respectively, indicating that antigenic properties of bacteria can modulate the APC instruction mechanism on Treg as well as adaptive T cells (Th1 and Th2) [32]. Therefore, the composition of bacterial flora in the periodontal crevice may affect the induction of Treg cells in the periodontal tissue. None the less, the present study demonstrated clearly that FoxP3/CD25 double-positive cells diminish in the bone resorption lesion of periodontal disease where abundant RANKL+ lymphocytes infiltrate. It is speculated that chemoattractant factors that recruit FoxP3/CD25 double-positive Treg cells are down-regulated, or antagonized, in the diseased gingival tissue and that this results in the decrease of FoxP3/CD25 double-positive Treg cells in the bone resorption lesion of periodontal disease.
In addition to Treg-associated IL-10 cytokine production, it is remarkable that IL-10 is also produced by Th2-type cells. Although it is considered a controversial finding in the literature, both Th1 and Th2 cell types are found in periodontally diseased tissues [33,34]. Interestingly, our preliminary in vitro studies demonstrated that another Th2 cytokine, IL-4, can up-regulate the sRANKL production by CD3/CD28-stimulated naive T cells isolated from both humans and mice (unpublished data). Therefore, it is plausible that IL-4 produced by Th2 type T cells may antagonize the suppression of RANKL-expression by IL-10. Consequently, the cellular source and expression pattern of IL-10 and IL-4 in periodontal lesions may play a key role in the regulation of RANKL production by the T effector cells.
In summary, the present study demonstrated that diminished FoxP3/CD25 double-positive Treg cells, in the context of periodontal diseased tissue, is associated with pathogenic RANKL expression by activated lymphocytes. Possible production of immune suppressive cytokine IL-10 by Treg cells also appeared to play a pivotal role in the regulation of such RANKL expression.
Acknowledgments
We thank Dr Colin Dunstan for OPG-Fc (Amgen Inc, Thousand Oak, CA, USA) and Dr Takashi Yanaoka and Mr Tadahiko Kounoike for their support of this study. This work was supported by grants DE-03420, DE-09018 and DE-14551 from the National Institute of Dental and Craniofacial Research.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima T, Ueki-Maruyama K, Oda T, et al. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res. 2005;84:639–43. doi: 10.1177/154405910508400711. [DOI] [PubMed] [Google Scholar]
- 3.Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–50. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 4.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 5.Valverde P, Kawai T, Taubman MA. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Miner Res. 2004;19:155–64. doi: 10.1359/JBMR.0301213. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–9. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 7.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 8.Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005;208:181–93. doi: 10.1111/j.0105-2896.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 9.Mackler BF, Frostad KB, Robertson PB, Levy BM. Immunoglobulin bearing lymphocytes and plasma cells in human periodontal disease. J Periodontal Res. 1977;12:37–45. doi: 10.1111/j.1600-0765.1977.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 10.Seymour GJ, Greenspan JS. The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease. J Periodont Res. 1979;14:39–46. doi: 10.1111/j.1600-0765.1979.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 11.Taubman MA, Stoufi ED, Ebersole JL, Smith DJ. Phenotypic studies of cells from periodontal disease tissues. J Periodont Res. 1984;19:587–90. doi: 10.1111/j.1600-0765.1984.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 12.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365–74. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malberg K, Molle A, Streuer D, Gangler P. Determination of lymphocyte populations and subpopulations extracted from chronically inflamed human periodontal tissues. J Clin Periodontol. 1992;19:155–8. doi: 10.1111/j.1600-051x.1992.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 14.Seymour GJ, Powell RN, Davies WI. The immunopathogenesis of progressive chronic inflammatory periodontal disease. J Oral Pathol. 1979;8:249–65. doi: 10.1111/j.1600-0714.1979.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Matsuyama T, Hosokawa Y, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–98. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006;176:625–31. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Honda T, Domon H, et al. Gene expression analysis of the CD4+ T-cell clones derived from gingival tissues of periodontitis patients. Oral Microbiol Immunol. 2005;20:382–6. doi: 10.1111/j.1399-302X.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 18.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai T, Ito H, Sakado N, Okada H. A novel approach for detecting an immunodominant antigen of Porphyromonas gingivalis in diagnosis of adult periodontitis. Clin Diagn Lab Immun. 1998;5:11–17. doi: 10.1128/cdli.5.1.11-17.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorska R, Laskus-Perendyk A, Gregorek H, Kowalski J. The influence of surgical treatment of periodontal disease on selected lymphocyte subpopulations important for cellular and humoral immune responses. J Periodontol. 2005;76:1304–10. doi: 10.1902/jop.2005.76.8.1304. [DOI] [PubMed] [Google Scholar]
- 21.Hasan A, Childerstone A, Pervin K, et al. Recognition of a unique peptide epitope of the mycobacterial and human heat shock protein 65–60 antigen by T cells of patients with recurrent oral ulcers. Clin Exp Immunol. 1995;99:392–7. doi: 10.1111/j.1365-2249.1995.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan A, Shinnick T, Mizushima Y, van der Zee R, Lehner T. Defining a T-cell epitope within HSP 65 in recurrent aphthous stomatitis. Clin Exp Immunol. 2002;128:318–25. doi: 10.1046/j.1365-2249.2002.01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun A, Chia JS, Chiang CP. Increased proliferative response of peripheral blood mononuclear cells and T cells to Streptococcus mutans and glucosyltransferase D antigens in the exacerbation stage of recurrent aphthous ulcerations. J Formos Med Assoc. 2002;101:560–6. [PubMed] [Google Scholar]
- 24.Lewkowicz N, Lewkowicz P, Banasik M, Kurnatowska A, Tchorzewski H. Predominance of type 1 cytokines and decreased number of CD4(+) CD25(+high) T regulatory cells in peripheral blood of patients with recurrent aphthous ulcerations. Immunol Lett. 2005;99:57–62. doi: 10.1016/j.imlet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 26.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 28.Morgan ME, van Bilsen JH, Bakker AM, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Teng YT. Interleukin-10 inhibits gram-negative-microbe-specific human receptor activator of NF-kappaB ligand-positive CD4+-Th1-cell-associated alveolar bone loss in vivo. Infect Immun. 2006;74:4927–31. doi: 10.1128/IAI.00491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodont Res. 2004;39:432–41. doi: 10.1111/j.1600-0765.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 31.Takai H, Kanematsu M, Yano K, et al. Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem. 1998;273:27091–6. doi: 10.1074/jbc.273.42.27091. [DOI] [PubMed] [Google Scholar]
- 32.Kopitar AN, Ihan Hren N, Ihan A. Commensal oral bacteria antigens prime human dendritic cells to induce Th1, Th2 or Treg differentiation. Oral Microbiol Immunol. 2006;21:1–5. doi: 10.1111/j.1399-302X.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 33.Salvi GE, Brown CE, Fujihashi K, et al. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J Periodont Res. 1998;33:212–25. doi: 10.1111/j.1600-0765.1998.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 34.Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profile of T lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79:1548–55. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]