Abstract
Coeliac disease (CD) is an immune-mediated enteropathy triggered by ingestion of wheat gluten and related cereals in genetically predisposed individuals. Circulating immunoglobulin A (IgA) class autoantibodies against tissue transglutaminase (IgA–TGA) are highly specific and sensitive serological markers for CD, which is ultimately confirmed by duodenal biopsy. Although CD is considered a life-long disorder, transient or fluctuating IgA–TGA seropositivity has been observed in asymptomatic individuals on a gluten-containing diet. We set out to explore possible differences in the maturation of IgA–TGA avidity between individuals progressing to CD and subjects remaining healthy despite occasional expression of autoantibodies. We developed a time-resolved fluorometric IgA–TGA assay based on human recombinant tissue transglutaminase (tTG), and further modified the method to also measure urea-dependent avidity of the autoantibodies. We measured the autoantibody titres and avidities of sequential serum samples from 10 children developing CD and 10 children presenting transient or fluctuating autoantibodies. Both the initial titres at seroconversion and peak values of transient/fluctuating IgA–TGA were significantly lower than corresponding autoantibody titres in samples drawn from individuals with progressing CD (P = 0·004 and P = 0·0002, respectively). However, there were no statistically significant differences in the initial or peak avidity index values between the two groups of children. The avidity index values increased during the follow-up period (P = 0·013 for both groups) with no significant difference in the rate of avidity maturation between children with transient/fluctuating IgA–TGA and children developing CD. According to our results, high autoantibody titres have a higher predictive value than avidity maturation of TGA-IgA in screening for CD.
Keywords: autoantibody, avidity, coeliac disease, tissue transglutaminase, transient IgA–TGA
Introduction
Coeliac disease (CD) is an inflammatory intestinal disorder triggered by dietary gliadin from wheat and similar prolamin proteins from related cereals. The disease is characterized by intestinal villous atrophy with a lymphocytic infiltrate and crypt hyperplasia resulting in impaired absorptive function [1]. Classical manifestations include diarrhoea, abdominal pain, weight loss and failure to thrive, but an increasing number of diagnosed CD patients exhibit predominantly extra-intestinal symptoms, i.e. osteoporosis, iron deficiency anaemia, vitamin deficiencies, delayed puberty and infertility [2,3]. In addition, asymptomatic individuals with villous atrophy on duodenal biopsy are detected occasionally via screening of high-risk individuals or by conducting biopsy for other reasons. As a rule, CD patients experience clinical and histological recovery after excluding gluten from their diet.
The prevalence of CD in western countries is estimated to be in the range of 0·5–1·0%, including both symptomatic and asymptomatic individuals [4–6]. However, first-degree relatives of biopsy-proven CD patients as well as people with selective immunoglobulin A (IgA)-deficiency, type 1 diabetes and other autoimmune disorders have an up to 10-fold increased prevalence of the disease [7–10]. Certain human leucocyte antigen (HLA)-alleles confer genetic susceptibility, with the HLA-DQ2 molecule being carried by the majority of CD patients and HLA-DQ8 by most of the remainder [11]. Because approximately 25–30% of the general population express the DQ2 molecule and only a small fraction of those individuals develop CD, additional factors, either genetic or environmental (other than gluten), are needed for disease precipitation.
Serological screening of CD is performed routinely by measuring circulating disease-associated antibodies, particularly autoantibodies against endomysium (EMA) and tissue transglutaminase (TGA). In fact, tissue transglutaminase (tTG) has been identified as the autoantigen for EMA [12], a discovery that has resulted in the development of sensitive and specific enzyme-linked immunosorbent (ELISA) assays using recombinant human tTG [13–16]. IgA class TGA have a higher positive predictive value for CD than have IgG autoantibodies [17]; nevertheless, detection of elevated IgG EMA and TGA is useful as a screening tool in patients with selective IgA deficiency [18–20]. Although the existence of a transient form of CD has been a matter of debate (for reviews, see Chartrand and Seidman [21] and Schmitz [22]), most observers now agree that CD is a permanent condition of gluten intolerance. Thus the clinical significance of transient or fluctuating IgA tTG autoantibodies while on a gluten-containing diet, described by Liu et al. [23], Mäki et al. [6] and Simell et al. [24] remains obscure.
Avidity assays are used widely for discrimination between the acute and chronic phases of microbial infections, and there are also implications of affinity maturation of antibodies associated with autoimmune diseases, i.e. systematic lupus erythematosus (SLE) [25] and type 1 diabetes [26,27]. In type 1 diabetes, affinity maturation seems to be dependent on the antigen specificity of the autoantibodies [28]. Also in CD, there is evidence of avidity maturation of disease-associated antibodies, as Saalman et al. [29] showed that IgG antibodies to gliadin (IgG–AGA) and β-lactoglobulin in children with untreated CD were of higher avidity than corresponding antibodies from healthy children. In addition, Vainio et al. [30] have reported significantly increased amounts of high-avidity IgA gliadin antibodies (IgA–AGA) during prolonged experience of the CD-associated condition dermatitis herpetiformis.
To our knowledge, this is the first report comparing the avidity maturation of IgA–TGA in samples from individuals progressing to CD and in autoantibody-positive subjects without overt disease. Our aim was to investigate whether knowledge of autoantibody avidity would add to the understanding of the clinical relevance of transient and/or fluctuating IgA–TGA positivity. As time-resolved fluorometric assays have proven highly sensitive and reliable in detection of autoantibodies associated with type 1 diabetes [31,32] and, in addition, lend themselves for antibody avidity determination [28], we established fluorometric IgA–TGA concentration and avidity assays and then compared the autoantibody levels and avidities in children developing CD with those in healthy individuals presenting transient or fluctuating autoantibodies.
Subjects and methods
Subjects
For the optimization of the IgA–TGA antibody and avidity fluorometric assays, we used 10 IgA–TGA positive and 10 negative clinical serum samples from patients suspected of CD. The cut-off level of the IgA–TGA assay was determined by analysing 100 serum samples from healthy young adults. After establishing the assays, we analysed a panel of 259 samples from 20 children participating in the Type 1 Diabetes Prediction and Prevention project (DIPP) [33]. The ethics committees of the participating university hospitals approved the study. DIPP-children carrying HLA-alleles particularly predisposing to CD were recruited to a study aimed at determining the natural history of CD-associated antibodies before the diagnosis of CD [24]. TGA was measured in serum samples taken between years 2000–03 using a recombinant human TGA kit (Celikey™; Pharmacia Diagnostics, Freiburg, Germany) with values 5–8 U/ml regarded as equivocal and > 8 U/ml as positive, as indicated by the manufacturer. If a sample was equivocal or positive for TGA, all samples collected since birth and also all forthcoming samples were analysed for TGA, IgA–anti-gliadin antibodies (AGA), IgG–AGA, EMA and anti-reticulin antibodies (ARA). For children with positive TGA value, upper gastrointestinal videoendoscopy with duodenal biopsies under anaesthesia was recommended. The development of CD-associated antibodies and disease in the children selected for the current study is presented in Table 1.
Table 1.
Age at seroconversion and diagnosis of coeliac disease (CD) in the children monitored for anti-tissue transglutaminase (tTG) immunoglobulin A (IgA) avidity.
| Age (years) at seroconversion to | |||||||
|---|---|---|---|---|---|---|---|
| Child no. | Age (years) at gastroscopy | Status | IgA-EMA+ | IgA-ARA+ | IgA-AGA+ | IgG-AGA+ | IgA-tTG+ |
| 3804 | 2·5b | 2·5 | 3·0a | 2·5a | 2·5 | 6·0 | CD |
| 8118 | 6·0 | 6·0 | 6·0 | 6·6 | 6·0 | 6·7 | CD |
| 11282 | 3·5 | 4·5 | 0·7b | 0·7 | 3·5 | 5·0 | CD |
| 13254 | 2·6 | 2·6 | 2·6b | 1·8 | 2·6 | 3·3 | CD |
| 13723 | 4·0 | 4·0 | 2·5b | 1·5 | 4·0 | 5·0/5·3 | n.s./CD |
| 14474 | 1·5 | 1·5 | 2·0 | 1·3 | 1·5 | 3·1 | CD |
| 15805 | 1·8 | 1·8b | 3·0b | 1·0 | 1·8 | 2·7/3·7 | n.s./CD |
| 18532 | 3·0 | 3·0 | 2·5 | 1·3 | 3·0 | 3·6 | CD |
| 19789 | 1·5 | 1·5 | 1·5 | 1·5 | 1·5 | 3·4 | CD |
| 20470 | 2·5 | 2·5 | 3·0 | 2·5 | 2·5 | 3·3 | CD |
| 132 | 6·0 | –c | 9·9 | –c | 6·5b | –d | n.d. |
| 7622 | 2·4b | –c | –c | –c | 3·5b | 5·0/6·1 | no CD |
| 7868 | 5·0a | 5·0a | –c | 5·0a | 5·0a | 5·8 | no CD |
| 11147 | 1·3a | 1·5a | –c | 1·0a | 1·3a | –d | n.d. |
| 11287 | 3·4b | 2·4b | –c | 3·4 | 3·4a | 4·0 | no CD |
| 14068 | 3·5 | –c | 6·3 | 5·6 | 4·0b | –d | n.d. |
| 14199 | 3·0a | –c | –c | –c | 3·5a | –d | n.d. |
| 17295 | 2·5b | 2·5b | –c | 1·5 | 2·5a | –d | n.d. |
| 22688 | 1·0a | 1·6a | –c | 1·0a | 1·0a | –d | n.d. |
| 24936 | 1·0b | 1·0a | 1·0a | 0·8a | 1·0a | –d | n.d. |
n.s.: Non-specific duodenal biopsy findings; n.d.; not determined.
Transient positivity
fluctuating positivity
no seroconversion
gastroscopy not performed. EMA: endomysium; ARA: anti-reticulin antibodies; AGA: antibodies to gliadin.
Of the children participating in our study, 10 (seven boys and three girls) were diagnosed with biopsy-proven CD and 10 children (five boys and five girls) had been TGA-positive with transient or fluctuating TGA values. Duodenal biopsies had been taken from three children in the latter group, with normal villous architecture. We analysed the IgA–TGA levels and avidities of series of serum samples collected from the study children since birth. In children who were diagnosed with CD, the average number of samples studied, with standard deviation (s.d.) and range in parenthesis, was 11·8 (±3·9; 7–19), and for the children with transient or fluctuating values, 14·1 (±4·0; 7–20). At the time of the diagnosis of CD, children were of a mean age of 4·4 (±1·3; 3·1–6·7) years. The last sample we analysed from the children with CD was taken at the following visit to the clinic after the diagnosis of CD. The samples had been drawn at a mean time of 5 (±1·5; 3·5–8·1) months after the duodenal biopsies had been taken. The children with transient or fluctuating TGA values were observed until the end of April, 2005. At the end of the follow-up time, the mean age of the children with transient or fluctuating TGA values was 6·3 (±2·1; 2·6–10) and of the children with CD 4·8 (±1·3; 3·5–7·1) years.
Biotinylation of tTG
Recombinant human tTG, expressed by baculovirus infection of Spodoptera frugiperda Sf9 insect cells, was purchased from Diarect AG, Freiburg, Germany. In the biotinylation reaction, which was carried out in 50 mM HEPES, 0·9% NaCl, pH 7·4, tTG was allowed to react with a 50-fold molar excess of biotinamidocaproic acid 3-sulpho-N-hydroxysuccinimide ester (Sigma-Aldrich, St Louis, MO, USA) for 4 h at room temperature. The biotinylated tTG (bio-tTG) was separated from unconjugated biotinylation reagent by gel filtration on a NAP-5 column (GE Healthcare Bio-Sciences, Uppsala, Sweden) using 50 mM HEPES, 0·9% NaCl and 0·05% NaN3, pH 7·4, as elution buffer.
Time-resolved fluorometric IgA–TGA assay
The reagents and equipment used in the IgA–TGA assay were from PerkinElmer Life and Analytical Sciences, Wallac Oy, Turku, Finland, unless stated otherwise. The assay was performed in streptavidin-coated 96-well microtitre plates (SA-plates), with all incubations performed at room temperature and all samples measured in duplicates. The mean is calculated automatically by the multicalc program and given in the graphs. Serum samples were normally diluted 1 : 1000 in Delfia assay buffer, but samples with IgA–TGA levels above the standard curve were diluted further in assay buffer and measured again. As a calibrator we used a human serum tTG IgA standard from Biofile Ltd (Turku, Finland), giving the results in arbitrary units (AU).
In the first step of the assay, 250 ng of bio-tTG in 120 µl Delfia assay buffer was added to each well and incubated for 45 min with slow shaking on a Delfia plate shake. After washing the wells once with Delfia wash solution in a Delfia plate wash, 100 µl diluted sample or calibrator was added to the wells and incubated for 45 min with slow shaking. The wells were washed twice, after which 100 µl of goat polyclonal anti-human IgA-Eu tracer (Biofile Ltd), diluted 1 : 25 in assay buffer, was added, and incubation continued for an additional 30 min. After four washing rounds, 150 µl of Delfia enhancement solution was added to the wells, and the plates were incubated for 15 min on a plate shake. The fluorescence was measured in a Delfia 1234 plate fluorometer, and the results were calculated with the multicalc computer program expressed as a mean of duplicate samples.
Competitive inhibition
Competitive inhibition studies were performed by adding increasing amounts of unlabelled recombinant human tTG (0–300 ng per well) in 10 µl of assay buffer to one IgA–TGA negative and five positive serum samples (range 20·3–8832 AU) in the second step of the fluorometric assay, in which autoantibodies are captured by bio-tTG. Otherwise the autoantibody assay was performed as described above.
IgA–TGA avidity assay
In the avidity assay, IgA tTG autoantibodies were first captured by bio-tTG bound to SA wells in a procedure identical to that of the quantitative fluorometric IgA–TGA assay. After two washing rounds, low-avidity autoantibodies were eluted by incubating the wells with 120 µl of assay buffer containing 5 M urea, pH 8·0, for 15 min on a plate shake. Parallel control wells were treated with assay buffer without urea for determination of the maximal fluorescence. After two washes, remaining IgA–TGA was detected as described for the autoantibody assay. The results were expressed as avidity indices (AI), i.e. the ratio fluorescence with urea/fluorescence without urea, in per cent.
Statistical analysis
The Mann–Whitney U-test was used for comparison of unpaired data, while the Wilcoxon signed-rank test was used for comparison of matched pairs. Correlation between the results obtained by immunofluorometric assay (IFMA) and enzyme-linked immunosorbent assay (ELISA) was calculated using Spearman's rank correlation. The analyses were performed using StatView® (SAS Institute, Inc., Cary, NC, USA).
Results
Performance characteristics of the fluorometric IgA–TGA assay
Cut-off value
On the basis of the 99th percentile of the assay performed by sera from 100 healthy university students, the cut-off limit was set at 13·0 AU.
Reproducibility
The variation studies were performed using four serum samples with IgA–TGA levels between 27 and 6530 AU. Interassay variation was studied by measuring the autoantibody levels in duplicates for five times on separate days, whereas intra-assay variation studies were performed by determining the IgA–TGA levels in duplicate at five random positions on a SA-plate. The reproducibility of the assay was excellent, with a mean intra-assay, interassay and total coefficient of variation (CV) of 2·5, 2·2 and 2·4%, respectively (Table 2).
Table 2.
Reproducibility of the fluorometric immunoglobulin A–tissue transglutaminase (IgA–TGA) assay.
| Coefficient of variation (CV) (%) | |||
|---|---|---|---|
| Coefficient of variation (CV) (%) | |||
| Mean IgA–TGA (AU) | Intra-assay | Interassay | Total |
| 27·3 | 2·4 | 3·1 | 3·0 |
| 102 | 3·2 | 2·3 | 2·6 |
| 709 | 1·5 | 1·1 | 1·6 |
| 6530 | 2·7 | 2·3 | 2·5 |
Linearity
We determined the linear measurement range of the fluorometric assay by analysing serial dilutions of a serum sample with a high IgA–TGA titre. The increase in signal was linear up to 1351 AU, and no high-dose hook effect was observed up to the highest measured concentration of 6394 AU.
Detection limit
The detection limit was defined as the mean signal of the zero calibrator + 2 s.d., and was calculated to be 0·33 AU on the basis of 27 separate assays performed during a period of 7 months.
Correlation between the fluorometric assay and ELISA
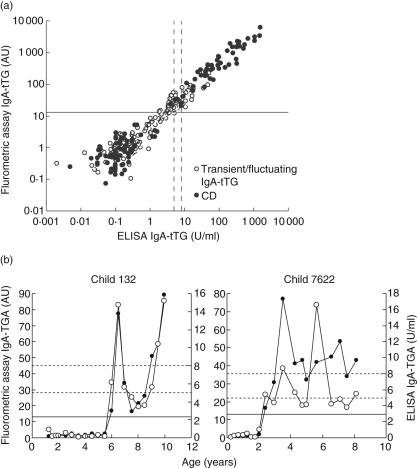
Scatter plot analysis of the IgA–TGA values obtained with the fluorometric assay and ELISA (Celikey™) showed a strong correlation between the methods (r = 0·93, Spearman's rank correlation) (Fig. 1a). There were, however, some discrepancies regarding the autoantibody positivity. All 67 samples that tested positive by the ELISA were also detected positive by the fluorometric assay, whereas altogether 22 samples of 179 with a negative result in the ELISA (one from a child diagnosed with CD) were found to be IgA–TGA positive by the fluorometric method. In addition, all 13 samples with equivocal ELISA IgA–TGA results (range 5–8 U), were autoantibody positive in the fluorometric assay. Due to the differently set cut-off levels of the assays, two children presenting fluctuating autoantibodies, as measured by ELISA, were continuously IgA–TGA positive from seroconversion onwards in the fluorometric assay (Fig. 1b).
Fig. 1.
(a,b) Correlation between the fluorometric assay and enzyme-linked immunosorbent assay (ELISA) (Celikey™). (a) The immunoglobulin A (IgA)–tissue transglutaminase (TGA) values of all the 259 follow-up samples analysed by the fluorometric assay were plotted against the corresponding ELISA results, R = 0·93. The cut-off level of the fluorometric assay is marked with a solid line, while the equivocal zone of the ELISA is indicated by dotted lines. (b) Two children showing fluctuating IgA–TGA in the ELISA (○) presented continuous autoantibodies from seroconversion onwards as measured in the fluorometric assay (•), cut-off levels marked as in (a).
Competitive inhibition
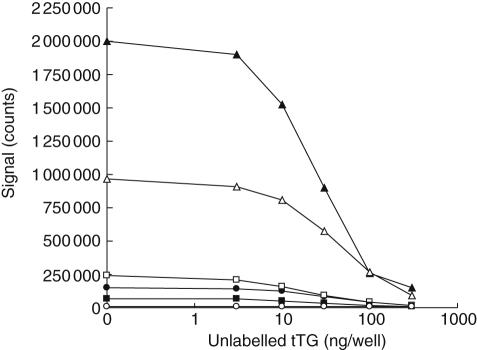
The specificity of the binding of IgA–TGA to the antigen-coated surface was studied by adding increasing amounts (0–300 ng per well) of unlabelled human recombinant tTG to one IgA–TGA negative and five positive serum samples in the autoantibody capturing step of the fluorometric assay. The signals of all IgA–TGA-positive samples decreased in a dose-dependent manner with 10% or less of the maximal signal remaining after addition of 300 ng of unlabelled tTG per well (Fig. 2), showing that the autoantibodies bind specifically to immobilized bio-tTG in the microtitre well.
Fig. 2.
Competitive inhibition of binding with unlabelled tissue transglutaminase (tTG) of immunoglobulin A (IgA) tGT autoantibodies. Increasing amounts of unlabelled tTG were added to one IgA-tissue transglutaminase (TGA)-negative (○) and five positive serum samples in the second incubation of the fluorometric assay, in which IgA–TGA is captured by bio-tTG in SA-wells.
Establishment of the IgA–TGA avidity assay
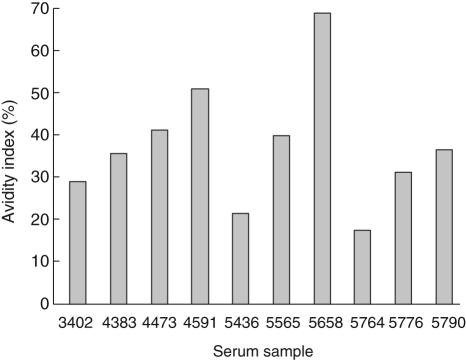
Based on our previous experience of establishing time-resolved immunofluorometric avidity assays on SA-plates [28], elution of low-avidity antibodies with urea was chosen as the approach for measuring IgA–TGA avidity. During an elution time of 15 min, a urea concentration of 5 M separated well 10 single serum samples according to their avidities (Fig. 3). The results from the avidity assay were not concentration-dependent, as scatter plots of avidity indices versus autoantibody titres showed no correlation (data not shown). Neither did the sample dilution affect the avidity index values as long as the IgA–TGA concentration remained above the cut-off level and within the linear range of the autoantibody assay, as different dilutions of two high-titre samples (8832 AU and 6035 AU, diluted 1 : 20000, 1 : 100000 and 1 : 500000) and one low-titre sample (69·8 AU, diluted 1 : 200, 1 : 1000 and 1 : 5000) gave similar results in the avidity assay (data not shown).
Fig. 3.
Identification of variations in the avidity index level of immunoglobulin A (IgA) tissue transglutaminase (tTG) autoantibodies in independent serum samples using urea. IgA–anti-tissue transglutaminase (TGA) from 10 serum samples from different individuals were bound to bio-tTG in SA-wells and then challenged with 5 M urea before detection. Avidity indices were calculated as the ratio remaining fluorescence/maximal fluorescence, in per cent. The corresponding IgA–TGA titres in AU for the different samples were 43·5 (no. 3402), 194 (no. 4383), 92·8 (no. 4473), 8832 (no. 4591), 589 (no. 5436), 62·0 (no. 5565), 6035 (no. 5658), 287 (no. 5764), 20·3 (no. 5776) and 69·8 (no. 5790).
Monitoring of IgA–TGA levels and avidity indices in children with genetic predisposition for CD
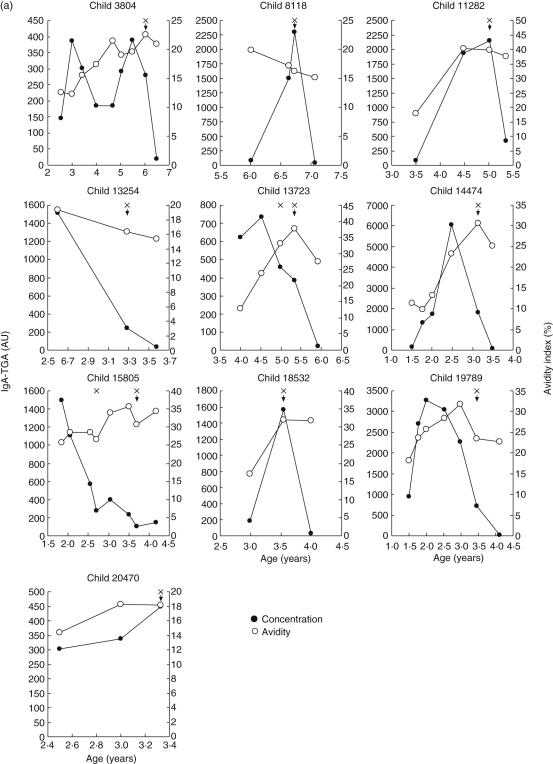
A serum sample panel consisting of 118 samples drawn at 1–8-month intervals from 10 children developing CD was analysed for IgA–TGA, and autoantibody-positive samples were tested further for avidity (Fig. 4a). The pattern of serum IgA–TGA concentration varied between the children. For some children, the autoantibody level increased substantially from seroconversion to diagnosis and then decreased dramatically upon introduction of a gluten-free diet, while other children showed declining IgA–TGA titres at the time of the CD confirming biopsy. One child had converted to IgA–TGA negativity when visiting the clinic for the first time after the diagnosis. Overall, the autoantibody levels varied between 107 AU and 2290 AU at gastroscopy and confirmation of CD. Also the avidity patterns showed individual variation. However, for most children the avidity indices increased during development of CD, and for five of them the increase in avidity index value was two- to threefold. At the time of diagnosis, the avidity indices were significantly higher than at seroconversion (range 16–40% and 11–26%, respectively; P = 0·013). The degree of avidity maturation was not dependent upon serum autoantibody concentration and duration of IgA–TGA positivity before diagnosis or the age of the children studied.
Fig. 4.
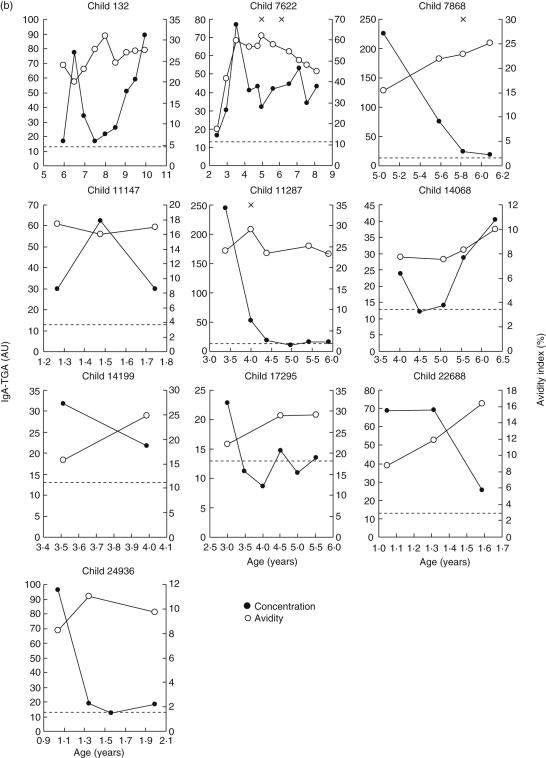
(a,b) Monitoring of immunoglobulin A (IgA) tissue transglutaminase (tTG) autoantibody levels and avidities in children genetically predisposed to coeliac disease (C). Serum samples from 10 children diagnosed with CD (a) were analysed for autoantibody level (•) and avidity (○) during a period extending from seroconversion to 3·5–8·1 months after duodenal biopsy (x), diagnosis of CD and starting gluten-free diet (arrow). In two children (no. 13723 and no. 15805), gluten-free diet was started after the second biopsy. Similarly, autoantibody titres and avidities of follow-up serum samples from 10 children with transient or fluctuating IgA–anti-tissue transglutaminase (TGA) values (b) were measured during the periods of seropositivity. For children with fluctuating autoantibodies, negative titres are also shown. The dotted lines indicate the cut-off value of the fluorometric IgA–TGA assay.
Similarly, the IgA–TGA levels and avidities of a serum sample panel, including 141 sequential samples from 10 children presenting transient or fluctuating autoantibodies according to a previous study [24], were determined (Fig. 4b). In this group, the IgA–TGA values remained below 250 AU, whereas a maximal antibody concentration between 389 and 6037 AU was seen in all children progressing to CD, the difference being statistically significant (P = 0·0002). Already at seroconversion to IgA–TGA positivity, the children developing CD presented higher autoantibody titres than the children exhibiting occasional IgA–TGA but remaining healthy (P = 0·004). Two of the children found to have fluctuating autoantibodies by ELISA (numbers 132 and 7622), were continuously IgA–TGA positive, from seroconversion to the last sample analysed in our assay. Most of the children presenting transient or fluctuating autoantibodies as measured by ELISA showed increasing IgA–TGA avidity index values during the seropositivity period (initial range 7·7–24%, range at the end of follow-up 9·7–45%, P = 0·013). Child no. 7622, who was supposed to have fluctuating IgA–TGA but presented continuous autoantibody positivity in this study, showed an almost 3·5-fold difference between the lowest and highest avidity index value (18 and 60%, respectively).
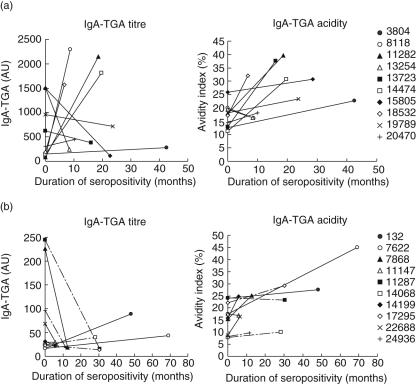
The tendency of the changes in IgA–TGA concentration and avidity during the period extending from seroconversion to diagnosis (children with confirmed CD) or to disappearance of autoantibodies (children with transient/fluctuating IgA–TGA) are presented in Fig. 5. There was no significant difference in the rate of increase in avidity index value between the two groups of children (P = 0·55).
Fig. 5.
(a,b) Tendency of changes in serum immunoglobulin A (IgA)–anti-tissue transglutaminase (TGA) concentration and avidity during seropostivity. For children with confirmed coeliac disease (C) (a), the autoantibody levels and avidity indices were measured at seroconversion and at diagnosis before introducing a gluten-free diet. Children with transient or fluctuating autoantibodies (b) were analysed at the first and last time-point of seropositivity. Children with fluctuating IgA–TGA positivity are indicated with dashed lines. The children marked with symbols (•) and (○) had fluctuating autoantibodies as determined by enzyme-linked immunosorbent assay (ELISA), but in the fluorometric assay they presented IgA–TGA positivity throughout the follow-up.
Discussion
Antibody avidity determinations are useful tools for discrimination between the acute and chronic phase of many infectious diseases. The clinical significance of avidity maturation of disease-associated autoantibodies is investigated less extensively. Nevertheless, there are reports showing a relationship between high-avidity antibodies against double-stranded DNA and renal damage in SLE [25], and between high-affinity immune responses to (pro)insulin and high risk for developing type 1 diabetes [26,27]. We studied previously the avidity maturation of autoantibodies against the protein tyrosine phosphatase-like IA-2 molecule and glutamic acid decarboxylase (GAD65) during progression to type 1 diabetes, but were not able to distinguish genetically predisposed prediabetic children from high-risk children remaining healthy on the basis of autoantibody avidity [28]. To our knowledge, investigations of affinity maturation of CD-associated antibodies have been focused hitherto on antibodies to dietary gliadin [29]. In this report, we studied the avidity maturation of autoantibodies against tissue transglutaminase, both in children progressing to CD and in subjects remaining healthy despite occasional presentation of IgA–TGA. Our aim was to explore whether the avidity index level might give additional information on the predictive value of transient and/or fluctuating IgA class tTG autoantibodies in healthy individuals exposed to dietary gluten, which have been described in at least three reports [6,23,24].
As in our previously established fluorometric assays for detection of type 1 diabetes-associated autoantibodies [31,32], in this study we preferred to use a streptavidin-coated surface and a biotin-labelled antigen to direct coating of the antigen, taking advantage of a biotinylation reagent providing a spacer between the antigen and the solid phase and thus preventing possible hiding of crucial epitopes or alterations in protein conformation. The fluorometric IgA–TGA assay detected specifically tTG autoantibodies (Fig. 2), showed good reproducibility (Table 2) and correlated well with a commercial ELISA-type assay (Celikey™) (Fig. 1a), and hence made an applicable starting-point for the establishment of the avidity assay. Challenging of IgA–TGA bound to bio-tTG in streptavidin-coated microtitre wells with 5 M urea for 15 min was chosen as the approach for measuring avidity, because this method detected pronounced differences in avidity between single serum samples from individuals with suspected CD (Fig. 3).
After optimizing the fluorometric IgA–TGA assay, we analysed a panel of 259 sequential serum samples from 10 children with progression to CD and 10 children without confirmed disease despite transient or fluctuating autoantibodies as measured by ELISA [24] (Fig. 4). Samples detected IgA–TGA-positive in the fluorometric assay were analysed further regarding urea-dependent avidity. We observed a clear difference in the IgA–TGA titres between the children with progression to CD and children presenting with transient or fluctuating autoantibodies. In the latter group, the IgA–TGA levels remained below 100 AU, except for one peak value of about 250 AU in two cases (no. 7868 and no. 11287), whereas most of the children progressing to CD showed several times higher autoantibody levels. This result is in concordance with the data of Liu et al. [23], which showed that in IgA–TGA-positive children autoantibody titres can vary with time and a higher IgA-level predicts an abnormal biopsy finding.
Saalman et al. [29] investigated the avidity maturation of IgG class antibodies to gliadin (IgG–AGA) and β-lactoglobulin during early childhood using a thiocyanate elution assay, and found that the avidities of these two dietary antibodies increased with age both in healthy children as well as in children with untreated CD. The process seemed to be accelerated in the latter group, as gliadin and β-lactoglobulin antibodies in children with untreated CD showed significantly higher avidities than those of healthy children of the same age. In our study, however, there was no correlation between avidity of IgA–TGA and the age at sampling for either of the two group (data not shown). In both groups, the avidity indices showed an increasing tendency during the follow-up period with no significant difference in the avidity maturation rate between the children developing overt disease and the children expressing transient or fluctuating autoantibodies.
Saalman et al. [29] also observed that the avidity of IgG–AGA was preserved during remission on a gluten-free diet, despite a marked decrease in serum antibody concentration. Upon gluten challenge, most children showed a further increase in antibody avidity together with an elevated IgG–AGA level. In accordance with that study, the avidity indices of IgA–TGA in this report remained approximately on the same level after introduction of a gluten-free diet (Fig. 4a), despite a conspicuous drop in serum antibody concentration in most of the cases. For only two children (no. 13723 and no. 14474), the urea-dependent avidity decreased with more than 10% after withdrawal of dietary gluten. For one child (no. 20470), the first sample drawn after diagnosis was already IgA–TGA negative, and hence avidity data after elimination of dietary gluten are missing for this subject. Because relapse on gluten challenge is no longer used for confirmation of CD diagnosis, there were no serum samples available for the study of IgA–TGA avidity development after reintroduction of gluten to the diet of the children described in this report.
Two of the children with fluctuating IgA–TGA positivity as measured by ELISA (no. 132 and no. 7622) were constantly autoantibody positive in the fluorometric assay, from seroconversion onwards (Fig. 1b). Their autoantibody titres showed a clearly increasing tendency during the study period, contrary to IgA–TGA titres of the other children in the same group (Fig. 5). We therefore suspect these two children of developing CD. The children are followed continuously in the context of the Finnish DIPP study, and for one child (no. 132), the IgA–TGA level has continued to increase since the last sample for this report was analysed. Consequently, duodenal biopsy has been recommended, but not yet conducted. Child no. 7622 has previously undergone videoendoscopy and biopsy twice, for the second time almost 3·5 years ago, with normal villous architecture.
Our results suggest that there are differences in serum IgA–TGA concentration, but not in avidity maturation, between genetically predisposed individuals developing CD and high-risk subjects remaining disease-free despite occasional presentation of CD-associated autoantibodies. Whether these findings could be of clinical importance, for instance in reducing the number of unnecessary duodenal biopsies with a normal outcome, remains to be evaluated by analysing larger cohorts of individuals seroconverting to IgA–TGA positivity.
Acknowledgments
This study was supported by the National Technology Agency of Finland (TEKES), PerkinElmer Life and Analytical Sciences, Wallac Oy, Turku, Finland and Biofile Ltd, Turku, Finland.
References
- 1.Dewar D, Pereira SP, Ciclitira PJ. The pathogenesis of coeliac disease. Int J Biochem Cell Biol. 2004;36:17–24. doi: 10.1016/s1357-2725(03)00239-5. [DOI] [PubMed] [Google Scholar]
- 2.Brandimarte G, Tursi A, Giorgetti GM. Changing trends in clinical form of celiac disease. Which is now the main form of celiac disease in clinical practice? Minerva Gastroenterol Dietol. 2002;48:121–30. [PubMed] [Google Scholar]
- 3.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 4.Csizmadia CG, Mearin ML, von Blomberg BM, Brand R, Verloove-Vanhorick SP. An iceberg of childhood coeliac disease in the Netherlands. Lancet. 1999;353:813–4. doi: 10.1016/S0140-6736(99)00243-3. [DOI] [PubMed] [Google Scholar]
- 5.Hill I, Fasano A, Schwartz R, Counts D, Glock M, Horvath K. The prevalence of celiac disease in at-risk groups of children in the United States. J Pediatr. 2000;136:86–90. doi: 10.1016/s0022-3476(00)90055-6. [DOI] [PubMed] [Google Scholar]
- 6.Mäki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–24. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 7.Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143–8. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Rajadhyaksha M, Wortsman J. Celiac disease-associated autoimmune endocrinopathies. Clin Diagn Lab Immunol. 2001;8:678–85. doi: 10.1128/CDLI.8.4.678-685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hervonen K, Hakanen M, Kaukinen K, Collin P, Reunala T. First-degree relatives are frequently affected in coeliac disease and dermatitis herpetiformis. Scand J Gastroenterol. 2002;37:51–5. doi: 10.1080/003655202753387356. [DOI] [PubMed] [Google Scholar]
- 10.Högberg L, Fälth-Magnusson K, Grodzinsky E, Stenhammar L. Familial prevalence of coeliac disease: a twenty-year follow-up study. Scand J Gastroenterol. 2003;38:61–5. doi: 10.1080/00365520310000456. [DOI] [PubMed] [Google Scholar]
- 11.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–55. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 12.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 13.Bürgin-Wolff A, Dahlbom I, Hadziselimovic F, Petersson CJ. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand J Gastroenterol. 2002;37:685–91. doi: 10.1080/00365520212496. [DOI] [PubMed] [Google Scholar]
- 14.Osman AA, Richter T, Stern M, et al. Production of recombinant human tissue transglutaminase using the baculovirus expression system, and its application for serological diagnosis of coeliac disease. Eur J Gastroenterol Hepatol. 2002;14:1217–23. doi: 10.1097/00042737-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez E, Riestra S, Rodrigo L, et al. Comparison of six human anti-transglutaminase ELISA-tests in the diagnosis of celiac disease in the Saharawi population. World J Gastroenterol. 2005;11:3762–6. doi: 10.3748/wjg.v11.i24.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zintzaras E, Germenis AE. Performance of antibodies against tissue transglutaminase for the diagnosis of celiac disease: meta-analysis. Clin Vaccine Immunol. 2006;13:187–92. doi: 10.1128/CVI.13.2.187-192.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troncone R, Maurano F, Rossi M, et al. IgA antibodies to tissue transglutaminase: an effective diagnostic test for celiac disease. J Pediatr. 1999;134:166–71. doi: 10.1016/s0022-3476(99)70410-5. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin A deficiency: how effective are the serological methods of diagnosis? Clin Diagn Lab Immunol. 2002;9:1295–300. doi: 10.1128/CDLI.9.6.1295-1300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korponay-Szabó IR, Dahlbom I, Laurila K, et al. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut. 2003;52:1567–71. doi: 10.1136/gut.52.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlbom I, Olsson M, Forooz NK, Sjoholm AG, Truedsson L, Hansson T. Immunoglobulin G (IgG) anti-tissue transglutaminase antibodies used as markers for IgA-deficient celiac disease patients. Clin Diagn Lab Immunol. 2005;12:254–8. doi: 10.1128/CDLI.12.2.254-258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chartrand LJ, Seidman EG. Celiac disease is a lifelong disorder. Clin Invest Med. 1996;19:357–61. [PubMed] [Google Scholar]
- 22.Schmitz J. Is celiac disease a lifelong disorder? Clin Invest Med. 1996;19:352–6. [PubMed] [Google Scholar]
- 23.Liu E, Bao F, Barriga K, et al. Fluctuating transglutaminase autoantibodies are related to histologic features of celiac disease. Clin Gastroenterol Hepatol. 2003;1:356–62. doi: 10.1053/s1542-3565(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 24.Simell S, Kupila A, Hoppu S, et al. Natural history of transglutaminase autoantibodies and mucosal changes in children carrying HLA-conferred celiac disease susceptibility. Scand J Gastroenterol. 2005;40:1182–91. doi: 10.1080/00365520510024034. [DOI] [PubMed] [Google Scholar]
- 25.Villalta D, Romelli PB, Savina C, et al. Anti-dsDNA antibody avidity determination by a simple reliable ELISA method for SLE diagnosis and monitoring. Lupus. 2003;12:31–6. doi: 10.1191/0961203303lu277oa. [DOI] [PubMed] [Google Scholar]
- 26.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114:589–97. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlosser M, Koczwara K, Kenk H, et al. In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia. 2005;48:1830–2. doi: 10.1007/s00125-005-1864-6. [DOI] [PubMed] [Google Scholar]
- 28.Westerlund A, Ankelo M, Ilonen J, Knip M, Simell O, Hinkkanen AE. Absence of avidity maturation of autoantibodies to the protein tyrosine phosphatase-like IA-2 molecule and glutamic acid decarboxylase (GAD65) during progression to type 1 diabetes. J Autoimmun. 2005;24:153–67. doi: 10.1016/j.jaut.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Saalman R, Dahlgren UI, Fallstrom SP, Hanson LA, Ahlstedt S, Wold AE. Avidity progression of dietary antibodies in healthy and coeliac children. Clin Exp Immunol. 2003;134:328–34. doi: 10.1046/j.1365-2249.2003.02296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vainio E, Kosnai I, Hallstrom O, Karpati S, Maki M, Reunala T. Antigliadin and antireticulin antibodies in children with dermatitis herpetiformis. J Pediatr Gastroenterol Nutr. 1986;5:735–9. doi: 10.1097/00005176-198609000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Ankelo M, Westerlund-Karlsson A, Ilonen J, et al. Time-resolved fluorometric assay for detection of autoantibodies to glutamic acid decarboxylase (GAD65) Clin Chem. 2003;49:908–15. doi: 10.1373/49.6.908. [DOI] [PubMed] [Google Scholar]
- 32.Westerlund-Karlsson A, Suonpää K, Ankelo M, Ilonen J, Knip M, Hinkkanen AE. Detection of autoantibodies to protein tyrosine phosphatase-like protein IA-2 with a novel time-resolved fluorimetric assay. Clin Chem. 2003;49:916–23. doi: 10.1373/49.6.916. [DOI] [PubMed] [Google Scholar]
- 33.Kupila A, Muona P, Simell T, et al. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia. 2001;44:290–7. doi: 10.1007/s001250051616. Juvenile Diabetes Research Foundation Centre for the Prevention of Type I Diabetes in Finland. [DOI] [PubMed] [Google Scholar]