Abstract
Transplantation of many tissues requires histocompatibility matching of human leukocyte antigens (HLA) to prevent graft rejection, to reduce the level of immunosuppression needed to maintain graft survival, and to minimize the risk of graft-versus-host disease, particularly in the case of bone marrow transplantation. However, recent advances in fields of gene delivery and genetic regulation technologies have opened the possibility of engineering grafts that display reduced levels of HLA expression. Suppression of HLA expression could help to overcome the limitations imposed by extensive HLA polymorphisms that restrict the availability of suitable donors, necessitate the maintenance of large donor registries, and complicate the logistics of procuring and delivering matched tissues and organs to the recipient. Accordingly, we investigated whether knockdown of HLA by RNA interference (RNAi), a ubiquitous regulatory system that can efficiently and selectively inhibit the expression of specific gene products, would enable allogeneic cells to evade immune recognition. For efficient and stable delivery of short hairpin-type RNAi constructs (shRNA), we employed lentivirus-based gene transfer vectors, which provide a delivery system that can achieve integration into genomic DNA, thereby permanently modifying transduced graft cells. Our results show that lentivirus-mediated delivery of shRNA targeting pan-Class I and allele-specific HLA can achieve efficient and dose-dependent reduction in surface expression of HLA in human cells, associated with enhanced resistance to alloreactive T lymphocyte-mediated cytotoxicity, while avoiding MHC-non-restricted killing. We hypothesize that RNAi-induced silencing of HLA expression has the potential to create histocompatibility-enhanced, and, eventually, perhaps “universally” compatible cellular grafts.
Immune responses against donor (i.e, non-self) antigens are the primary cause of allogeneic transplant rejection resulting in graft failure. To date, the primary strategies for avoiding rejection have been to minimize antigenic differences between donor and recipient by matching HLA alleles and by subjecting the transplant recipient to potent immunosuppression. Of the several gene loci encoding HLA antigens, the most important for graft survival are the Class I antigens A and B and the Class II antigen DR. However, HLA is remarkably polymorphic, with more than 220, 460, 110, and 360 molecularly defined epitopes for HLA-A, B, C, and DR, respectively. Mismatching of the serological antigens is enough to increase the probability of graft failure in bone marrow transplantation,1 and even when serology is matched, small molecular genetic differences may cause transplant rejection.2 Similarly, recent studies have provided evidence that Class I HLA matching at the triplet level can benefit kidney transplant outcome,3 and HLA mismatches are associated with a higher incidence of chronic rejection, a major cause of late allograft loss after renal transplantation.4,5 Anti-HLA antibodies also play a crucial role for renal graft survival,4 and several HLA antibody specificities detected in recipients after organ transplantation are associated with an increased incidence of rejection and graft failure.6 Thus, histocompatibility matching is imperative to achieve an optimal therapeutic outcome for many types of transplants. However, the need to match donors and recipients among populations harboring extensive HLA polymorphisms restricts the availability of compatible donors, necessitates the maintenance of large registries to match suitable donors with waiting recipients, and complicates the logistics of procuring and delivering matched tissues and organs across long distances to the recipient.
Recent advances in techniques for gene delivery and gene therapy and in understanding molecular mechanisms involved in regulation of gene expression have opened the possibility of engineering grafts in which HLA expression is minimized or even eliminated, either globally or in an allele-specific manner. Such suppression of HLA expression could help to overcome the limitations to tissue and organ transplantation imposed by HLA polymorphism. Furthermore, as HLA polymorphism is the most relevant immunologic barrier to organ transplantation, HLA incompatibilities will also be a major barrier to allogeneic stem cell-based regenerative therapies.7,8 Although embryonic stem cells do not display HLA, expression progressively increases as the cells differentiate.7 Thus, HLA suppression could overcome this limitation by inducing immunologic tolerance and decreasing the risk of rejection.
In particular, RNA interference (RNAi) has recently emerged as a prevailing genetic tool for silencing gene expression by triggering posttranscriptional degradation of homologous transcripts through a multi-step mechanism involving double-stranded small interfering RNA (siRNA).9,10 Thus, we hypothesized that RNAi-induced silencing of HLA expression has the potential to create histocompatibility-enhanced, and, potentially, “universally” compatible cellular grafts that can be transplanted without the need to find matching donors. For delivery of HLA-targeting precursor short hairpin-type RNA (shRNA) constructs, which are subsequently processed into siRNA,10 we employed a lentivirus-based gene delivery vector system. Lentiviral vectors are attractive tools for this purpose as they offer the ability to efficiently transduce a wide variety of primary human cells, whether proliferating or quiescent, and can achieve permanent integration into the genomic DNA of transduced cells, thereby enabling long-term modification of cellular phenotype with a single procedure.11–17 Therefore, to demonstrate the feasibility of this strategy for cellular engineering of grafts to achieve HLA silencing and reduce alloreactive immunogenicity, we constructed and tested lentiviral vectors expressing HLA-A allele-specific shRNA constructs, as well as pan-Class I-specific shRNA constructs directed against conserved sequences in HLA-A,B,C.
MATERIALS AND METHODS
HLA Class I-Targeted Lentiviral Vectors
Class I pan-specific shRNA constructs targeted against highly conserved HLA-A,B,C sequences and allele-specific shRNAs targeted against unique sequences in HLA-A0201 were designed and cloned into plasmid pLentiLox-DsRed, which encodes an HIV-derived lentiviral vector containing a multiple cloning site for insertion of shRNA constructs to be driven by an upstream U6 promoter and a downstream CMV promoter-DsRed fluorescent protein (marker gene) cassette flanked by loxP sites. Different shRNA-encoding lentiviral vector constructs were first screened by plasmid lipofection into 293T human embryonic kidney-derived cells (ATCC #CRL-11268) with FuGene-6 (Roche Diagnostics. Indianapolis, Ind) according to the manufacturer’s instructions. Lentivirus preparations were produced with a standard third-generation packaging system as previously described18; briefly, packaging plasmids (pMD.G encoding VSV-G envelope, pMDLg/p encoding HIV gag-pol, and pRSV-REV encoding HIV rev) were cotransfected along with each pLentiLox-DsRed vector plasmid into 293T cells by calcium phosphate precipitation, and 48 hours later the virus-containing supernatant medium was collected, filtered, and concentrated by ultracentrifugation. Vector titers were determined by infection of 293T cells with serial dilutions of the concentrated virus preparation, followed by FACS analysis of DsRed expression 48 hours later using an EPICS-XL flow cytometer (Beckman Coulter, Miami, Fla).
FACS Analysis of HLA Silencing
Lentiviral vectors were used to transduce naïve 293T cells, and allele-specific and pan-specific anti-HLA shRNA constructs were screened for silencing efficiency by two-color FACS analysis, confirming transduction efficiency with DsRed expression and examining cell surface HLA expression with FITC-conjugated antibodies that were pan-specific for HLA-ABC (Clone G46-2.6) or allele-specific for HLA-A2 (Clone BB7.2) or isotype controls (BD Biosciences, San Jose, Calif). HLA typing of 293T cells was performed using a standard complement lysis assay.
Alloreactive Cytotoxic T Lymphocytes and Lymphokine-Activated Killer Cells
Primary alloreactive cytotoxic T lymphocytes (alloCTL) were isolated from peripheral blood mononuclear cells (PBMC) as previously described,19 using leukopheresis samples purchased from the San Diego Blood Bank without patient identifiers, following an IRB-approved exemption protocol. Precursor PBMC were typed to select samples expressing HLA antigens different from those on stimulator cells. For these studies, human U-87MG cells (ATCC #HTB-14) were employed as stimulator cells, and for optimal HLA antigen presentation were cultured in RPMI-1640 medium containing 500 IU/mL of recombinant human IFN-gamma and treated with mitomycin C prior to incubation with precursor donor lymphocytes at responder-to-stimulator ratios of 10:1 in RPMI-1640 medium containing 60 IU/mL of recombinant human IL-2 (R&D systems, Minneapolis, Minn). To generate non-MHC-restricted lymphokine-activated killer cells (LAK),20,21 PBMC were resuspended at 10e6/mL in RPMI-1640 medium containing 600 U/mL of IL-2 for 5 to 6 days.
AlloCTL and LAK Cytotoxicity Assays
Lentiviral vector-transduced 293T cells were analyzed for sensitivity to alloCTL-mediated and LAK cell-mediated lysis, respectively, by coculture with alloCTL or LAK cells at a ratio of 10:1 (effector: target) for 48 hours, and viability of adherent cells after washing was determined by MTS assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, CellTiter 96 Non-Radioactive Cell Proliferation Assay, Promega, Madison, Wisc). All treated wells were analyzed at least in triplicate, and results expressed as the mean percentage of surviving cells compared to that of the untreated control sample. Statistical analysis was performed using Graph-Pad Prism (GraphPad Software, San Diego, Calif). Cell viability results determined by MTS assays between the different groups were compared using one-way ANOVA, and P < .05 were considered significant.
RESULTS
Development of Lentiviral Vectors for Silencing of HLA Class I Expression
Candidate siRNA sequences directed against unique sequences in human HLA-A0201 (HLA-A2 allele-specific) and against common sequences conserved among Class I loci (HLA-ABC pan-specific) were designed as hairpin loop structures and cloned into the pLentiLox-DsRed vector construct. The highest silencing activities, as determined by FACS analysis for cell surface HLA expression after transient transfection of the vector construct alone, were obtained with a HLA-A,B,C pan-specific shRNA construct targeting the conserved HLA Class I sequence 5′-GCTACTACAACCAGAGCGAG-3′, and an allele-specific shRNA construct targeting the unique HLA-A0201 sequence 5′-GGATTACATCGCCCTGAAAG-3′ (data not shown). These vector constructs were selected for virus production and further testing. Virus production was performed using a standard third-generation lentiviral packaging system, and, upon infection of target cells, the resultant viruses deliver both the U6 promoter-driven shRNA cassette and a CMV promoter-driven DsRed fluorescent marker gene cassette. Virus titers, as determined by FACS analysis for expression of the co-expressed DsRed fluorescent marker protein, were generally in the range of approximately 2 to 10 × 10e8 transducing units per mL.
Lentiviral Gene Transfer for Pan-Specific and Allele-Specific Inhibition of HLA Expression
Lentiviral vectors for shRNA targeting pan-specific and allele-specific HLA sequences were used to transduce naïve 293T target cells, which normally express predominantly the HLA Class I antigen HLA-A2, as well as lower levels of HLA-B7,Cw7, at increasing multiplicities of infection (MOI; ie, virus-to-cell ratio). As shown in Fig 1, successfully transduced cells show an increase in mean fluorescence level in the red channel (Y axis) due to co-expression of DsRed. Concomitantly, these cells also show a dose-dependent reduction in HLA levels due to shRNA expression, which can be detected as a reduction in fluorescence in the green channel (X axis) compared to untransduced (no lentivirus) and negative control (transduced with DsRed lentivirus only) target cells upon binding HLA-specific antibodies conjugated with fluorescein isothiocyanate (FITC). After transduction at higher MOI (10–30) with allele-specific and pan-specific shRNA vectors, cell surface expression of HLA-A2 and HLA-ABC was reduced by up to 50% and over 80%, respectively, compared to HLA expression levels in cells transduced with lentivirus expressing DsRed only (Fig 1).
Fig 1.
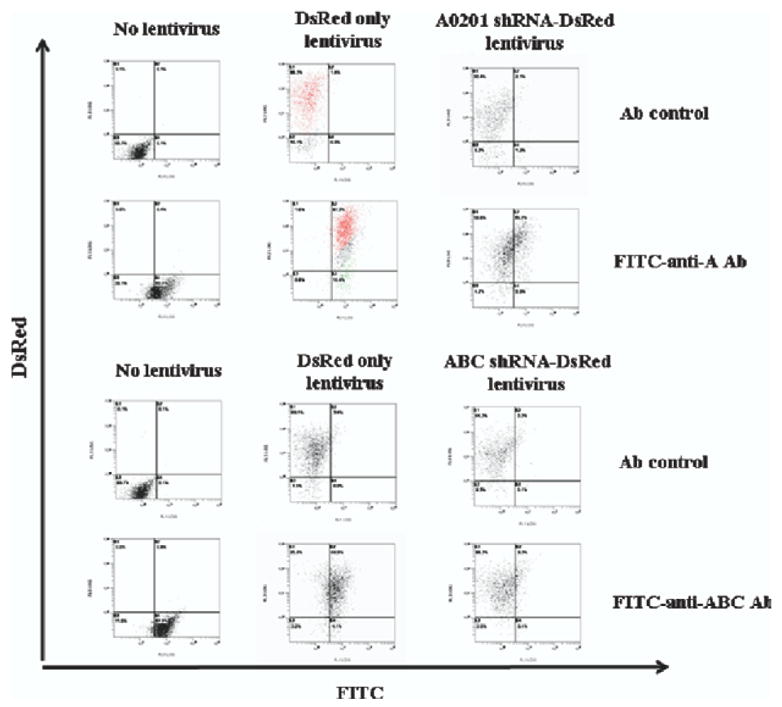
HLA knockdown by lentiviral shRNA vectors. The control lentiviral vector expresses the DsRed marker gene only (DsRed only lentivirus), while the A0201 shRNA-DsRed lentiviral vector expresses an HLA-A0201 allele-specific shRNA in addition to DsRed, and the ABC shRNA-DsRed lentiviral vector expresses a pan-Class I HLA-ABC-specific shRNA in addition to DsRed. Cell surface HLA expression was detected by FACS analysis using a fluorescein isothiocyanate (FITC)-conjugated anti-HLA-A antibody (FITC-anti-A2 Ab) or anti-HLA-ABC antibody (FITC-anti-ABC Ab). Note that with no lentivirus infection and isotype control antibody (Ab control), the events are all in the lower left quadrant. DsRed-only lentivirus infection with control antibody causes the entire population (highlighted in red) to shift only along the DsRed axis (Y-axis) to the upper left quadrant with no shift along the FITC axis (X-axis). DsRed-only lentivirus-infected cells stained with FITC-conjugated HLA antibodies show a shift in both DsRed fluorescence (Y axis) and FITC fluorescence (X-axis) to the upper right quadrant (also highlighted in red). In contrast, with HLA-A0201- or HLA-ABC-specific shRNA-DsRed infection in the presence of FITC antibodies, there is only a shift up the DsRed axis and very little shift along the FITC axis, indicating that there is nothing for the anti-HLA antibodies to bind to even when present and demonstrating that successful knockdown has been achieved.
Resistance to Alloreactive Cytotoxic T Lymphocyte (alloCTL)-Mediated Cell Killing
Alloreactive human effector T cells (alloCTL) were generated against human stimulator cells expressing the Class I antigens HLA-A2, B44, and C5. Lentiviral vector-transduced 293T target cells were analyzed for sensitivity to cytolysis after incubation with these HLA-activated alloCTL preparations. Target cells transduced with either HLA-A2 allele-specific or HLA-ABC pan-specific shRNA vectors, as well as pLentiLox-DsRed control vector transduced cells, were co-cultured with alloCTL with a ratio of 10:1 (effector: target cell ratio) for 48 hours, and the viability of adherent cells remaining after washing was determined by MTS assay. As shown in Fig 2, HLA-ABC shRNA and HLA-A0201 shRNA vectors both conferred significantly enhanced resistance to alloCTL-mediated killing compared to cells transduced with control vector expressing DsRed only (P < .05 for both comparisons). While complete loss of HLA expression may not only enhance resistance to HLA-restricted alloCTL killing but also increase sensitivity to killing by non-HLA-restricted effector cells, we observed no significant difference in the survival of HLA-ABC shRNA or HLA-A0201 shRNA vector-transduced target cells compared to DsRed only-transduced control cells after incubation with lymphokine-activated killer cells derived from the same donor PBMC (data not shown).
Fig 2.
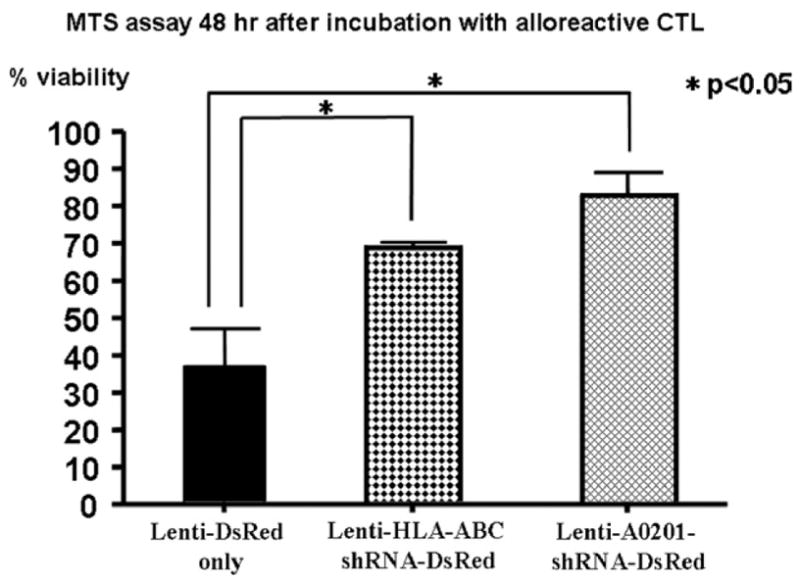
Alloreactive CTL-mediated cytotoxicity after lentivirus-mediated shRNA knockdown of HLA expression. 293T target cells transduced with control lentivirus vectors expressing DsRed only (Lenti-DsRed only) continue to express HLA-A2 and B7,Cw7 and show <40% viability 48 hours after incubation with alloreactive CTL that had been activated against stimulator cells expressing HLA-A2/B44/C5. In contrast, significantly increased viability is observed in target cells transduced with pan-specific (Lenti-ABC-shRNA-DsRed) or allele-specific (Lenti-A0201-shRNA-DsRed) vectors (P < .05 for both compared to control, as indicated). Results are expressed as mean values ± SEM in percent viability.
DISCUSSION
In this study, we have demonstrated that lentiviral vectors expressing pan-Class I specific shRNA constructs directed against conserved sequences in HLA-A,B,C, as well as HLA-A-specific shRNA constructs can achieve MOI (dose)-dependent knockdown of HLA levels in human cells, associated with induction of resistance to killing by alloreactive T effector cells, without incurring significant sensitivity to non-HLA-restricted killer cells.
In previous studies, Mhashilkar et al and Beyer et al22,23 reported that an antihuman MHC I single-chain intrabody expressed within cells from an adenovirus vector was reported to achieve phenotypic knockout of Class I HLA in human primary keratinocytes and in endothelial (HUVEC) cells, and intrabody-transduced HUVEC were protected from lysis by allogeneic sensitized CTL.23 Subsequently, Gonzalez et al24 demonstrated that stably transfected plasmids containing siRNA expression cassettes could achieve HLA down-regulation in cultured and primary T cells, and that this downregulation provided protection from CTL-mediated cytolysis. While these studies have established proof-of-concept for targeted inhibition of HLA expression leading to reduced immunogenicity, adenovirus vectors generally remain episomal and do not integrate stably in the host cell, therefore HLA downregulation using this approach is unlikely to have long-term benefit for graft survival. Similarly, while stable transformants expressing high copy numbers of siRNA sequences could be isolated after plasmid transfection, this was possible only through the use of an immortalized Jurkat T-cell line, which allowed stable selection with cotransfected antibiotic resistance markers. This methodology would not be possible for stable transfection of most primary cells, particularly quiescent stem cells. Figueiredo and colleagues25 have most recently reported the use of lentivirus vectors carrying shRNA cassettes targeting HLA-A and beta2-microglobulin in HeLa and immortalized B cells, which they demonstrated was effective using surrogate assays showing prevention of HLA-A-specific antibody-mediated, complement-dependent cytotoxicity and reduced CD8+ T-cell proliferation and interferon-gamma secretion. In the present study, we confirmed and extended these results using a different set of HLA-A0201-specific and HLA-ABC pan-Class I specific shRNA constructs delivered by lentivirus vectors and a more direct demonstration of their effectiveness in conferring resistance to alloreactive CTL by measurement of target cell survival.
Thus, we postulate that lentivirus-mediated delivery of shRNA constructs targeting HLA can be useful for long-term modulation of the alloimmunogenicity of transplanted cells and tissues. Moreover, RNAi-mediated silencing can be designed to be HLA allele-specific to nullify individual mismatches in otherwise well-matched donor tissues, and it may be advantageous that HLA knockdown with these particular pan-Class I shRNA constructs did not result in complete loss of expression even at high MOI, as this may be why sensitivity to non-HLA-restricted killer cell activity was not observed. Lowering expression of HLA may be as effective as utilization of powerful nonspecific immunosuppressive agents and would represent a fundamental shift in the concept of achieving histocompatibility by engineering the graft rather than immunosuppressing the host. While efficient transduction of entire solid organs remains a technical hurdle, application of this strategy can be readily envisioned for ex vivo transduction of cellular transplants such as bone marrow and stem cells, pancreatic islet cells, and keratinocytes.
Acknowledgments
This work was supported in part by a Pilot Seed Grant from the UCLA Center of Biological Radioprotectors (U19 AI067769). The authors would like to thank Dr. William McBride, Dr. Richard Gatti, and Dr. Karin Gaensler for helpful discussion, and acknowledge the UCLA JCCC/CURE Vector Core for assistance with lentiviral vector production.
Footnotes
K.H. and N.A.L. must be considered primary co-authors owing to equal participation in this study. N.K. and J.C.C. also participated equally as senior co-authors.
References
- 1.Petersdorf EW, Anasetti C, Martin PJ, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 2.Fleischhauer K, Kernan NA, O’Reilly RJ, et al. Bone marrow-allograft rejection by T lymphocytes recognizing a single amino acid difference in HLA-B44. N Engl J Med. 1990;323:1818. doi: 10.1056/NEJM199012273232607. [DOI] [PubMed] [Google Scholar]
- 3.Haririan A, Fagoaga O, Daneshvar H, et al. Predictive value of human leucocyte antigen epitope matching using HLAMatch-maker for graft outcomes in a predominantly African-American renal transplant cohort. Clin Transplant. 2006;20:226. doi: 10.1111/j.1399-0012.2005.00473.x. [DOI] [PubMed] [Google Scholar]
- 4.Cicciarelli J. HLA typing immunogenetics and transplantation. Curr Opin Organ Transpl. 2004;9:1. [Google Scholar]
- 5.Cicciarelli J, Aswad S, Mendez R. Significant HLA matching effect in a large urban transplant center composed primarily of minorities. Transplant Proc. 2005;37:658. doi: 10.1016/j.transproceed.2004.12.214. [DOI] [PubMed] [Google Scholar]
- 6.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136. doi: 10.1016/j.tibtech.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 9.Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Genet. 2001;2:110. doi: 10.1038/35052556. [DOI] [PubMed] [Google Scholar]
- 10.McManus MT, Petersen CP, Haines BB, et al. Gene silencing using micro-RNA designed hairpins. RNA. 2002;8:842. doi: 10.1017/s1355838202024032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borok Z, Harboe-Schmidt JE, Brody SL, et al. Vesicular stomatitis virus G-pseudotyped lentivirus vectors mediate efficient apical transduction of polarized quiescent primary alveolar epithelial cells. J Virol. 2001;75:11747. doi: 10.1128/JVI.75.23.11747-11754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Case SS, Price MA, Jordan CT, et al. Stable transduction of quiescent CD34(+)CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M, Kasahara N, Keene DR, et al. Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa. Nat Genet. 2002;32:670. doi: 10.1038/ng1041. [DOI] [PubMed] [Google Scholar]
- 14.Follenzi A, Sabatino G, Lombardo A, et al. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum Gene Ther. 2002;13:243. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- 15.Koya RC, Weber JS, Kasahara N, et al. Making dendritic cells from the inside out: lentiviral vector-mediated gene delivery of granulocyte-macrophage colony-stimulating factor and interleukin 4 into CD14+ monocytes generates dendritic cells in vitro. Hum Gene Ther. 2004;15:733. doi: 10.1089/1043034041648381. [DOI] [PubMed] [Google Scholar]
- 16.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 17.Sakoda T, Kasahara N, Hamamori Y, et al. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol. 1999;31:2037. doi: 10.1006/jmcc.1999.1035. [DOI] [PubMed] [Google Scholar]
- 18.Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse CA, Beck LT. Artificial-capillary-system development of human alloreactive cytotoxic T-lymphocytes that lyse brain tumours. Biotechnol Appl Biochem. 1997;25:197. [PubMed] [Google Scholar]
- 20.Rees RC. MHC restricted and non-restricted killer lymphocytes. Blood Rev. 1990;4:204. doi: 10.1016/0268-960x(90)90049-x. [DOI] [PubMed] [Google Scholar]
- 21.Hoskin DW, Stankova J, Anderson SK, et al. A functional and phenotypic comparison of murine natural killer (NK) cells and lymphokine-activated killer (LAK) cells. Int J Cancer. 1989;43:940. doi: 10.1002/ijc.2910430536. [DOI] [PubMed] [Google Scholar]
- 22.Mhashilkar AM, Doebis C, Seifert M, et al. Intrabody-mediated phenotypic knockout of major histocompatibility complex class I expression in human and monkey cell lines and in primary human keratinocytes. Gene Ther. 2002;9:307. doi: 10.1038/sj.gt.3301656. [DOI] [PubMed] [Google Scholar]
- 23.Beyer F, Doebis C, Busch A, et al. Decline of surface MHC I by adenoviral gene transfer of anti-MHC I intrabodies in human endothelial cells-new perspectives for the generation of universal donor cells for tissue transplantation. J Gene Med. 2004;6:616. doi: 10.1002/jgm.548. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez S, Castanotto D, Li H, et al. Amplification of RNAi-targeting HLA mRNAs. Mol Ther. 2005;11:811. doi: 10.1016/j.ymthe.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Figueiredo C, Seltsam A, Blasczyk R. Class-, gene-, and group-specific HLA silencing by lentiviral shRNA delivery. J Mol Med. 2006;84:425. doi: 10.1007/s00109-005-0024-2. [DOI] [PubMed] [Google Scholar]


