Abstract
β-Arrestin 2 (βarr2) is a multifunctional protein that regulates numerous aspects of G-protein-coupled receptor function. However, its possible involvement in developmental processes is poorly understood. In this work, we examined the potential role of βarr2 during Xenopus early development. Gain- and loss-of-function studies showed that Xenopus βarr2 (xβarr2) is required for proper convergent extension (CE) movements, and normal cell polarization and intercalation without affecting cell fate. Moreover, for CE movements, βarr2 acts as an essential regulator of dishevelled-mediated PCP (planar cell polarity) signaling, but not G-protein-mediated Ca2+ signaling. Notably, xβarr2 is localized with the same distribution as the dishevelled protein, which is reasonable, as xβarr2 is required for dishevelled activation of RhoA. Furthermore, xβarr2 interacts with the N-terminal quarter of Daam1 and RhoA proteins, but not Rac1, and regulates RhoA activation through Daam1 activation for CE movements. We provide evidence that the endocytic activity of xβarr2 is essential for control of CE movements. Taken together, our results suggest that βarr2 has a pivotal role in the regulation of Xenopus CE movements.
Keywords: β-arrestin 2, convergent extension movements, planar cell polarity, Wnt pathway, Xenopus development
Introduction
Morphogenetic movements in gastrulation are essential for establishing basic germ layers and body axis during early vertebrate development. The major driving forces for this process include convergent extension (CE) movements, by which cells polarize and elongate along the mediolateral axis and intercalate toward the midline (convergence), leading to extension of the anterior/posterior axis (reviewed by Wallingford et al, 2002; Veeman et al, 2003). Although the precise molecular mechanisms of CE movements are not clearly understood, the noncanonical Wnt pathway is known to be important in the control of CE movements (reviewed by Kuhl, 2002; Myers et al, 2002; Wallingford and Habas, 2005). The Wnt signaling pathway, which is mediated by interaction between frizzled (fz), a member of another class of trimeric G-protein-coupled receptor (GPCR) (Wodarz and Nusse, 1998; Malbon, 2004), and members of the Wnt family of secreted glycoproteins, consists of two pathways; the first is the canonical Wnt pathway (Wnt/β-catenin pathway) that mediates induction of a secondary axis in Xenopus embryos and epithelial transformation (Miller et al, 1999a; Polakis, 2000) by stabilization of β-catenin and nuclear expression of specific target genes such as Siamois and Xnr3 (Lemaire et al, 1995; Axelrod et al, 1998; Tada and Smith, 2000). The second type is the noncanonical Wnt pathway that regulates cell shape, cell polarity, and cell adhesion without induction of a secondary axis. This pathway is further subdivided into the Wnt/Ca2+ pathway and the Wnt/planar cell polarity (PCP) pathway (Veeman et al, 2003). The former acts via a trimeric G protein to stimulate intracellular calcium release, activate protein kinase Cα (PKCα) (Slusarski et al, 1997a, 1997b; Sheldahl et al, 1999) and Cdc42 (Choi and Han, 2002; Penzo-Mendez et al, 2003). The latter PCP pathway is transmitted via dishevelled, which has a dual role in the regulation of both the Wnt/β-catenin and PCP pathways (Wharton, 2003; Wallingford and Habas, 2005), and requires Daam1, RhoA, Rac1, Rho-associated kinase α, and Jun N-terminal kinase (JNK) (Myers et al, 2002; Wallingford and Habas, 2005). In the PCP pathway, phosphorylation of dishevelled is important for its translocation to the cell membrane and this translocation is a prerequisite for functional signaling activation (Rothbacher et al, 2000; Tada and Smith, 2000; Kinoshita et al, 2003; Ossipova et al, 2005; Park et al, 2005).
β-arrestins, β-arrestin 1 and 2, are multifunctional adaptors that regulate numerous aspects of GPCR, also called seven-transmembrane receptor, functions (reviewed by Luttrell and Lefkowitz, 2002; Lefkowitz and Shenoy, 2005). Classically, β-arrestins have been known for their critical roles in GPCR desensitization by sterically blocking receptor–G protein interaction and by internalization formed by clathrin-coated pits. Recent evidence, however, suggests that β-arrestins mediate a variety of receptor signals, regulatory processes, and crosstalk between signaling pathways via interaction with various β-arrestin-interacting proteins (Perry and Lefkowitz, 2002; Lefkowitz and Shenoy, 2005). In addition, β-arrestins have also been known to mediate the endocytosis of a number of other receptors such as Fz receptor, TGFβ receptor, and IGF1 receptor (Spiegel, 2003; Lefkowitz and Whalen, 2004). However, the physiological and functional importance of these proteins during embryonic development remains unclear.
Results
Cloning and expression pattern of β-arrestin 2 in Xenopus laevis
A partial clone for the Xenopus β-arrestin 2 (xβarr2) was identified in a Xenopus EST database, and a full-length cDNA of xβarr2 was isolated from a gastrula cDNA library using a PCR-based approach (GenBank accession number: DQ397537). The encoded protein is highly similar to orthologs from other species (human, 85% identity; mouse/rat, 84% identity; zebrafish, 84% identity; Figure 1A). To elucidate the potential role of xβarr2 in developmental processes, the spatiotemporal expression pattern of β-arrestin 2 (βarr2) was first analyzed during Xenopus embryogenesis. Temporally, xβarr2 is expressed both maternally and zygotically throughout early development (Figure 1B and C). Spatially, βarr2 is visible in the animal hemisphere of the embryo during the cleavage and blastula stages. At the gastrula stages, βarr2 is first expressed in the dorsal marginal zone (DMZ), but later expands laterally and ventrally. At the neurula stages, it shows restricted expression in anterior tissues containing the anterior neural plate. At later stages, βarr2 expression was localized in the cement gland, brain, and otic vesicle. In addition, βarr2, like visual arrestin, was detected in the retina (Figure 1D). These data indicate that xβarr2 has a dynamic expression pattern including expression in the dorsal mesoderm and ectoderm tissues that undergo CE movements.
Figure 1.
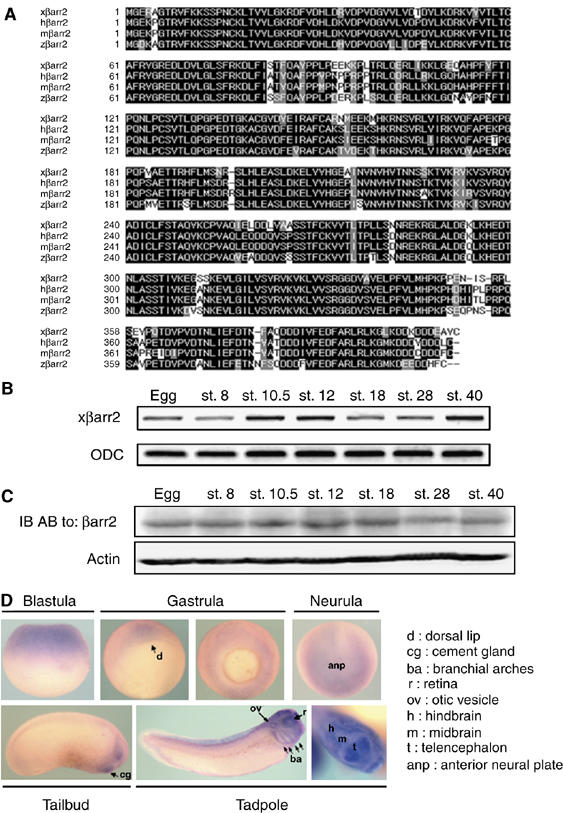
Sequence and expression of xβarr2. (A) Comparison of the protein sequences of xβarr2 and βarr2 orthologs from other species. Amino-acid identities are indicated by the shaded background. The alignment was generated using the CLUSTAL method. (B) RT–PCR analysis of temporal mRNA expression of xβarr2. Developmental stages are indicated above each lane. ODC (ornithine decarboxylase) served as a loading control. (C) Immunoblotting (IB) analysis of temporal protein expression of xβarr2. Actin was used as a loading control. (D) In situ hybridization showing the spatial expression of βarr2 during early Xenopus development.
βarr2 is required for CE movements in Xenopus development
Antisense morpholino oligonucleotides (MOs) are extremely useful as efficient blockers of specific mRNA translation (Heasman, 2002). To investigate the endogenous role of βarr2 during Xenopus early embryogenesis, a MO was designed to be complementary to the translation initiation sequence of xβarr2 mRNA (xβarr2 MO; Supplementary Figure S1A). The xβarr2 MO effectively blocked translation of mRNA containing the 5′-UTR sequences complementary to its MO, but translation of mRNA containing only the open reading frame of the protein was not affected. Control MO (Co MO) had no effect on translation of the βarr2 protein, and neither xβarr2 MO nor Co MO inhibited translation of the control, actin (Supplementary Figure S1B). Likewise, xβarr2 MO dramatically reduced the endogenous levels of βarr2 protein in DMZ tissues, where CE occurs (Supplementary Figure S1C). Interestingly, depletion of xβarr2 in Xenopus resulted in CE movements-defective phenotypes (Figure 2B and R), which included the delay of blastopore closure, the failure of neural tube closure and anterior/posterior axis formation, and the significant inhibition of the elongation of DMZ explants (Figure 2D and S). Additionally, xβarr2 overexpression caused defects of CE movements that were indistinguishable from those of the xβarr2 knockdown (Figure 2R and S; Supplementary Figure S2B and D).
Figure 2.
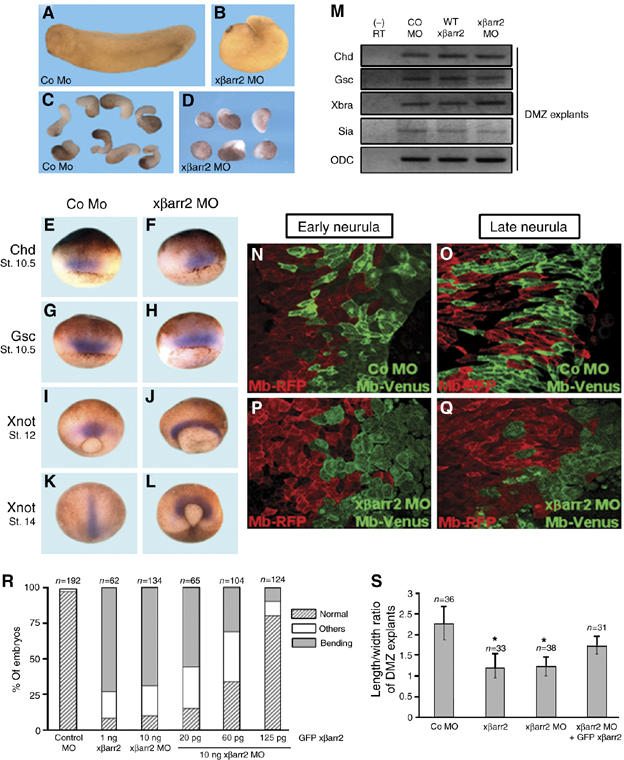
xβarr2 is essential for CE movements. (A–D) Functional depletion of xβarr2 causes the disruption of CE movements. Two blastomeres of four-cell stage embryos were injected at the dorsal equatorial region with xβarr2 MO or Co MO (10 ng). (A, B) Compared to the Co MO-injected embryos, embryos expressing xβarr2 MO at stage 33 showed dorsal flexure, and failure to straighten the anterioposterior axis. (C, D) DMZ explants from Co MO-injected embryos elongated, but the explants expressing xβarr2 MO were significantly inhibited. (E–L) Whole mount in situ hybridization with Chd, Gsc, and Xnot as probes. Before the onset of CE movements (stage 10.5), both Co MO- (E, G) and xβarr2 MO-injected embryos (F, H) exhibited normal expression of Chd and Gsc. Convergent and extended localization of Xnot in the notochord (I, K) was altered in mid-gastrula (J) and early-neurula (L) embryos expressing xβarr2 MO, whereas Xnot expression levels remained the same. (M) RT–PCR analysis showed that xβarr2 does not affect dorsal cell fate specification. DMZ explants expressing xβarr2 (1 ng), xβarr2 MO, or Co MO (10 ng) were isolated at stage 10.5 and analyzed by RT–PCR, using primers specific for Chd, Gsc, Xbra, and Sia. ODC, a loading control. (−) RT, minus reverse transcription control sample. (N–Q) xβarr2 depletion inhibited intercalation in the dorsal mesoderm. xβarr2 MO or Co MO (10 ng) was coinjected with membrane-bound Venus (Mb-Venus) into one of the two dorsal blastomeres at the four-cell stage. Mb-RFP was injected into the other dorsal blastomere. Monitoring of the intercalation progress was conducted in the same DMZ explants at stages 14 and 15, and 18 and 19. (R, S) Rescue of xβarr2 knockdown by coinjection of GFP xβarr2 mRNA. Quantitative assays of rescue in whole embryos (R) and DMZ elongation assays (S) were each performed more than three times. n, total number of embryos and explants. (R) Others indicate a truncated and mild kinked axis. (S) Error bars indicate the mean±s.d. *P<0.01 versus Co MO or rescue.
Dorsoventral patterning of the mesoderm affects CE movements indirectly by impacting cell fates in DMZ (Keller and Danilchik, 1988). To examine whether the phenotypes resulting from xβarr2 MO were caused by a defect in mesodermal differentiation, whole mount in situ hybridization was performed using several mesodermal genes. Before the onset of CE movements, xβarr2 MO-injected embryos (Figure 2F and H) exhibited normal expression of dorsal mesodermal markers, chordin (Chd) and goosecoid (Gsc) (Figure 2E and G). However, convergent and extended localization of Xnot in the notochord (Figure 2I and K) was blocked in mid-gastrula (Figure 2J) and early neurula (Figure 2L) embryos expressing xβarr2 MO, but Xnot expression levels remained the same. In addition, RT–PCR analysis demonstrated that xβarr2 does not affect the endogenous expression of dorsal mesoderm genes (Figure 2M) nor induce expression of these genes regulated by activin and Wnt/β-catenin signaling (Supplementary Figure S2E and F). We further determined the effect of xβarr2 MO on CE movements at the cellular level. To observe the intercalating cells in DMZ tissues, we used two lineage tracers, membrane-bound (Mb)-Venus and Mb-RFP, and injected these into each of the two dorsal blastomeres at the four-cell stage, as described previously (Hyodo-Miura et al, 2006). The observation of intercalation progress was conducted in the same DMZ explants at stages 14 and 15, and 18 and 19. The Co MO-injected side showed the same dynamic intercalation and spindle-shaped morphology for the developmental stages as the control side (Figure 2N and O). However, in the xβarr2 MO-injected side, normal polarization and intercalation were both inhibited compared to the opposite side (Figure 2P and Q). To verify the specificity of the xβarr2 knockdown in CE movements, a rescue assay was performed with a GFP xβarr2 construct that is unable to be inhibited by the xβarr2 MO. Coinjection of xβarr2 MO with GFP xβarr2 mRNA rescued the CE defects caused by xβarr2 MO in a dose-dependent manner (Figure 2R and S). Overall, these results support the idea that βarr2 is required for proper CE movements, and normal cell polarization and intercalation without affecting cell fate during Xenopus gastrulation.
xβarr2 is involved in the Wnt/PCP pathway, but not in the Wnt/Ca2+ pathway
The physiological defects induced by βarr2 in Xenopus CE movements were reminiscent of those caused by noncanonical Wnt pathway components (Myers et al, 2002; Veeman et al, 2003). A fundamental event in the noncanonical Wnt pathway is that the noncanonical fz receptor induces the translocation of downstream molecules including dishevelled, RhoA, and PKCs to the cell membrane in animal cap cells of Xenopus embryos (Axelrod et al, 1998; Sheldahl et al, 1999; Medina and Steinbeisser, 2000; Kinoshita et al, 2003; Park et al, 2006). Therefore, it is possible that the noncanonical fz receptor regulates the subcellular localization of βarr2. When GFP xβarr2 is expressed alone, βarr2 is diffusely distributed in the Xenopus animal cap cells (Figure 3A). Interestingly, expression together with Xenopus fz7 (Xfz7) and Rat fz2 (Rfz2), the well-known noncanonical fz receptors, led to redistribution of xβarr2 from the cytoplasm to the plasma membrane (Figure 3B and C). Moreover, βarr2, like dishevelled (Wallingford et al, 2000), localized to the cell membrane in Xenopus DMZ tissues that were actively engaged in CE movements (Figure 3D). In light of theses findings, we decided to determine whether the βarr2 distribution to the membrane by noncanonical fz receptors was due to a mutual interaction. A co-immunoprecipitation analysis showed that xβarr2 is physically associated with both Xfz7 and Rfz2 (Figure 3E).
Figure 3.
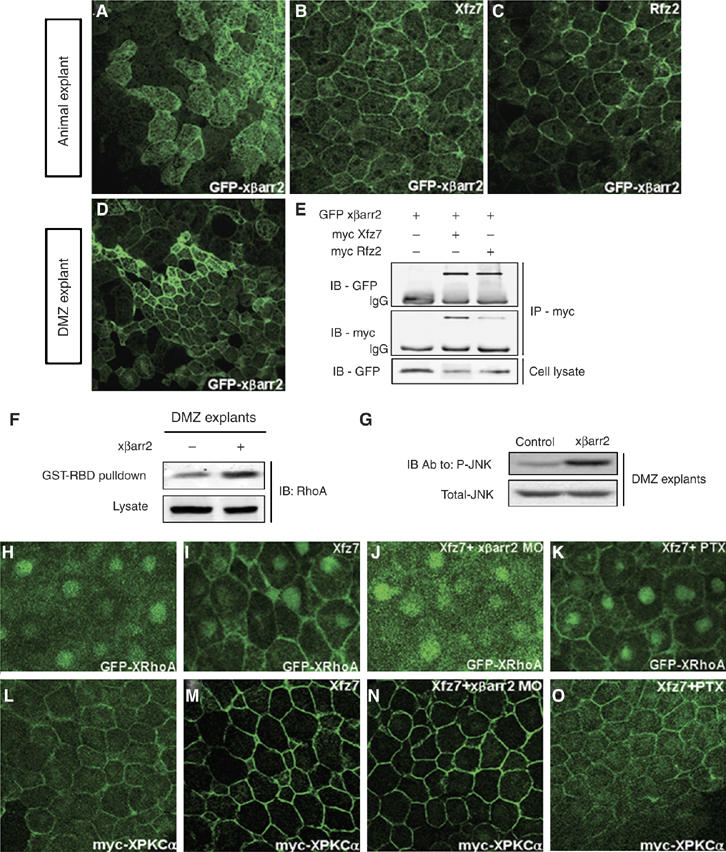
xβarr2 is involved in the Wnt/PCP pathway, but not Wnt/Ca2+ pathway. (A–C) xβarr2 distribution in cytoplasm was translocated by Xfz7 and Rfz2 to the cell membrane in animal cap cells. GFP xβarr2 (500 pg), Xfz7 (2 ng), and Rfz2 (2 ng) were injected, either alone or in the combinations indicated, into the animal regions of all blastomeres at the four-cell stage. Animal caps were dissected at stages 9 and 10, immediately fixed and analyzed under a microscope. (D) In DMZ cells, xβarr2 was localized to the cell membrane. DMZ explants expressing GFP xβarr2 (500 pg) were dissected at stages 11 and 11.5. (E) xβarr2 physically interacted with Xfz7 and Rfz2 in vivo. HEK293FT cells were transfected for 48 h with GFP-xβarr2, myc-Xfz7, or myc-Rfz2, either alone or in combination as indicated. Cell lysates were immunoprecipitated with anti-myc antibody and the immunocomplexes blotted with specific antibodies. (F, G) Two blastomeres of four-cell stage embryos were injected at the dorsal marginal region with xβarr2 (2 ng). The DMZ explants were dissected at stage 10.25 and cultured until stage 12. (F) xβarr2 activated RhoA in DMZ tissues during gastrulation. GTP-bound RhoA in DMZ lysates was precipitated using GST-RBD and visualized by immunoblotting with anti-RhoA antibody. (G) xβarr2 increased JNK phosphorylation in CE movements. The explant lysates were blotted with antiphospho JNK and anti-JNK antibodies. (H–O) Embryos were microinjected into the animal regions of all blastomeres at the four-cell stage with the indicated mRNAs (GFP xβarr2, 500 pg, myc XPKCα, 500 pg; Xfz7, 1.5 ng; xβarr2 MO, 10 ng; PTX, 1 ng). (H–K) xβarr2 MO, but not PTX, inhibited translocation of XRhoA to the membrane induced by Xfz7 in animal caps. (L–O) xβarr2 depletion could not block the membrane accumulation of XPKCα induced by Xfz7, whereas PTX was inhibitory.
The noncanonical Wnt/fz signals, including Wnt/PCP or Wnt/Ca2+ pathway, regulate CE movements (Myers et al, 2002; Veeman et al, 2003). To elucidate which of these pathways involves βarr2 in the control of CE movements, we first measured the activity of RhoA by using a GST-RBD fusion protein that recognizes the GTP-bound active RhoA (Ren et al, 1999) and the phosphorylated level of JNK in CE movements. With these assays, we found that xβarr2 activates RhoA (Figure 3F) and increases JNK phosphorylation in DMZ explants (Figure 3G). We then proceeded to determine whether βarr2 is essential for the Xfz7-dependent membrane localization of RhoA in Xenopus animal cap cells (Park et al, 2006). Intriguingly, xβarr2 knockdown (Figure 3J) inhibited the membrane accumulation of XRhoA induced by Xfz7 in response to Wnt/PCP signaling (Figure 3I). In contrast to RhoA, the xβarr2 MO (Figure 3N) could not block the Xfz7-dependent membrane localization of PKCα (Figure 3M), which indicates the activation of the trimeric G-protein-mediated Wnt/Ca2+ pathway (Sheldahl et al, 1999; Medina et al, 2000). However, pertussis toxin (PTX; Gα and Gβγ inhibitor of the heterotrimeric Gi family) did not inhibit RhoA membrane localization (Figure 3K), although it blocked PKCα (Figure 3O). Together, these results robustly suggest that xβarr2 is essential for the Wnt/PCP pathway, but not the Wnt/Ca2+ pathway.
βarr2 acts as an essential mediator of dishevelled-mediated PCP signaling in Xenopus CE movements
Many of receptors including GPCR directly interact with βarr2 and translocate βarr2 to the cell membrane (Luttrell and Lefkowitz, 2002; Lefkowitz and Shenoy, 2005). A recent report, however, claimed that in cultured cells, fz receptor perhaps recruits βarr2 via dishevelled indirectly (Chen et al, 2003). Considering this argument, the above finding of noncanonical fz-mediated βarr2 translocation to the membrane prompted us to determine physiologically the hierarchical order between βarr2 and dishevelled in Xenopus CE movements. We observed that, unlike Xfz7 (Kinoshita et al, 2003), xβarr2 could not alter a normal punctate localization pattern of Xdsh at various subcellular sites (Figure 4A and C) nor induce the hyperphosphorylation of Xdsh (data not shown) in animal cap cells. Likewise, xβarr2 depletion did not inhibit the membrane accumulation of Dsh induced by Xfz7 (Figure 4B and D). Expression of Xdsh-MA, a construct fused with the mitochondrial-membrane-anchoring sequence, disrupts the normal punctate distribution (Park et al, 2005) (data not shown) and the Xfz7-dependent membrane localization of Xdsh (Figure 4E), and results in a relocalization to intracellular clusters in animal cap cells of Xenopus embryos. If Xfz7-induced translocation of xβarr2 to the cell membrane is directly regulated by Xdsh but not Xfz7, then xβarr2 should be sequestered from the membrane by Xdsh-MA. Surprisingly, in the presence of Xdsh-MA, the normal or Xfz7-induced distribution of xβarr2 is relocalized to clusters in the center of animal cap cells (Figure 4F–H) and xβarr2-induced RhoA activation is reduced in DMZ cells (Figure 4I). Moreover, depletion of Xdsh inhibited xβarr2 membrane localization in DMZ cells (Figure 4J and K). We further demonstrated that the CE movements-defective phenotypes caused by Xdsh are rescued by xβarr2 MO (Figure 4L) and that depletion of xβarr2 blocks Xdsh-induced RhoA activation in ventral marginal zone (VMZ) cells (Figure 4M). From these results, we conclude that βarr2 acts as an essential mediator of dishevelled-mediated PCP signaling in Xenopus CE movements.
Figure 4.
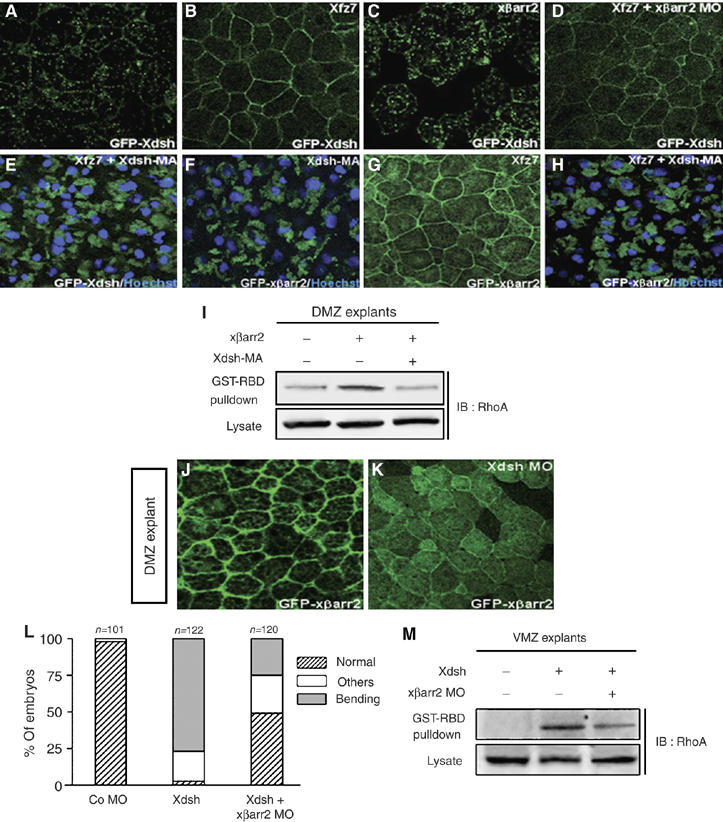
βarr2 acts downstream of dishevelled in PCP signaling. (A–L) Four-cell stage embryos were microinjected into the animal regions of all blastomeres. The amount of injected mRNAs: GFP Xdsh, 500 pg; xβarr2, 1–2 ng; Xfz7, 1.5 ng; xβarr2 MO, 5–10 ng; GFP xβarr2, 500 pg; Xdsh-MA, 500 pg; Xdsh MO, 40 ng; Xdsh, 1 ng. (A) Xdsh was distributed in a punctuate fashion in animal cap cells. Xfz7 led Xdsh to the cell membrane (B), but xβarr2 did not change the Xdsh distribution (C). (D) xβarr2 depletion did not alter the membrane accumulation of Xdsh induced by Xfz7. (E) Xfz7-dependent membrane localization of Xdsh was relocalized by Xdsh-MA to the intracellular cluster. (F–H) The normal (F) or Xfz7-induced distribution of xβarr2 (G, H) was relocalized by Xdsh-MA to the intracellular cluster. (I) Xdsh-MA reduced the RhoA activation induced by xβarr2 in DMZ cells. GTP-bound RhoA in DMZ lysates was precipitated using GST-RBD and monitored by immunoblotting with anti-RhoA antibody. (J, K) The membrane localization of xβarr2 in DMZ was blocked by Xdsh MO. (L) The CE movements-defective phenotypes caused by Xdsh were rescued by xβarr2 MO. Quantitative rescue assays were performed more than three times. n, total number of embryos. Others indicate a truncated and mild kinked axis. (M) xβarr2 depletion reduced the RhoA activation induced by Xdsh in VMZ tissues.
Dishevelled comprises three highly conserved domains that are important for interacting with dishevelled-associated proteins: the amino DIX (dishevelled/axin) domain, the central PDZ (PSD-95, DLG, ZO1) domain, and the carboxyl DEP (dishevelled, EGL-10, pleckstrin) domain (Wharton, 2003; Wallingford and Habas, 2005). As xβarr2 interacts with Xdsh (Figure 5A, lane 2), as previously reported for mammalian βarr2 (Chen et al, 2003), we used deletion mutants of Xdsh to map the region that interacts with xβarr2. Co-immunoprecipitation experiments showed that xβarr2 is capable of binding to Xdsh-ΔDIX, Xdsh-ΔPDZ, and Xdsh-ΔDEP (Figure 5A and B). Interestingly, xβarr2 did not interact with the PDZ domain, but did interact with the DIX domain (Figure 5C), indicating that the amino and carboxyl regions containing the DIX and DEP domain are necessary for the interaction with βarr2.
Figure 5.
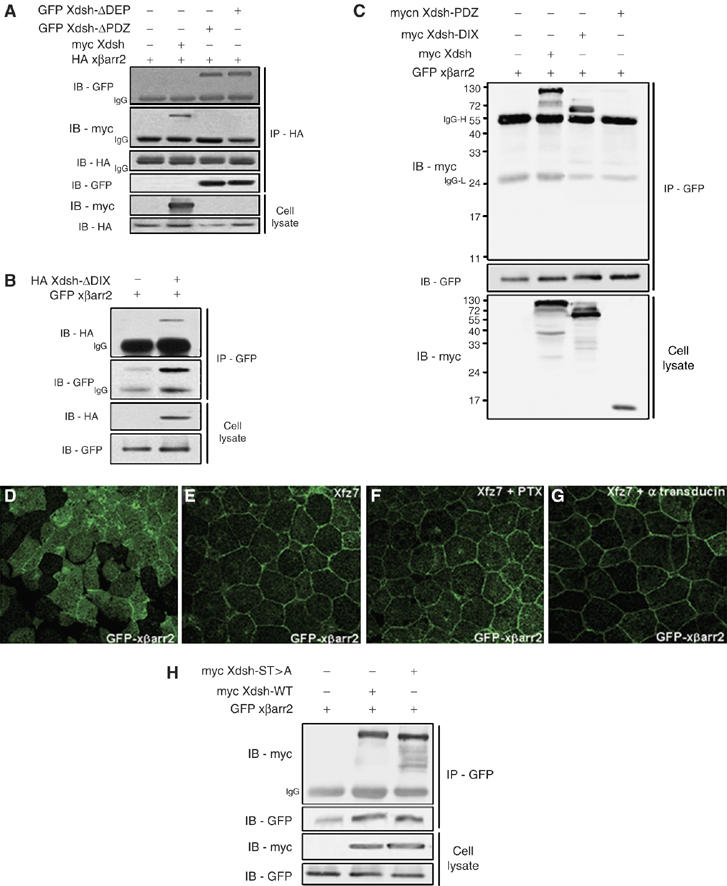
Biochemical interaction of xβarr2 with dishevelled. (A–C) HEK293FT cells were transfected for 48 h with various constructs, either alone or in combination as indicated. (A, B) xβarr2 was capable of binding to Xdsh-ΔDIX, Xdsh-ΔPDZ, and Xdsh-ΔDEP. (C) xβarr2 bound to the DIX domain, but not the PDZ domain. (D–G) Four-cell stage embryos were microinjected into the animal regions of all blastomeres. The amount of injected mRNAs: Xfz7, 1.5 ng; GFP xβarr2, 500 pg; PTX, 1 ng; α-transducin, 1 ng. PTX (F) and α-transducin (G) did not alter the membrane accumulation of xβarr2 induced by Xfz7 (E) in animal cap cells. (H) The binding level between xβarr2 and Xdsh-ST>A did not reduce compared with the level between xβarr2 and Xdsh.
We observed that xβarr2, like mammalian βarr2 (Chen et al, 2003), preferentially interacts with phosphorylated Xdsh (data not shown; Figure 6K, lanes 3 and 4). A recent report has shown that PKC activity is important for the membrane localization of βarr2 in cultured cells and PKCα phosphorylation of dishevelled stimulates βarr2 binding in vitro, although it is not known for mechanism of PKCα activation (Chen et al, 2003). As the Wnt/Ca2+ pathway activates PKCα through Giβγ signaling in Xenopus (Sheldahl et al, 1999), we assessed the effect of PTX and α-transducin (Gαt), a Giβγ signaling inhibitor, on the translocation of βarr2 to the plasma membrane. As shown in Figure 5D–G, these inhibitors did not affect the membrane accumulation of Xfz7-induced xβarr2 in animal cap cells. In addition, α-transducin could not rescue the CE defects caused by xβarr2 (Supplementary Figure S3), implying that G-protein-mediated PKCα activity is not essential for βarr2 function in Xenopus embryos. Recently, it was shown that mutating Par-1 phosphorylation sites in Xdsh (Xdsh-ST>A) by deleting six serine and two threonine residues in the front region rear to PDZ domain significantly reduced Xdsh phosphorylation and also affected membrane translocation of Xdsh (Ossipova et al, 2005). We then tested whether Par-1 phosphorylation sites in dishevelled are sufficient for the binding of dishevelled to βarr2. As shown in Figure 5H, the binding level between xβarr2 and Xdsh-ST>A was not reduced compared with the level between xβarr2 and Xdsh. In addition, xβarr2 did not bind to xPar-1A (data not shown). This result indicates that xβarr2 interacts with phosphorylated dishevelled via other phosphorylation sites.
Figure 6.
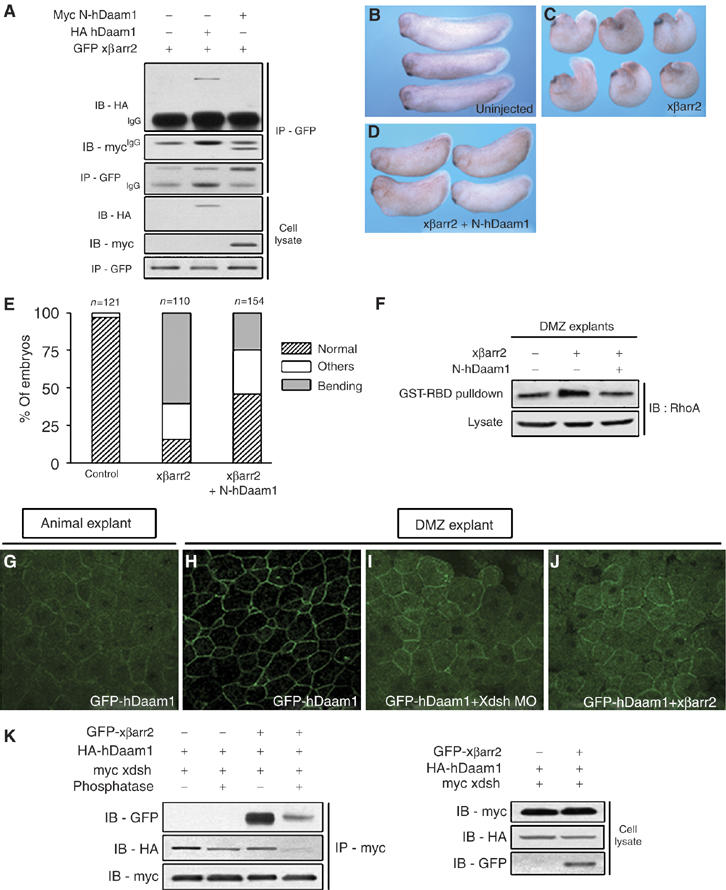
βarr2 is required for Daam1 to regulate RhoA activation in PCP signaling. (A) The N-terminal region of hDaam1 especially interacted with xβarr2. HEK293FT cells were transfected for 48 h with GFP xβarr2 and HA hDaam1 or myc N-hDaam1. (B–F) Rescue assays using βarr2 and a DN form of Daam1 at biological and biochemical levels. The CE-defective phenotypes caused by xβarr2 (1 ng; C) were rescued by N-hDaam1 (250 pg; D). (E) Quantitative rescue assays were performed more than three times. n, total number of embryos. Others indicate a truncated and mild kinked axis. (F) N-hDaam1 blocked xβarr2-induced RhoA activation. The amount of injected mRNAs: xβarr2, 2 ng; N-hDaam1, 1 ng. (G, H) hDaam1 was diffusely distributed in animal cap cells, whereas in DMZ cells, it was localized in cell membrane. (I, J) The membrane distribution of hDaam1 in DMZ was blocked by Xdsh and xβarr2 MO. GFP hDaam1 (500 pg), Xdsh MO (40 ng), and xβarr2 MO (10 ng) are injected, either alone or in combinations as indicated. (K) The interaction between hDaam1 and Xdsh was reduced by phosphatase treatment. HEK293TF cell extracts were treated with PAP at 30°C for 1 h and immunoprecipitated with anti-myc antibody (for Xdsh). Immunocomplexes were blotted with anti-HA (for hDaam1) and anti-GFP antibodies (for xβarr2).
βarr2 specifically mediates the PCP/RhoA pathway by controlling Daam1 in Xenopus CE movements
Although βarr2 is required for both activation and membrane recruitment of RhoA in noncanonical fz/dishevelled signaling, it does not harbor an identifiable GEF motif for RhoA activation. Daam1, a formin-homology (FH) protein, is a scaffold protein involved in RhoA activation downstream of dishevelled in the PCP pathway, likely via the recruitment of a Rho-GEF (Habas et al, 2001). To study the relationship between βarr2 and Daam1 at the molecular and physiological levels, we first tested whether βarr2 interacts with Daam1. Strikingly, xβarr2 bound to hDaam1, especially its N-terminal region (N-hDaam1; Figure 6A). Moreover, dishevelled formed a ternary complex with βarr2 and Daam1 (Figure 6K, lane 3). To analyze whether βarr2 is involved in Xenopus CE movements via Daam1, biological and biochemical experiments using βarr2 and N-hDaam1, a dominant-negative (DN) form of Daam1, were performed. Interestingly, the CE-defective phenotypes caused by xβarr2 (Figure 6C and E) were rescued by N-hDaam1 (Figure 6D and E), and N-hDaam1 blocked xβarr2-induced RhoA activation (Figure 6F). Next, we determined whether βarr2 regulates the subcellular localization of Daam1. When GFP hDaam1 is expressed alone, Daam1 is diffusely distributed in the Xenopus animal cap cells (Figure 6G), whereas in DMZ cells, it is localized to the cell membrane (Figure 6H). Its distribution in DMZ was blocked by both Xdsh and xβarr2 MO (Figure 6I and J), indicating that βarr2 mediates the PCP/RhoA pathway by controlling Daam1 in CE movements. We further assessed if the phosphorylated form of dishevelled is required for its own interaction with Daam1. Phosphatase treatment did have an effect on the interaction between Xdsh and hDaam1 (Figure 6K, lanes 1 and 2). In the presence of xβarr2, binding between these proteins was dramatically reduced by phosphatase treatment (Figure 6K, lanes 3 and 4).
Next, we wanted to determine the relationship between βarr2 and the Rho GTPases in the PCP pathway. We first investigated the interaction between βarr2 and the Rho GTPases that are regulated by dishevelled, RhoA, and Rac1. Co-immunoprecipitation experiments demonstrated that xβarr2 binds to XRhoA, but not to XRac1 (Figure 7A; Supplementary Figure S4). We further examined whether βarr2 distribution is affected by variants of dishevelled that differ in the ability to activate RhoA and Rac1 (Park et al, 2005). The DEP domain of dishevelled is required for membrane localization of dishevelled in the presence of Fz and Rac1 activation, but not RhoA. These significances were also illustrated by a KM [K (Lys) → M (Met)] mutation in the DEP domain that disrupts membrane localization of dishevelled and reduces its ability to activate Rac1 (Axelrod et al, 1998; Boutros et al, 1998; Habas et al, 2003). Intriguingly, although Xdsh did not alter the subcellular localization of xβarr2 that was evenly distributed (Figure 7B), mDvl1-KM-CAAX (the farnesylation signal of Ras), which harbors this point mutation but is constitutively localized to the cell membrane, activates RhoA but not Rac1 (Park et al, 2005) and relocalizes xβarr2 to the cell membrane (Figure 7C). This relocalization of xβarr2 was identical to that caused by mDvl1-CAAX (Figure 7D), which activates both Rac1 and RhoA (Park et al, 2005). However, Xdsh-MA, which blocks both RhoA and Rac1 activation and is defective in PCP but not β-catenin signaling (Park et al, 2005), translocated xβarr2 to intracellular clusters (Figure 7E). Taken together, these observations suggest that dishevelled activation of RhoA requires the membrane translocation of βarr2 and the signaling property of DEP domain's specific sequence, which is important for Rac1 activation but not directly involved in βarr2 function.
Figure 7.
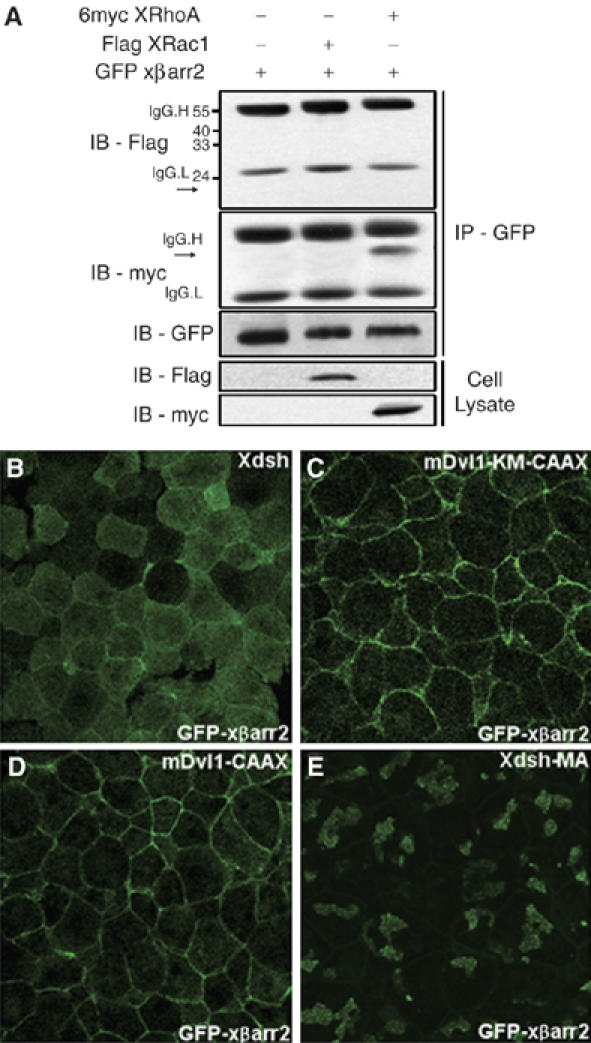
βarr2 mediates the specific signaling property of dishevelled that is important for RhoA activation, but not Rac1. (A) xβarr2 bound to XRhoA, but not to XRac1. HEK293FT cells were transfected for 48 h with GFP xβarr2 and 6myc XRhoA or Flag XRac1. Arrows indicate a size of epitope-tagged XRhoA or XRac1. (B–E) Four-cell stage embryos were microinjected into the animal regions of all blastomeres. (B) Xdsh did not alter the subcellular localization of xβarr2 that was evenly distributed. (C, D) βarr2 was translocated to the cell membrane by mDvl1-CAAX and mDvl1-KM-CAAX. (E) Xdsh-MA could translocate xβarr2 to intracellular clusters.
The endocytic function of βarr2 is essential for Xenopus CE movements
βarr2 acts as an adapter protein that targets receptors to clathrin-coated pits for endocytosis by interaction with components of the cellular endocytic machinery (Luttrell and Lefkowitz, 2002; Shenoy and Lefkowitz, 2003). Indeed, βarr2 binds directly to the clathrin and β2 adaptin subunit of the heterotetrameric AP2 (adapter protein 2) adapter complex through a LIEF sequence (Krupnick et al, 1997) and RxR sequence (Laporte et al, 2000), respectively. In addition, three basic residues within residues 233–251 of βarr2 (Lys233, Arg237, and Lys251), which bind to phosphoinositides, have been known to be important for clathrin-coated pits recruitment and receptor internalization (Gaidarov et al, 1999). To test the endocytic role of βarr2 on CE movements, we used the xβarr2 constructs mutating LIEF (LIEF → AAEA), RxR (RLR → ALA), and phosphoinositide-binding (KRK → 3Q) sequences used in a previously reported mutagenesis study (Shenoy and Lefkowitz, 2003) (Supplementary Figure S5). Strikingly, mutation of these sequences caused CE movements-defective phenotypes and significant inhibition of elongation of DMZ explants (Figure 8A and B), and inhibited both RhoA and JNK activation in CE movements (Figure 8C and D). Likewise, the CE defects induced by these mutants were rescued by wild-type (WT) xβarr2 (Figure 8A and B), but not xβarr2 MO (Figure 8A), indicating that endocytic activity of βarr2 is essential for control of Xenopus CE movements. We further determined if xβarr2 colocalizes with the cellular endocytic element in DMZ tissues during gastrulation. As shown in Figure 8E–G, endogenous β2 adaptin did not colocalize with xβarr2 in the cytoplasm, but partly overlapped in its colocalization to the cell membrane.
Figure 8.
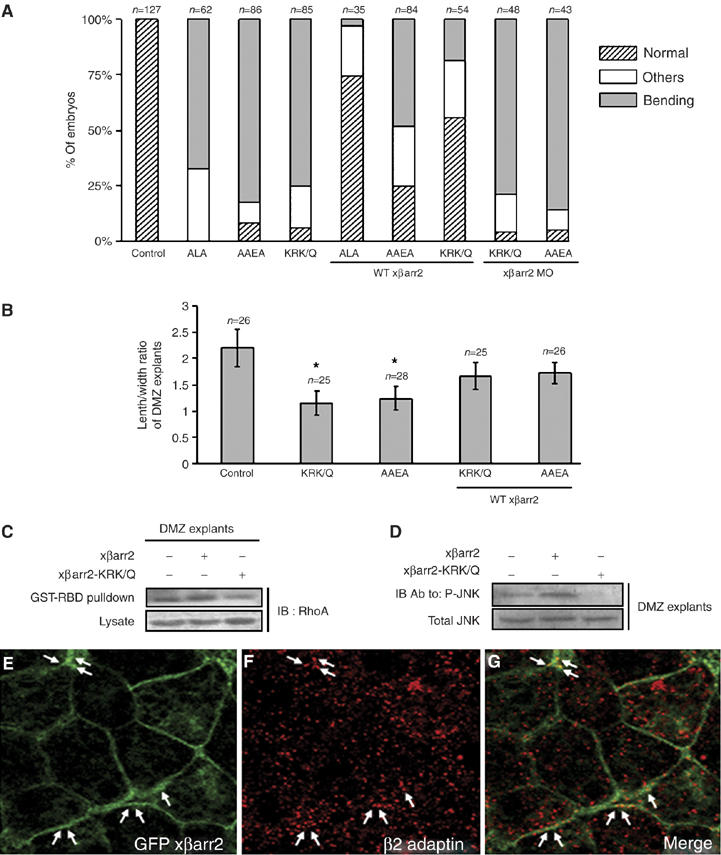
Endocytic function of βarr2 is required for Xenopus CE movements. (A–G) Four-cell stage embryos were microinjected into the animal regions of all blastomeres. The amount of injected mRNAs: xβarr2-AAEA, 1 ng; xβarr2-ALA, 1 ng; xβarr2-KRK/Q, 1 ng; xβarr2, 1–2 ng; xβarr2 MO, 10 ng; GFP xβarr2, 500 pg. (A, B) The CE defects induced by the endocytic mutants of xβarr2 were rescued by WT xβarr2, but not xβarr2 MO. Quantitative assays of rescue in whole embryos (A) and DMZ elongation assay (B) were performed more than two times. n, total number of embryos and explants. (A) Others indicate a truncated and mild kinked axis. (B) Error bars indicate the mean±s.d. *P<0.01 versus control or rescue. (C) xβarr2-KRK/Q inhibited RhoA activation in DMZ tissues during gastrulation. GTP-bound RhoA in DMZ lysates was precipitated using GST-RBD and visualized by immunoblotting with anti-RhoA antibody. (D) xβarr2-KRK/Q reduced JNK phosphorylation in CE movements. DMZ explant lysates were blotted with antiphospho JNK and anti-JNK antibodies. (E–G) In DMZ cells, endogenous β2 adaptin did not colocalize with xβarr2 in the cytoplasm, but partly overlapped in its colocalization to the cell membrane. DMZ explants expressing GFP xβarr2 were dissected at stages 11 and 11.5. Arrow indicates the colocalization of xβarr2 and β2 adaptin.
Discussion
βarr2 is traditionally associated with the GPCR signaling termination and receptor internalization (Attramadal et al, 1992; Ferguson et al, 1996), and also acts as a positive mediator leading to the activation of specific signals by interaction with various β-arrestin-interacting proteins in cell culture (Lefkowitz and Shenoy, 2005). However, the relevance of βarr2-mediated signaling in developmental processes was poorly understood. Our data suggest that βarr2 acts as an essential component of the dishevelled-mediated PCP/RhoA pathway to regulate CE movements in Xenopus development. First, xβarr2 was expressed in the dorsal mesoderm and ectoderm tissues that undergo CE movements. Second, gain- and loss-of-function studies showed that xβarr2 is required for proper CE movements, and normal cell polarization and intercalation without affecting cell differentiation. Third, xβarr2 was required for both activation and membrane recruitment of RhoA in noncanonical fz/dishevelled signaling. Fourth, xβarr2 interacted with the N-terminal quarter of Daam1 and RhoA, and activated RhoA by controlling Daam1 in CE movements. Finally, xβarr2 was relocalized with the specific variants of dishevelled that are important for RhoA activation.
Phosphorylation of dishevelled is necessary for its translocation to the cell membrane and is a prerequisite for noncanonical Wnt signaling activation (Rothbacher et al, 2000; Tada and Smith, 2000; Kinoshita et al, 2003; Ossipova et al, 2005; Park et al, 2005). However, how the phosphorylation and membrane localization of dishevelled are involved in noncanonical Wnt signaling activation was unknown. Given this, our investigation describes for the first time that βarr2 in the noncanonical PCP pathway acts as a mediator recognizing both the phosphorylation and membrane localization of dishevelled, and that this recognition activates Daam1, which in turn regulates RhoA activation in Xenopus CE movements. However, βarr2 in Wnt/PCP signaling is not necessary for the signaling role of dishevelled that regulates Rac1 activation. Consistent with this idea, recent reports claim that RhoA and Rac1 function in parallel to downstream of dishevelled in the noncanonical Wnt/PCP pathway (Habas et al, 2001, 2003) and a KM mutation might be not required for the βarr2 function related to dishevelled (Yu et al, 2007). In addition, xβarr2 preferentially interacted with phosphorylated Xdsh, and the DIX and DEP domains of Xdsh were the regions important for interaction with xβarr2. These results suggest that these regions of dishevelled are phosphorylated by some protein kinases in correlation with βarr2 in CE movements, although PKCα and Par-1 are not involved. As dishevelled is known to interact with a number of protein kinases (Wharton, 2003; Wallingford and Habas, 2005), it would be interesting to determine the phosphorylation sites in dishevelled and the protein kinases required for its interaction with βarr2.
βarr2 plays a pivotal role in desensitization of receptor–G protein coupling and signaling (Attramadal et al, 1992; Ferguson et al, 1996). In Xenopus, the Wnt/Ca2+ pathway is required for G-protein-mediated PKCα activation (Sheldahl et al, 1999; Choi and Han, 2002; Penzo-Mendez et al, 2003). However, we showed that xβarr2 depletion could not block the membrane accumulation of XPKCα induced by Xfz7. Furthermore, inhibition of Wnt/Ca2+ signaling using PTX and α-transducin could not block the membrane accumulation of xβarr2 induced by Xfz7 and could not rescue CE defects caused by βarr2 overexpression. These results suggest that βarr2 is not involved in noncanonical fz-mediated Ca2+ signaling.
The FH proteins, including Daam1, exhibit autoinhibitory regulation; the inactive state is maintained by intramolecular binding between the N-terminal region of the diaphanous inhibitory domain (DID) and the carboxyl region of the diaphanous autoregulatory domain (DAD) (Higgs, 2005). Indeed, C-Daam1, the carboxyl region including the FH domains and DAD, appears constitutively active, but N-Daam1 containing only the DID functions as a DN form (Habas et al, 2001). In particular, the proteins binding to the N-terminal domain disrupt the DID–DAD interaction, thus activating FH protein function (Higgs, 2005). However, the precise molecular mechanism by which Daam1 activation is regulated in the noncanonical PCP pathway remains unknown. Our study provided the novel finding that (1) xβarr2 interacts with the N-terminal quarters of hDaam1; (2) the defects in CE movements caused by xβarr2 are rescued by N-hDaam1; (3) N-hDaam1 blocks xβarr2-induced RhoA activation; (4) the membrane localization of Daam1 in DMZ is inhibited by xβarr2 depletion; (5) in the presence of xβarr2, phosphatase treatment can dramatically reduce binding between hDaam1 and Xdsh. Taken together, these findings suggest that βarr2 regulates RhoA activation by controlling Daam1 autoinhibition in Xenopus CE movements and βarr2 binding to phosphorylated dishevelled in the cell membrane is at least partly involved in the recruitment of Daam1 to dishevelled.
βarr2 directly activates JNK3 by acting as a scaffold that interacts with ASK, MKK4, and JNK3, but not JNK1 (McDonald et al, 2000). We demonstrated that βarr2 stimulates JNK phosphorylation in Xenopus, resulting in CE movements. However, xβarr2 did not interact with the Xenopus JNK1 that functions in the noncanonical Wnt signaling pathway to regulate Xenopus CE movements (Yamanaka et al, 2002) (data not shown). βarr2 mediated the dishevelled-mediated RhoA activation, and interacted with Daam1 and RhoA, but not Rac1. These results raise the possibility that βarr2-mediated activation of JNK in CE movements is mediated by RhoA, but not JNK3, which has a more limited pattern of expression (Davis, 2000), or Rac1. Supporting this idea, previous work demonstrated that JNK1 activation is regulated by RhoA in Xenopus and results in CE movements (Unterseher et al, 2004; Kim and Han, 2005) and in Drosophila tissue polarity (Strutt et al, 1997; Boutros et al, 1998; Fanto et al, 2000; Weber et al, 2000).
Two patterns of β-arrestin trafficking within the cell have been described (Luttrell and Lefkowitz, 2002). ‘Class A' receptors preferentially bind to βarr2 over βarr1 and dissociate from the βarr2 before internalization. In contrast, ‘class B' receptors bind equally well to both βarr1 and βarr2, and remain associated with them upon internalization. The formation of a transient receptor–β-arrestin complex (class A) favors recycling to the cell membrane, whereas the formation of a stable receptor-β-arrestin complex (class B) favors routing of the receptor to lysosomes for degradation (Luttrell and Lefkowitz, 2002). Recently, it has been shown that class B receptors lead to robust and persistent activation of ERK1/2 proteins localized on endosomes (DeFea et al, 2000; Tohgo et al, 2002, 2003). However, it is not known whether endocytosis of class A receptors also facilitate other aspects of cellular signaling. In cultured cells, βarr2 does not overlap with the cellular endocytic components in the cytoplasm. Moreover, like class A GPCRs, Fz4 dissociates from βarr2 before internalization, although what role this endocytosis plays is unclear (Chen et al, 2003). In this study, we demonstrated that inhibition of xβarr2 endocytosis negatively regulates xβarr2 function in CE movements that leads to activation of Wnt/PCP signaling. Likewise, in Xenopus DMZ tissues that were actively occupied in noncanonical Wnt/fz signaling, endogenous β2 adaptin and GFP xβarr2 colocalized at the plasma membrane, but not in the cytoplasm. Therefore, our combined results suggest that the physiological role of βarr2 is that it mediates clathrin-coated endocytosis of the Wnt/Fz complex in Xenopus CE movements and that fz internalization mediated by βarr2 has a role in promoting productive signal transduction. Wnt/Fz internalization in signal propagation is in agreement with recent studies in Drosophila, where endosomal trafficking facilitates the Wg signaling cascade (Rives et al, 2006; Seto and Bellen, 2006), although the role of βarr2 in this internalization remains unclear. In support of this possibility, we have a preliminary result that stimulation by noncanonical Wnt ligands (e.g. Xwnt11 and Xwnt5a) leads to internalization of Xfz7 in animal cap cells of Xenopus embryos (unpublished data).
In conclusion, our finding provides direct evidence for the physiological and functional importance of βarr2-mediated noncanonical Wnt signaling in morphogenetic movements during Xenopus gastrulation. On the molecular and physiological levels, this is the first study to demonstrate βarr2-mediated receptor regulation where βarr2 is recruited to receptors indirectly by the involvement of other adaptor proteins (Spiegel, 2003). From this, we propose a molecular mechanism linking noncanonical PCP signaling from fz to RhoA: (1) noncanonical Wnt protein activates the fz receptor resulting in hyperphosphorylation of dishevelled, followed by translocation of dishevelled to the cell membrane; (2) βarr2 preferentially interacts with phosphorylated dishevelled and specifically recruits Daam1 to dishevelled; and (3) Daam1 activates RhoA by recruitment of a currently undefined RhoA-GEF. Future experiments are warranted to elucidate further the molecular mechanisms underlying CE movements by identification and characterization of proteins that functionally interact with βarr2 and/or Daam1 in noncanonical Wnt signaling.
Materials and methods
Xenopus embryos and microinjection
Xenopus laevis was purchased from Xenopus I and Nasco. Eggs were obtained from Xenopus laevis primed with 800 U of human chorionic gonadotropin (Sigma). In vitro fertilization was performed as described previously (Newport and Kirschner, 1982), and developmental stages of the embryos were determined according to Nieuwkoop and Faber (1967). Microinjection was carried out in 0.33 × Modified Ringer (MR) containing 4% Ficoll-400 (GE healthcare) using a Nanoliter Injector (WPI). Injected embryos were cultured in 0.33 × MR until stage 8 and then transferred to 0.1 × MR until they had reached the appropriate stage for the experimentation outlined below.
Plasmids, RNA synthesis, and MOs
For expression in Xenopus embryos, the entire coding region of xβarr2 was cloned into the XhoI and XbaI sites of the pCS2+, HA-pCS2+, and EGFP-pCS2+ vector. pCS2+ and GFP xβarr2 were linearized with Asp718. GFP-hDaam1 was constructed as N-terminal fusion of hDaam1 cDNA (Habas et al, 2001) with EGFP-pCS2+ vector. Antisense MOs were obtained from Gene Tools. The MO sequences were, for xβarr2 MO, 5′-GAAGATGGGGGAGAGGGCGGGGACC-3′, and, for Co MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′. The MO of Xdsh used was as described previously (Sheldahl et al, 2003). For specificity assay of xβarr2 MO, xβarr2 was cloned into the BamHI and ClaI sites of the myc-pCS2+ vector. myc xβarr2 was linearized with Asp718. Capped mRNAs were synthesized from linearized plasmids using the mMessagae mMachine kit (Ambion).
In situ hybridization and RT–PCR
Whole-mount in situ hybridization was performed with digoxigenin-labeled probes as described by Harland (1991). An antisense in situ probe against xβarr2 was generated by linearizing the pGEM-T xβarr2 construct with HindIII and transcribing with the SP6 RNA polymerase. RT–PCR analyses were carried out as described elsewhere (Kim and Han, 2005). The forward and reverse primers for xβarr2 were 5′-ACACCCTGACCCCTCTGCTGT-3′ and 5′-GTCGGAATACCCTCAGACAGATGT-3′, respectively. Primers for Chordin, Xbra, and Goosecoid were used as described online by De Robertis (www.hhmi.ucla.edu/derobertis/index.html). Primers for ODC, Siamois, and Xnr3 were also used as described online (Xenbase, www.xenbase.org/xmmr/Marker_pages/primers.html).
Immunoprecipitation and immunoblotting
HEK293FT cells (Invitrogen) were transfected with the indicated constructs for 48 h using Lipofectamine-Plus Reagent (Invitrogen) and then resuspended in immunoprecipitation (IP) buffer (50 mM HEPES/NaOH (pH 7.5), 3 mM EDTA, 3 mM CaCl2, 80 mM NaCl, 1% Triton X-100, 5 mM DTT). Cells were disrupted and centrifuged so as to remove insoluble debris. The indicated antibodies were added to the supernatants and incubated at 4°C for 6 h. Protein A sepharose (Zymed) was added and the mixture was incubated at 4°C for 3 h. The immunocomplexes bound to Protein A beads were washed five times with IP buffer. Mouse anti-myc monoclonal, mouse anti-GFP monoclonal, mouse anti-HA monoclonal, mouse anti-βarr2 monoclonal (Santa Cruz Biotechnology), and mouse anti-Flag monoclonal antibodies (Sigma) were used for IP and immunoblot analysis. To dephosphorylate cell extracts, lysates were treated with 5 U of potato acid phosphatase (PAP; Sigma) at 37°C for 1 h and then immunoprecipitated as described above.
RhoA activity assay
Isolation of activated RhoA from DMZ or VMZ tissues was performed by affinity for the protein-binding domain of Rhotekin (GST-C21). These tissues were dissected at stage 10.5, cultured until stage 12, and lysed in the lysis buffer. GST-RBD fusion protein was produced from the bacteria transformed with pGEX3X-GST-RBD. GST-RBD binding assay was performed as described (Park et al, 2006). The quantity of protein in each sample was determined using BCA protein assay reagent (Pierce).
Immunofluorescence microscopy and immunostaining
The subcellular localization of proteins was monitored using an assay described previously (Miller et al, 1999b). Dissected animal caps and DMZ cells fixed in 4% paraformaldehyde in PBS for 2 h and then rinsed in PBS and directly mounted for GFP-tagged proteins. Alternatively, they were permeabilized in ice-cold Dent's fix (80% MeOH and 20% DMSO) and incubated in PBSTB (PBS, 0.1% Triton X-100, and 2% BSA) to block nonspecific binding, followed by a standard immunostaining procedure. Image analysis was performed using a confocal laser-scanning microscope (Olympus, FluoView (FV) 1000). The antibodies for immunofluorescence were mouse anti-myc (1:500 dilution, Santa Cruz) and mouse anti-β2 adaptin (1:1000 dilution, BD Biosciences) primary antibodies, and fluorescein isothiocyanate-labeled (1:200 dilution, Sigma) and Alexa Fluor 594 (1:150 dilution, Molecular Probes) goat antimouse secondary antibodies.
The procedure for observing cells during CE movements was basically carried out as described (Wallingford et al, 2000) with some modification. Explants were isolated at stage 10.25 and cultured on a coverglass coated with fibronectin (0.1 mg/ml F1141; Sigma-Aldrich). The observation was performed in the stages, 14 and 15 and 18 and 19 using a confocal laser-scanning microscope (Olympus, FluoView (FV) 1000).
DMZ elongation assay
Embryos were injected with mRNA into either DMZ at the four-cell stage embryos. DMZ explants were excised at stage 10.5 and were cultured in 1 × MR containing 10 μg/ml of bovine serum albumin, 50 μg/ml of gentamycin, and 5 μg/ml of streptomycin, until stage 18.
Statistical analysis
Data were analyzed by two-tailed t-test. Values in graph were expressed as mean±s.d.
Supplementary Material
Supplementary Figures
Acknowledgments
We are grateful to Dr R Moon, J Wallingford, J Green, X He, I Daar, H Steinbeisser, N Kinoshita, and N Ueno for the generous gifts of plasmids. We also thank Dr Yunje Cho and Jeong-Ho Chang for purification of GST fusion protein. Finally, we acknowledge the other members of our laboratory for reading this manuscript and providing constructive criticism. This work was supported by the Advanced Basic Research Laboratory Program (R14-2002-012-01001-0) and Pure Basic Research Group (070-2005-C00115) of the KRF, and Brain Korea 21 project.
References
- Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ (1992) Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem 267: 17882–17890 [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N (1998) Differential recruitment of dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev 12: 2610–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94: 109–118 [DOI] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ (2003) Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of frizzled 4. Science 301: 1391–1394 [DOI] [PubMed] [Google Scholar]
- Choi SC, Han JK (2002) Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol 244: 342–357 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW (2000) beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148: 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, Weber U, Strutt DI, Mlodzik M (2000) Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol 10: 979–988 [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE III, Colapietro AM, Barak LS, Menard L, Caron MG (1996) Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271: 363–366 [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH (1999) Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J 18: 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/frizzled signaling is required for vertebrate gastrulation. Genes Dev 17: 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X (2001) Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel formin homology protein Daam1. Cell 107: 843–854 [DOI] [PubMed] [Google Scholar]
- Harland RM (1991) In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol 36: 685–695 [DOI] [PubMed] [Google Scholar]
- Heasman J (2002) Morpholino oligos: making sense of antisense? Dev Biol 243: 209–214 [DOI] [PubMed] [Google Scholar]
- Higgs HN (2005) Formin proteins: a domain-based approach. Trends Biochem Sci 30: 342–353 [DOI] [PubMed] [Google Scholar]
- Hyodo-Miura J, Yamamoto TS, Hyodo AC, Iemura S, Kusakabe M, Nishida E, Natsume T, Ueno N (2006) XGAP, an ArfGAP, is required for polarized localization of PAR proteins and cell polarity in Xenopus gastrulation. Dev Cell 11: 69–79 [DOI] [PubMed] [Google Scholar]
- Keller R, Danilchik M (1988) Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development 103: 193–209 [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK (2005) JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn 232: 958–968 [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N (2003) PKC delta is essential for dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev 17: 1663–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Goodman OB Jr, Keen JH, Benovic JL (1997) Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem 272: 15011–15016 [DOI] [PubMed] [Google Scholar]
- Kuhl M (2002) Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Semin Cell Dev Biol 13: 243–249 [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG (2000) The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem 275: 23120–23126 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by beta-arrestins. Science 308: 512–517 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Whalen EJ (2004) beta-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol 16: 162–168 [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB (1995) Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81: 85–94 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ (2002) The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115: 455–465 [DOI] [PubMed] [Google Scholar]
- Malbon CC (2004) Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front Biosci 9: 1048–1058 [DOI] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ (2000) Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290: 1574–1577 [DOI] [PubMed] [Google Scholar]
- Medina A, Reintsch W, Steinbeisser H (2000) Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech Dev 92: 227–237 [DOI] [PubMed] [Google Scholar]
- Medina A, Steinbeisser H (2000) Interaction of frizzled 7 and dishevelled in Xenopus. Dev Dyn 218: 671–680 [DOI] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT (1999a) Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene 18: 7860–7872 [DOI] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT (1999b) Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol 146: 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L (2002) Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet 18: 447–455 [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M (1982) A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30: 675–686 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J (1967) Normal Table of Xenopus laevis (Daudin). Amsterdam: North-Holland [Google Scholar]
- Ossipova O, Dhawan S, Sokol S, Green JB (2005) Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev Cell 8: 829–841 [DOI] [PubMed] [Google Scholar]
- Park E, Kim GH, Choi SC, Han JK (2006) Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation movements. Dev Biol 292: 344–357 [DOI] [PubMed] [Google Scholar]
- Park TJ, Gray RS, Sato A, Habas R, Wallingford JB (2005) Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr Biol 15: 1039–1044 [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez A, Umbhauer M, Djiane A, Boucaut JC, Riou JF (2003) Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev Biol 257: 302–314 [DOI] [PubMed] [Google Scholar]
- Perry SJ, Lefkowitz RJ (2002) Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol 12: 130–138 [DOI] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 18: 578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S (2006) Endocytic trafficking of Wingless and its receptors, arrow and DFrizzled-2, in the Drosophila wing. Dev Biol 293: 268–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE (2000) Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J 19: 1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ (2006) Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol 173: 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT (1999) Protein kinase C is differentially stimulated by Wnt and frizzled homologs in a G-protein-dependent manner. Curr Biol 9: 695–698 [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT (2003) Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 161: 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ (2003) Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J 375: 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT (1997a) Interaction of Wnt and a frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390: 410–413 [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT (1997b) Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol 182: 114–120 [DOI] [PubMed] [Google Scholar]
- Spiegel A (2003) Cell signaling. beta-arrestin—not just for G protein-coupled receptors. Science 301: 1338–1339 [DOI] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M (1997) The role of RhoA in tissue polarity and frizzled signalling. Nature 387: 292–295 [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC (2000) Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via dishevelled, but not through the canonical Wnt pathway. Development 127: 2227–2238 [DOI] [PubMed] [Google Scholar]
- Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM (2003) The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem 278: 6258–6267 [DOI] [PubMed] [Google Scholar]
- Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM (2002) beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem 277: 9429–9436 [DOI] [PubMed] [Google Scholar]
- Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, Schambony A (2004) Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. EMBO J 23: 3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM (2002) Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell 2: 695–706 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R (2005) The developmental biology of dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132: 4421–4436 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405: 81–85 [DOI] [PubMed] [Google Scholar]
- Weber U, Paricio N, Mlodzik M (2000) Jun mediates frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development 127: 3619–3629 [DOI] [PubMed] [Google Scholar]
- Wharton KA Jr (2003) Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol 253: 1–17 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14: 59–88 [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E (2002) JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3: 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T (2007) Association of dishevelled with the clathrin AP-2 adaptor is required for frizzled endocytosis and planar cell polarity signaling. Dev Cell 12: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures


