Abstract
Patch-clamp recording has revolutionized the study of ion channels, transporters, and the electrical activity of small cells. Vital to this method is formation of a tight seal between glass recording pipette and cell membrane. To better understand seal formation and improve practical application of this technique, we examine the effects of divalent ions, protons, ionic strength, and membrane proteins on adhesion of membrane to glass and on seal resistance using both patch-clamp recording and atomic force microscopy. We find that H+, Ca2+, and Mg2+ increase adhesion force between glass and membrane (lipid and cellular), decrease the time required to form a tight seal, and increase seal resistance. In the absence of H+ (10−10 M) and divalent cations (<10−8 M), adhesion forces are greatly reduced and tight seals are not formed. H+ (10−7 M) promotes seal formation in the absence of divalent cations. A positive correlation between adhesion force and seal formation indicates that high resistance seals are associated with increased adhesion between membrane and glass. A similar ionic dependence of the adhesion of lipid membranes and cell membranes to glass indicates that lipid membranes without proteins are sufficient for the action of ions on adhesion.
INTRODUCTION
The widely used patch-clamp recording technique allows currents to be recorded from single ion channels in small patches of membrane (single-channel recording) and also from small cells (whole cell recording) (1). In this technique, a glass pipette with a tip diameter of typically 0.5–2 μm is touched to the surface of a cell and a weak suction applied. After typically <1 min, a seal is formed between the cell membrane and the glass pipette with a resistance of >1 gigaohm (GΩ). This tight seal is often referred to as a giga seal. In forming the seal, the membrane patch usually moves 10–50 μm down the inside of the pipette, giving a large contact surface between membrane and glass (2–7). The seal forces are so great that the patch of membrane in the pipette can be excised from the cell for inside out recording or disrupted with high suction (or voltage) for whole cell recording without compromising the tight seal (1). Because of the high resistance of the seal, currents <1 pA are easily measured.
Although the patch-clamp technique is widely used, little information is available about the nature of the molecular interactions underlying the generation of the seal between membrane and glass. Forces that could influence the glass-membrane interaction include electrostatic forces, van der Waals forces, hydration forces, and steric forces (8–12). A better understanding of the factors involved in forming tight seals may give some insight into the mechanism underlying tight seal formation and also serve as a practical guide to facilitate patch-clamp recording.
In this study we examine the effects of divalent ions, pH, and ionic strength on interactions between patch pipette glass and membranes using two different techniques. In the first approach, we use the atomic force microscope (AFM) to measure the force holding membrane to glass. In the second, we measure the time to tight seal formation and the resistance of the tight seals using the patch-clamp technique. Both of these rather different approaches indicate that seals are tighter in the presence of Ca2+, Mg2+, and H+. Seals are not formed in the absence of divalent ions (<10−8 M) and H+ (10−10 M). When Ca2+ and Mg2+ are buffered to low levels, H+ at 10−7 M (pH 7) is sufficient to allow seals to be formed. A positive correlation between total adhesion force and seal formation indicates that high resistance seals are associated with increased total adhesion between membrane and glass.
MATERIALS AND METHODS
AFM force measurements
A custom-built AFM (13,14) was used to measure the mechanical force interaction between glass pipettes and either cell membranes or lipids. The base of the force-measuring cantilever was mounted on a piezoelectric translator equipped with electronic feedback to control the vertical position and movement of the cantilever (Physik Instruments, Karlsruhe, Germany; www.physikinstrumente.com). The force apparatus was combined with an inverted light microscope to permit visualization of the cantilever and glass pipette. The interaction between the tip of the cantilever coated with either lipid or a cell, and the glass of a pulled patch pipette (1.5 mm outside diameter, 0.86 mm inside diameter borosilicate glass; Harvard Apparatus, Holliston, MA) was determined from the deflection of the cantilever. Contact was made with the glass near, but not touching, the tip of the pulled pipette in case the pulling process itself changed the properties of the glass. A laser spot was reflected off the top side of the cantilever into a two-segment photodiode. Custom software was used to control the movement of the piezoelectric translator and timing of the measurements.
For measurements of the interaction between cell membrane and pulled glass pipette, the tip of the cantilever was coated with HEK 293 or NIH 3T3 cells. Two methods were used to coat the cantilever tip with a cultured cell: 1) Cantilevers were first rinsed with ethanol, coated with poly-L-lysine, and then placed in the bottom of culture dishes. NIH 3T3 cells were then grown in the culture dishes and on the cantilevers for 4–6 days (maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin 50 units/ml, and streptomycin 50 μg/ml). Five different cantilevers were imaged using scanning electron microscopy, and in all cases the scan revealed the presence of cells on the cantilever shafts and tips. 2) Alternatively, individual suspended HEK 293 cells were captured on the tip of a polylysine-coated cantilever. In this approach, HEK 293 cells were first cultured separately from the cantilevers. Using the inverted microscope of the chamber apparatus, a single cell was then selected and picked up on the tip of the cantilever by lowering it onto the surface of the selected cell. The cantilever was retracted after a latency of 3 s. The position of the cell on the cantilever was then verified using the inverted microscope of the force apparatus. Both gold-coated and transparent Si3N4 cantilevers (Veeco Instruments, Santa Barbara, CA) with a length of 200 μm, width of 40 μm, and radius tip of 20–60 nm were used. Exposure to solutions at pH 4 and 10 was limited by using a fast solution exchange to avoid possible damage to cells under conditions of extreme pH.
For measurements of glass-lipid interactions, L-alpha-phosphatidylcholine (egg), phosphatidylinositol, or phosphatidylserin (Avanti Polar Lipids, Alabaster, AL) were dissolved with one part lipid and four parts chloroform (v/v), and the solvent was evaporated under N2. A phosphate buffer solution (150 mM NaCl plus 10 mM 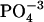 (as Na+), adjusted to pH 7.3) was added to dilute the lipid film to a final concentration of 0.2 mg/ml and thoroughly sonicated before 50 μl of the suspension was applied onto the cantilever. After 20 min, the cantilever was dipped in dionized water to dispose of excess lipid.
(as Na+), adjusted to pH 7.3) was added to dilute the lipid film to a final concentration of 0.2 mg/ml and thoroughly sonicated before 50 μl of the suspension was applied onto the cantilever. After 20 min, the cantilever was dipped in dionized water to dispose of excess lipid.
Analysis of the force data from the AFM was performed using custom software (13,14). Each AFM cycle determined the force versus distance relationship between the cantilever and the glass during a single approach and retraction of the cantilever. Typical approach and retraction speed was ∼1.3 μm/s. In each experiment, typically 10 force measurement cycles were averaged for each solution, with a 5–10-s delay between each cycle. For each AFM experiment, measurements were made using the same cantilever touching the same pipette for the different bath solutions to remove variability that could occur among different cantilevers, presumably due to differences in the coating of the cantilevers with cells or lipids. We found no significant difference in AFM adhesive force for first and second measurements between coated cantilevers and glass (see Results) or after small lateral movements of the cantilevers so that contact was made in a different place with the glass. Thus, unlike patch-clamping where a pipette can typically be used only once, a measurement of adhesive force has little effect on the properties for additional measurements.
Measurement of tight seal formation
The time required to form a tight seal with the patch-clamp technique and the tightness of the seal, as measured in ohms, was determined for the formation of on-cell seals between borosilicate glass pipettes and HEK 293 cells. The pipette was filled with the test solution and the cell was superfused with the same solution just before seal formation. An effort was made to control the visual shape of the patch pipettes and also the size and shape of the cells used in the experiments for uniformity. In addition, the magnitude of the suction was controlled and measured with a water manometer. A new pipette was used for each seal test.
Solutions
For experiments examining the effects of H+ on adhesion force and seal resistance, the solutions contained (mM): 150 KCl, 5 TES (N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid) pH buffer, and 2 EGTA. For experiments examining the effects of Ca2+ and Mg2+, the solutions contained (mM): 150 KCl, 5 TES pH buffer, 1 EGTA, 1 N-(2-hydroxyethyl)ethylenedi-amine-N,N′,N′-triacetic acid (HEDTA), and 0 or 10 CaCl2 or MgCl2; the KCl was reduced to 1 mM when 100 mM CaCl2 or MgCl2 was examined. The free concentrations of the divalent ions listed below were calculated with Maxchelator (WINMAXC v2.50, http://www.stanford.edu/∼cpatton/maxc.html), which takes the pH dependence of EGTA and HEDTA into account. For no added divalents at pH 7.0 and 10.0 the free concentration of Ca2+ was <10−8 M and of Mg2+ was <10−6 M when taking into account estimated contaminant divalents. For no added divalents at pH 4 the free concentration of Ca2+ and Mg2+ was ∼10−6 M. For 10 mM added Ca2+, the free concentration of Ca2+ at both pH 7.0 and 10.0 was 8.0 mM, and for 10 mM added Mg2+, the free concentration of Mg2+ at pH 7.0 and 10.0 was 8.8 and 8.0 mM, respectively. For pH 4.0 with 10 or 100 mM added Ca2+ or Mg2+ and also for pH 7.0 and 10.0 with 100 mM added Ca2+ or Mg2+, the free concentration of divalents was within 2% of the added concentrations. References in the text to solutions with 10 and 100 mM added divalent will be to the amount of added divalent. For experiments to examine the effect of ionic strength on lipid-glass interaction, the solutions contained (mM): 5 TES and 2 EGTA and 0.1–1000 NaCl, with the pH adjusted to 7.0.
Data are presented as mean ± SE; groups were compared with Student's t-test. Experiments were carried out at room temperatures of 21°C–23°C.
RESULTS
H+ and Ca2+ increase the attractive force between glass and cell membranes
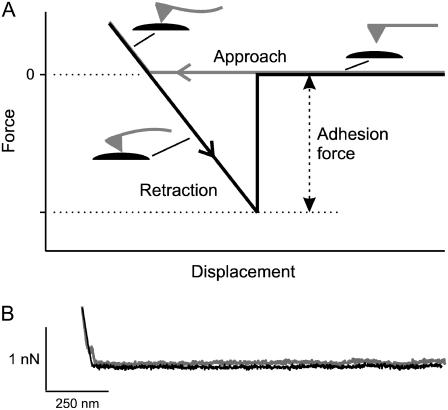
The AFM was used to measure the strength of the attraction force between glass and membrane to investigate factors involved in formation of tight seals with the patch-clamp technique. This was done by coating the cantilever of the AFM either with lipids or with cells, touching the coated cantilever to the glass near the tip of the pulled pipette, and then retracting the cantilever to measure the force to rupture the contact between membrane and glass. Fig. 1 A presents a schematic of a single cycle of an AFM measurement. The cantilever is moved downward to contact the glass (approach). Before contact the force level is zero, but once contact is made, there is an increasing positive force as the approach continues, progressively bending the cantilever upward. The cantilever is then retracted at a steady rate, which first relieves the positive force and then results in a negative force due to the adhesion between glass and membrane, which progressively bends the cantilever downward. When the force applied to the cantilever exceeds the adhesion force, the contact between membrane and glass ruptures, with a return of the cantilever to the zero force level. The difference between the force at the rupture and the force of the approach scan before contact is made indicates the adhesion force between the membrane and glass. Fig. 1 B shows traces from an experiment in which a bare cantilever was used, demonstrating that bare cantilevers (no coat of membrane) do not adhere to glass, as there was no adhesion force when the cantilever was retracted from the glass after contact was made.
FIGURE 1.
Measuring the adhesion force between membrane and glass with an AFM. (A) Schematic representation of a theoretical force-displacement curve recorded with an AFM for contact of a membrane-coated cantilever tip with a glass pipette. The adhesion force between the membrane and glass is taken as the maximum force reached (at the time the membrane and glass separate) as the cantilever tip is retracted. The force during the approach of the cantilever tip to the glass is plotted in gray, and the force during the retraction of the cantilever tip is plotted in black. See text for further details. (B) The adhesion force between a cantilever tip without membrane coating and the glass of a patch pipette is negligible because no increase in force (no adhesion) is seen when the cantilever tip is retracted from the pipette. In this example, [H+] was 10−7 M and [Ca2+] was 10 mM. The black trace was displaced downward slightly to avoid overlap so that it can be seen.
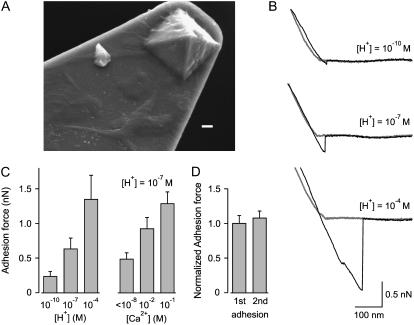
To examine the interaction between cell membranes and glass, we cultured cells on the cantilevers and then repeated the experiment shown in Fig. 1 B. Fig. 2 A shows a scanning electron micrograph of the tip of a cantilever on which NIH 3T3 cells were cultured so that the cantilever and its tip were covered with cells and their processes. Fig. 2 B presents AFM force displacement scans for contact between cell membrane and glass to measure the effect of three H+ concentrations on adhesion forces in the absence of Ca2+. It is practical knowledge that Ca2+ in the pipette can increase seal formation (8), perhaps by interacting with negative charges on the surface of the glass (8). If such interactions are required for membrane-glass adhesion, then removing divalent cations from the solution together with H+, which is highly effective at negating charge, even at very low concentrations (15), might be expected to reduce or eliminate membrane-glass adhesion. Indeed, with 10−10 M H+, the adhesion force was small (0.075 nN). Increasing the [H+] to 10−7 and 10−4 M then increased the adhesion force to 0.31 and 1.4 nN, respectively. Results from seven experiments are shown in Fig. 2 C (left panel). Increasing [H+] from 10−10 M to 10−4 M increased the mean adhesion force ∼5.6-fold (p < 0.003, n = 7). The effects of hydrogen ions on membrane-glass interaction were reversible, indicating that the membrane was not damaged during the experiment. If increased adhesive forces facilitate seal formation, then the observations in Fig. 2, B and C, suggest that increasing [H+] should also facilitate seal formation. This is shown to be the case in a later section of the work.
FIGURE 2.
H+ and Ca2+ increase the adhesive force between cell membranes and glass. Cantilever tips covered with cell membranes were touched to the glass of a pulled glass pipette and retracted using an AFM. (A) Scanning electron micrograph of a cantilever tip on which NIH 3T3 cells were cultured. Cell bodies and cell processes on the cantilever and tip can be seen. Bar = 1 μm. (B) Representative force-displacement curves for 10−10, 10−7, and 10−4 M H+. The adhesion force was small for 10−10 H+ (0.075 nN) and increased to 0.31 and 1.4 nN as the [H+] was increased to 10−7 and 10−4 M, respectively. (C) Increasing [H+] or [Ca2+] increases the adhesion force between cell membrane and glass. Each histogram bar presents the average response from 7 experiments (left panel) and 7–9 experiments (right panel). (D) Adhesive force between cell membrane and glass is not significantly different for first and second contacts. Data are normalized to the force for the first contact and present the average of 10 experiments. In this and the following figures, the [Ca2+] is <10−8 M and the Mg2+ is ≤10−6 M unless specifically indicated.
As mentioned above, it is practical experience that the presence of divalent cations in the solution can facilitate the formation of gigaohm seals when patch-clamping (8). If the adhesion forces between membrane and glass are related to seal resistance in patch-clamp recording, it might be expected that divalent cations will increase the adhesion force. Experiments examining the effect of Ca2+ on adhesion forces between cell membranes and glass are summarized in Fig. 2 C (right panel). Increasing [Ca2+] from 10−8 to 0.1 M at pH 7.0 increased the mean adhesion force ∼2.6-fold (p < 0.001, n = 7–9). The increased adhesion between cell membrane and glass with increased [Ca2+] gives a possible explanation for the practical observation that Ca2+ facilitates seal formation. Notice in Fig. 2 C that raising [H+] only ∼0.1 mM, from 10−7 to 10−4 M, has about the same effect on increasing adhesion force as raising [Ca2+] ∼100 mM, from <10−8 to 0.1 M.
The question arises as to whether the first contact between membrane and glass gives the same adhesive force as a second contact, as it is known from patch-clamping that it is much more difficult, if not impossible, to form a gigaohm seal a second time with the same pipette. To examine this question we examined AFM adhesion force for first and second contacts between cell membrane and glass. As shown in Fig. 2 D, which presents the average from 10 experiments, there was no significant difference, indicating that contact between glass and cell membrane followed by forceful detachment does not change the properties of the next cell membrane-glass interaction.
H+, Ca2+, and Mg2+ increase the attractive force between glass and lipids
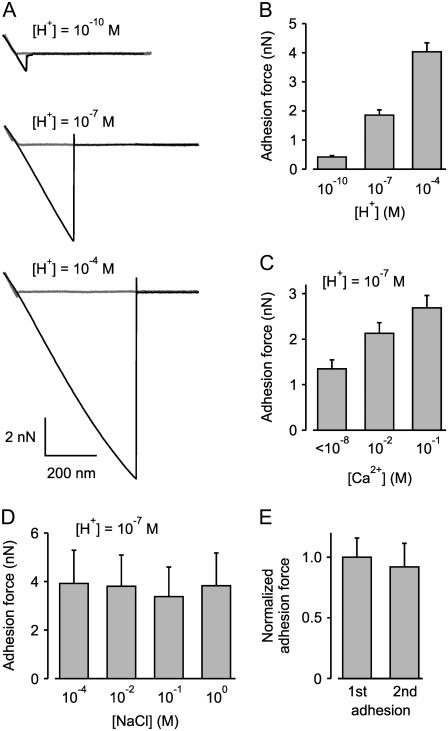
The above data indicate that Ca2+ and H+ increase the adhesion between cell membranes and glass. Corey and Stevens (8) suggested that it is the lipids in the cell membranes that are involved in the formation of the tight seal, with the proteins interfering in tight seal formation. Indicating lipid-glass interaction is that tight seals can be formed between patch pipettes and pure lipid membranes (16–18). If Ca2+ and H+ are acting to increase the attraction between the lipids of the cell membrane and glass, then it might be expected that they would have similar actions on lipid membranes without proteins. To examine this possibility, we coated the cantilevers and their tips with phosphatidylcholine and then examined the adhesion force after contact of the tips of the coated cantilevers with the glass of pulled patch pipettes. Results are shown in Fig. 3. Increasing [H+] from 10−10 to 10−4 M with no added divalent ions increased the mean adhesion force ∼9.7-fold (p < 0.01, n = 8), and increasing [Ca2+] from <10−8 to 0.1 M at pH 7.0 increased the mean adhesion force ∼1.8-fold (p < 0.04, n = 6–10). A [Ca2+] of 1 mM at pH 7.0 gave results similar to that of 10 mM (n = 5; data not shown). If Ca2+ acts through a general rather than specific effect to increase adhesion force, then it might be expected that Mg2+ should be able to replace Ca2+ in this action. Consistent with this idea, increasing [Mg2+] from ∼10−6 to 0.1 M at pH of 7.0 increased the mean adhesion force ∼5.8-fold (p < 0.005, n = 3; data not shown). When cantilevers were coated with either phosphatidylinositol or phosphatidylserine instead of phosphotidylcholine, increasing [H+] or [Ca2+] also increased the adhesion force in the 2–4 examined experiments for each lipid, but this was not studied in further detail. The increases in adhesive force between lipids and glass in the presence of H+, Ca2+, and Mg2+ were reversible.
FIGURE 3.
H+ and Ca2+ increase the adhesive force between lipid membranes and glass. Cantilevers and their tips were coated with phophatidylcholine (see Materials and Methods) and touched to the glass of a pulled glass pipette and then retracted using an AFM. (A) Representative force-displacement curves obtained with an AFM for 10−10, 10−7, and 10−4 M H+. The adhesion force increased from 0.87 nN to 5.0 and 9.7 nN as the [H+] was increased from 10−10 to 10−7 and 10−4 M, respectively. (B and C) Increasing [H+] or [Ca2+] increases the adhesion force between lipid membrane and glass. (D) Changing ionic strength by changing [NaCl] from 10−4 to 1 M has little effect on adhesion force, suggesting the effects of H+ and Ca2+ are not from changes in ionic strength of the solutions. (E) Adhesive force between lipid membrane and glass is not significantly different for first and second contacts. Data are normalized to the force for the first contact and present the average of seven experiments.
Increasing Ca2+ or Mg2+ to 100 mM would lead to increases in ionic strength. To examine whether it is the increases in ionic strength or specific action of the divalent ions that increase the adhesion, we examined the effect of increasing NaCl on adhesion forces. Fig. 3 D shows that the adhesion force between phosphatidylcholine and glass of pulled patch pipettes is independent of ionic strength for [NaCl] ranging from 10−4 to 1 M (p ∼ 0.3, n = 5–6). Thus, the observed effects of increasing Ca2+ and Mg2+ on adhesion force between membrane and glass (Figs. 2 and 3) are related to their specific properties and are not due to changes in ionic strength.
Fig. 3 E shows that a first contact between lipid and glass followed by forceful detachment does not change the properties of the next lipid-glass interaction. This observation is similar to that shown previously in Fig. 2 D for interaction between cell membrane and glass.
H+, Ca2+, and Mg2+ facilitate seal formation
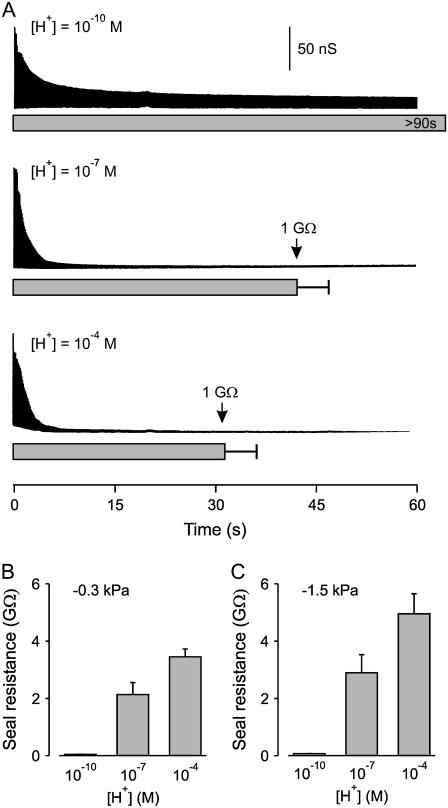
The observations in the previous sections indicate that H+, Ca2+, and Mg2+ increase adhesion forces between membrane and glass. If stronger adhesion forces contribute to tighter seals, then it might be expected that these ions would also facilitate seal formation and increase the resistance of the seal in a manner parallel to their effects on adhesion force. To examine whether H+, Ca2+, and Mg2+ facilitate seal formation, the time required to achieve a 1.0-GΩ seal to cell membrane was measured using standard patch-clamp recording techniques. When contact was first made between the patch pipette and cell membrane (defined as time zero and determined by an increase in pipette resistance), a constant suction of 0.3 kPa was applied to the pipette. Fig. 4 A shows the silhouette of current envelope response to a 5-mV square wave used to test seal resistance. When the [H+] in the solutions was 10−10 M with no added Ca2+ or Mg2+ (top silhouette), giga seal formation was not observed for examined times >90 s, as the resistance remained <0.05 GΩ (n = 21). Increasing the [H+] to 10−7 M then led to giga seal formation in 42 ± 5 s (Fig. 4 A, middle silhouette, p < 0.001, n = 23). Increasing [H+] to 10−4 M further reduced the time for giga seal formation to 31 ± 5 s (Fig. 4 A, lower silhouette, n = 24), although the reduction in time with increasing the [H+] from 10−7 to 10−4 M was not significant (p > 0.1).
FIGURE 4.
Increasing [H+] in the solutions decreases the time to seal formation and increases the seal resistance. (A) Plots of the silhouette of the current envelope resulting from a 5-mV square wave applied to the patch pipette after touching the patch pipette to the membrane surface of HEK 293 cells and applying suction of 0.3 kPa. With 10−10 M H+ the seal resistance decreased over time, but giga seals were not formed during >90 s of observation (n = 21). Increasing [H+] to 10−7 or 10−4 M lead to giga seal formation in 42 ± 5 and 31 ± 5 s, respectively. (B and C) The resistance of the seal can be increased by increasing the negative pressure applied to the patch pipette. Histograms of seal resistance observed after a 90-s application of 0.3 kPa (B) and 1.5 kPa (C) suction to the patch pipette at the indicated [H+]. Giga seals were not formed at either suction with 10−10 M H+. The higher suction increased the resistance 35% and 43% at 10−7 and 10−4 M H+, respectively, when compared to the resistance at the lower suction, with a significant increase at 10−4 M H+ (p = 0.03, n = 27 and 19).
To determine whether increasing [H+] also increased the resistance of the seal, maximum seal resistance after 90 s of 0.3 kPa suction was measured as a function of [H+] and plotted as a histogram in Fig. 4 B. The resistance of the seal increased significantly from 0.045 ± 0.004 to 2.1 ± 0.4 GΩ when [H+] was raised from 10−10 to 10−7 M (p < 0.0001, n = 21 and 28), and there was a further significant increase in seal resistance to 3.5 ± 0.3 GΩ when [H+] was raised further from 10−7 to 10−4 M (p < 0.015, n = 28 and 27).
The resistance of the seal depended also on the magnitude of suction applied to the patch pipette. We compared seal resistance as a function of [H+] with suction of either 0.3 kPa (Fig. 4 B) or 1.5 kPa (Fig. 4 C). The fivefold increase in suction to 1.5 kPa was still not sufficient to form giga seals at 10−10 M H+ but did increase the seal resistance ∼40% for both 10−7 and 10−4 M H+ (compare Fig. 4 B to Fig. 4 C).
We next examined whether Ca2+ would decrease the time to giga seal formation, as H+ did. A significant decrease in time to giga seal formation was observed when [Ca2+] was increased from a very low concentration (<10−8 M) to higher concentrations. The times to giga seal formation were 42 ± 5 s for <10−8 Ca2+ (n = 23), 16 ± 3 s for 0.01 M Ca2+ (n = 30), and 20 ± 3 s for 0.1 M Ca2+ (n = 30) (data not plotted). The decrease in times to giga seal formation for elevating [Ca2+] from <10−8 to either 0.01 or 0.1 M were significant (p < 0.0001 for both, n ≥ 23). The formation of gigaohm seals at the lowest Ca2+ (<10−8 M) examined in these experiments was expected because the experiments were carried out at pH 7.0, which is sufficient to promote giga seal formation with Ca2+ of <10−8 M (Fig. 4). It was shown in Fig. 4 A (upper silhouette) that giga seals were not formed in the absence of added Ca2+, Mg2+ (<10−8 M), and H+ (10−10 M).
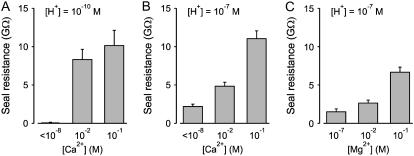
The decrease in time to gigaohm seal formation with increased Ca2+ was also associated with an increased maximum seal resistance. Seal resistance for 10−10 and 10−7 M H+ at three different [Ca2+] are compared in Fig. 5. With 10−10 M H+, giga seals were not formed with <10−8 M Ca2+ but were formed with 0.01 and 0.1 M Ca2+ (Fig. 5 A), with the resistance of the gigaohm seal for 0.1 M Ca2+ somewhat higher (10.2 ± 2.0 GΩ) than for 0.01 Ca2+ (8.3 ± 1.3 GΩ), but not significantly so (p > 0.4, n ≥ 13). With 10−7 M H+, gigaohm seals were formed at all three [Ca2+] examined, with the resistance increasing significantly (p < 0.001, n ≥ 33 for each increment in [Ca2+]) (Fig. 5 B).
FIGURE 5.
Increasing [Ca2+] or [Mg2+] in the solutions increases seal resistance. (A–C) Histograms of seal resistance for the indicated ionic conditions (n ranged from 10 to 35 for the various determinations). Giga seals were not formed with 10−10 M H+ and <10−8 M Ca2+. In A, seal resistance with 0.01 and 0.1 M Ca2+ was significantly greater than with <10−8 M Ca2+ (p < 0.0003) but were not significantly different from each other (p > 0.4). In B, each increase in [Ca2+] led to a significantly greater increase in seal resistance (p < 0.0001). In C, an increase in [Mg2+] from 0.01 to 0.1 M led to a significant increase in seal resistance (p < 0.0001).
If, as observed for adhesion force between lipid and glass, Ca2+ acts through a general rather than specific effect to increase seal resistance, then it might be expected that Mg2+ should be able to replace Ca2+ in tight-seal formation. Fig. 5 C shows that Mg2+ increased seal resistance in a manner similar to that of Ca2+ (compare to Fig. 5 B), but with smaller values for the maximum seal resistance.
Seal formation near physiological pH
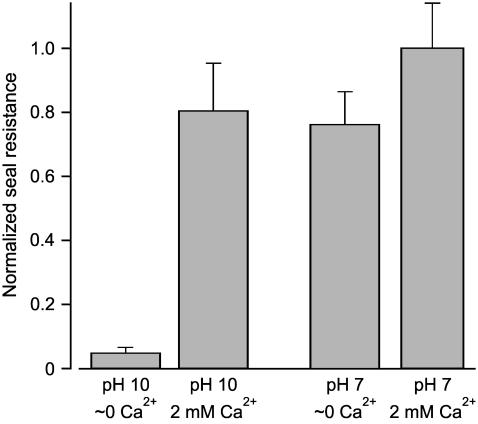
The above sections show that either H+ or Ca2+ is required for tight seal formation in the absence of Mg2+. The question arises as to the relative contribution of these two ions to seal formation under experimental conditions often used for patch-clamping in which Mg2+ is omitted. Results are shown in Fig. 6, where seal resistance is plotted as a fraction of the maximum seal resistance. With essentially no Ca2+, Mg2+ (<10−8 M), or H+ (10−10 M), tight seals were not formed; 2 mM Ca2+ or 10−7 M H+ led to tight seal formation with similar efficacy. The presence of 10−7 M H+ and 2 mM Ca2+ together gave a further small increase in seal resistance that was not statistically significant (p > 0.19, n ≥ 19). Therefore, both H+ and Ca2+ contribute to seal resistance for conditions often used for patch-clamping.
FIGURE 6.
Under physiological conditions, both H+ and Ca2+ contribute to the seal formation. Histograms of normalized seal resistance for various experimental conditions. At pH 10 (10−10 M H+) and ∼0 Ca2+ and Mg2+ (<10−8 M) giga seals were not formed. Increasing either [Ca2+] (2 mM) or [H+] (10−7 M) in the solutions then led to seal formation with equal efficacy. The combination of 2 mM Ca2+ and 10−7 M H+ increased seal resistance further, but not significantly.
DISCUSSION
This study compared the adhesion force between membrane and glass of patch pipettes with the resistance of the seals formed during patch-clamping and also with the time to formation of tight (gigaohm) seals. We found that H+, Ca2+, and Mg2+ increased adhesion forces between membranes (both lipid and cellular) and glass, decreased the time required to form a gigaohm seal, and increased seal resistance. At low concentrations of these ions (10−10 H+, <10−8 Ca2+, and Mg2+), tight seals were not formed and the adhesion forces were greatly reduced. Our experiments quantify the practical observations that divalent cations can facilitate seal formation (8) and also show that very low concentrations of H+ can substitute for divalent cations. It is the protons at pH 7.0 that allow seal formation in the absence of divalent cations. These results suggest that increased protons might prove useful to facilitate seal formation under conditions where seals are difficult to form or divalent ions must be excluded.
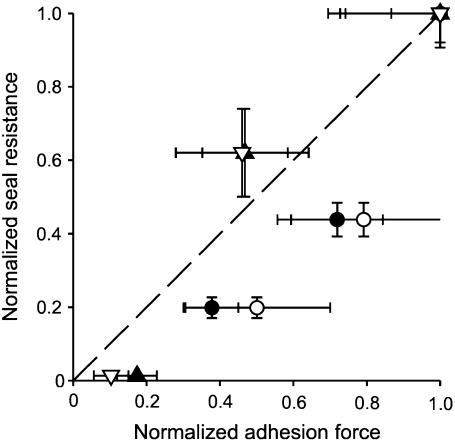
Our observations in Figs. 2–5 suggest a correlation between adhesion force and seal resistance. To examine this in greater detail, we plotted normalized seal resistance versus normalized adhesion force for each of the various conditions examined in our experiments. Results are shown in Fig. 7, indicating that ionic conditions that gave tighter seals were also generally associated with stronger adhesion forces. We cannot distinguish whether the greater adhesion force arises from a greater force per unit area of seal formation or from a greater contact area, as either might be expected to increase the total adhesion force and also the seal resistance. Nevertheless, the positive correlation between membrane-glass adhesion force and tight seal formation indicates that high resistance seals are associated with increased total attractive force between membrane and glass.
FIGURE 7.
Ionic conditions that increase adhesion force between membrane and pipette also increase seal resistance, suggesting that adhesive force contributes to seal resistance. Plot of normalized seal resistance against normalized adhesive force between pipette and cell-coated cantilevers (solid symbols) or lipid-coated cantilevers (open symbols). For each plotted point, the resistance data and adhesion data were obtained in different experiments but with the same ionic conditions: triangles, 10−10, 10−7, and 10−4 M [H+] all with no added Ca2+ or Mg2+; circles, <10−8, 0.01, and 0.1 M [Ca2+] all with 10−7 M H+. For each of the ions investigated (change in [H+] or [Ca2+]) the measured responses (adhesion force or patch-clamp seal resistance) were normalized to the response obtained with the highest concentration of that specific ion. The dashed line indicates a correlation of 1.0.
Our observation that the ionic dependence of AFM measured adhesive force was the same for lipid membranes and cell membranes (Figs. 2 and 3) indicates that membrane proteins were not required for the increased adhesive effects of H+ and Ca2+. It is already established that proteins are not necessary for seal formation, as indicated by tight seal formation between pure lipid membranes and glass (8,16–18).
In forming the tight seal for patch-clamping, the patch of membrane typically moves 10–50 μm down the inside of the pipette, giving a potentially large contact surface between membrane and glass (2–7). The seal is thought to be distributed over this area of contact and not localized at the tip of the pipette, as the membrane can be destroyed at the tip of the pipette and the seal is retained (3). The opposition of membrane to glass over the large area of seal must be close, with molecular dimensions ≤1–2 nm (12), to account for the high resistance of the seal (8). Consistent with close opposition and a large distributed area of the seal, the lateral diffusion of nystatin from the cell membrane outside of the patch pipette to the patch of membrane in the patch pipette is prevented by the patch-clamp seal between membrane and glass (19).
Although the membrane and glass are in close opposition in the distributed area of the seal, they do not adhere directly to each other, as there is a thin layer of water several molecules thick between lipid bilayer and glass (20–22). This thin layer of lubricating water allows lipid bilayers to spread along the surface of glass substrates (12). This thin layer of lubricating water would also enable the formation of gigaohm seals by allowing the membrane to flow up the inner surface of the glass pipette as the membrane patch is displaced into the pipette during patch formation. The farther the patch of membrane is displaced into the pipette, the greater would be the length of opposition between membrane and glass, giving a greater seal resistance (3). Hence, tight seal formation depends on a slippery (so the membrane can flow along the glass) but close (to increase the resistance) opposition of membrane and glass. Factors that would facilitate the membrane spreading along the glass surface might then be expected to facilitate seal formation. Cremer and Boxer (12) found that low pH facilitated lipid bilayer spreading on glass regardless of the net charge on the bilayer and suggested that the spreading process was driven by van der Waals forces. Our observations that low pH facilitated both seal formation and membrane-glass adhesion would be consistent with the possibility that low pH enhances the spread of the membrane into the pipette, increasing the length of the contact between membrane and glass.
The relative contributions of the various factors involved in forming a close opposition between membrane and glass and in the spreading of membrane over glass are unclear. Electrostatic forces, van der Waals forces, hydration forces, and steric forces may all contribute (8–12). The structure of the surface of glass with −OH and −O groups and possible factors involved in attraction of glass and phospholipids headgroups have been discussed by Corey and Stevens (8) and will not be repeated here. Our results give little information about the relative contributions of the various possible forces, but an extension of our approach over wider experimental conditions may help to provide such information. What we have done in this study is quantify the effects of divalent cations and H+ on membrane glass adhesion forces and seal formation, which can have immediate practical applications when performing patch-clamping.
It should be cautioned that the resistance of the patch of membrane and the resistance of the seal between the membrane and glass act in parallel to contribute to seal resistance as measured in our studies. Thus, treatments that decrease the leakage of the patch of membrane would increase the apparent seal resistance, even though they may have little effect on the actual seal between membrane and glass. Raising [H+] increases the conductance of lipid membranes (23), so the greater seal resistance induced by H+ in our studies (excluding effects on possible ion channels) would be through changes in the length or specific resistance of the membrane-glass seal and not from changing the resistance of the patch of membrane. The positive correlation between adhesion force and seal resistance for increases in [Ca2+] in our studies would also suggest, although indirectly, that the increase in seal resistance with increasing Ca2+ may also be mainly through changes in the membrane-glass seal. Nevertheless, although our experiments do not directly distinguish between the effects of the various ions on membrane patch resistance and membrane-glass seal resistance, they do measure “seal resistance” in the manner traditionally used during patch-clamp recording. Consequently, the findings presented here will be practically applicable to the formation of tighter seals when patch-clamping.
Acknowledgments
We thank Miodrag Micic for carrying out the scanning EM of the cantilevers and Felix Rico for performing some of the AFM experiments.
This work was supported by grants from The Israel Science Foundation founded by the Israel Academy of Sciences and Humanities and the Zlotowski Center for Neuroscience to S.D.S., from the National Institutes of Health (GM55611) to V.T.M., and from the National Institutes of Health (AR32805) and the Muscular Dystrophy Association to K.L.M. Z.G. was a recipient of the Kreitman Doctoral Fellowship and the Folks Foundation Graduate Fellowship.
Avi Priel and Ziv Gil contributed equally to this work.
Ziv Gil's present address is Dept. of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY 10021.
Shai D. Silberberg's present address is Molecular Physiology and Biophysics Section, Porter Neuroscience Research Center, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892.
References
- 1.Hamill, O. P., A. Marty, E. Neher, B. Sakmann, and F. J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- 2.Milton, R. L., and J. H. Caldwell. 1990. How do patch clamp seals form? A lipid bleb model. Pflugers Arch. 416:758–762. [DOI] [PubMed] [Google Scholar]
- 3.Sokabe, M., and F. Sachs. 1990. The structure and dynamics of patch-clamped membranes: a study using differential interference contrast light microscopy. J. Cell Biol. 111:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokabe, M., F. Sachs, and Z. Q. Jing. 1991. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys. J. 59:722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruknudin, A., M. J. Song, and F. Sachs. 1991. The ultrastructure of patch-clamped membranes: a study using high voltage electron microscopy. J. Cell Biol. 112:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinlaja, J., and F. Sachs. 1998. The breakdown of cell membranes by electrical and mechanical stress. Biophys. J. 75:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil, Z., S. D. Silberberg, and K. L. Magleby. 1999. Voltage-induced membrane displacement in patch pipettes activates mechanosensitive channels. Proc. Natl. Acad. Sci. USA. 96:14594–14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey, D. P., and C. F. Stevens. 1983. Science and technology of patch-recording electrodes. In Single-Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press, New York. 53–68.
- 9.Israelachvili, J. 1992. Intermolecular and Surface Forces. Academic Press, New York.
- 10.Israelachvili, J. N., and P. M. McGuiggan. 1988. Forces between surfaces in liquids. Science. 241:795–800. [DOI] [PubMed] [Google Scholar]
- 11.Israelachvili, J., and H. Wennerstrom. 1996. Role of hydration and water structure in biological and colloidal interactions. Nature. 379:219–225. [DOI] [PubMed] [Google Scholar]
- 12.Cremer, P. S., and S. G. Boxer. 1999. Formation and spreading of lipid bilayers on planar glass supports. J. Phys. Chem. B. 103:2554–2559. [Google Scholar]
- 13.Zhang, X. H., E. Wojcikiewicz, and V. T. Moy. 2002. Force spectroscopy of the leukocyte functional-associated antigen-1 (LFA-1)/intercellular adhesion moledule-1 (ICAM-1) interaction. Biophys. J. 83:2270–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, X., A. Chen, E. Wojcikiewicz, and V. T. Moy. 2005. Probing ligand-receptor interactions with atomic force microscopy. In A Molecular Cloning Manual. E. A. Golemis, editor. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 399–413.
- 15.Hille, B., A. M. Woodhull, and B. I. Shapiro. 1975. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos. Trans. R. Soc. Lond. B Biol. Sci. 270:301–318. [DOI] [PubMed] [Google Scholar]
- 16.Coronado, R., and R. Latorre. 1983. Phospholipid bilayers made from monolayers on patch-clamp pipettes. Biophys. J. 43:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tank, D. W., and C. Miller. 1983. Patch-clamped liposomes: recording reconstituted ion channels. In Single-Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press, New York. 91–105.
- 18.Opsahl, L. R., and W. W. Webb. 1994. Lipid-glass adhesion in giga-sealed patch-clamped membranes. Biophys. J. 66:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn, R. 1991. Diffusion of nystatin in plasma membrane is inhibited by a glass-membrane seal. Biophys. J. 60:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayerl, T. M., and M. Bloom. 1990. Physical properties of single phospholipid bilayers adsorbed to micro glass beads. A new vesicular model system studied by 2H-nuclear magnetic resonance. Biophys. J. 58:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, S. J., T. M. Bayerl, D. C. McDermott, G. W. Adam, A. R. Rennie, R. K. Thomas, and E. Sackmann. 1991. Structure of an adsorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys. J. 59:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig, B. W., S. Kruger, W. J. Orts, C. F. Majkrzak, N. F. Berk, J. V. Silverton, and K. Gawrisch. 1996. Neutron reflectivity and atomic force microscopy studies of a lipid bilayer in water adsorbed to the surface of a silicon single crystal. Langmuir. 12:1343–1350. [Google Scholar]
- 23.Gutknecht, J. 1984. Proton/hydroxide conductance through lipid bilayer membranes. J. Membr. Biol. 82:105–112. [DOI] [PubMed] [Google Scholar]