Abstract
Selenocysteine insertion during decoding of eukaryotic selenoprotein mRNA requires several trans-acting factors and a cis-acting selenocysteine insertion sequence (SECIS) usually located in the 3′ UTR. A second cis-acting selenocysteine codon redefinition element (SRE) has recently been described that resides near the UGA-Sec codon of selenoprotein N (SEPN1). Similar phylogenetically conserved elements can be predicted in a subset of eukaryotic selenoprotein mRNAs. Previous experimental analysis of the SEPN1 SRE revealed it to have a stimulatory effect on readthrough of the UGA-Sec codon, which was not dependent upon the presence of a SECIS element in the 3′ UTR; although, as expected, readthrough efficiency was further elevated by inclusion of a SECIS. In order to examine the nature of the redefinition event stimulated by the SEPN1 SRE, we have modified an experimentally tractable in vitro translation system that recapitulates efficient selenocysteine insertion. The results presented here illustrate that the SRE element has a stimulatory effect on decoding of the UGA-Sec codon by both the methylated and unmethylated isoforms of Sec tRNA[Ser]Sec, and confirm that efficient selenocysteine insertion is dependent on the presence of a 3′-UTR SECIS. The variation in recoding elements predicted near UGA-Sec codons implies that these elements may play a differential role in determining the amount of selenoprotein produced by acting as controllers of UGA decoding efficiency.
Keywords: selenocysteine, selenoprotein, SEPN1, readthrough, SRE, recoding
INTRODUCTION
Selenium is cotranslationally incorporated as the 21st amino acid selenocysteine into a subset of proteins in all three lineages of life (Bock et al. 1991; Rother et al. 2001; Hatfield and Gladyshev 2002). This is accomplished by the expansion of the genetic code to include a dual meaning for the codon UGA, either translational termination or selenocysteine insertion. It is generally believed that the evolutionary force driving the genetic code to accommodate selenocysteine is provided by the catalytic advantage conferred on enzymes when the sulfur of a catalytic cysteine site is replaced by selenium (Berry et al. 1992; Sun et al. 1999; Zhang et al. 2006). The lower pKa and redox potential of selenocysteine relative to cysteine produces a fully ionized and highly reactive group at physiological pH. Consequently, most known selenoproteins are oxidoreductases with selenocysteine in the active site.
In eukaryotes, selenocysteine insertion requires a selenocysteine insertion sequence (SECIS) RNA element, usually located in the 3′ UTR, a SECIS-binding protein (SBP2), a selenocysteine-specific elongation factor (eEFSec), and the selenocysteine-charged Sec tRNA[Ser]Sec (Lee et al. 1989; Copeland and Driscoll 1999; Tujebajeva et al. 2000). In addition, the expression of a subset of selenoproteins has been shown to depend upon the levels of two Sec tRNA[Ser]Sec isoforms (differing by a single methyl group at U34) (Moustafa et al. 2001; Carlson et al. 2005). The ratio of Um34 to U34 Sec tRNA[Ser]Sec, which varies in response to available selenium levels (Diamond et al. 1993), may in part be responsible for the observed hierarchical expression of selenoproteins under selenium-limiting conditions. Recent evidence suggests that key events that prime mRNAs for selenocysteine decoding may occur in the nucleus as well as the cytoplasm (Papp et al. 2006; Small-Howard et al. 2006), although the nature of these nuclear events remain speculative. While our understanding of the trans-acting factors involved in SECIS recognition and selenocysteine insertion in eukaryotes is growing rapidly, the integral mechanism by which the SECIS acts from a distance with these factors to affect reprogramming of the ribosome and redefinition of UGA codons remains elusive.
In one model, the SECIS structure recruits SBP2 and the eEFSec:Sec tRNA[Ser]Sec complex to the 3′ UTR of selenoprotein mRNAs. During decoding of the UGA-Sec codon, SBP2 brings eEFSec to the ribosome, causing a conformational change that triggers Sec tRNA[Ser]Sec decoding of the UGA codon. Alternatively, it has been proposed that SBP2 may be associated with ribosomes prior to interacting with the SECIS element, based on the observation that most cytoplasmic SBP2 are in association with ribosomes (for review, see Caban and Copeland 2006). In the latter case, the role of the SECIS might be to alter ribosome conformation by interacting with ribosome-bound SBP2 during UGA redefinition. In addition, the recent observation that the ribosomal protein L30 can also bind directly to the SECIS structure in competition with SBP2 has provoked the idea that L30 may act as a molecular switch to alter the SECIS (and possibly ribosome) conformation to affect the events leading to Sec tRNA[Ser]Sec decoding of UGA-Sec codons (Chavatte et al. 2005).
Bacterial SECIS elements are known to occur immediately downstream from the UGA-Sec codon (Zinoni et al. 1990; Berg et al. 1991; Huttenhofer et al. 1996; Zhang and Gladyshev 2005). In a recent search to determine if eukaryotic cis-acting signals might also reside near UGA-Sec codons, it was discovered that phylogenetically conserved cis-acting selenocysteine codon redefinition elements, termed SREs, can be identified just downstream from a subset of UGA-Sec codons (Howard et al. 2005; M.T. Howard, unpubl.). An independent bioinformatic search for deeply conserved functional RNA secondary structures identified the same elements downstream from the eukaryotic Selenoprotein T and SEPN1 UGA-Sec codons (Pedersen et al. 2006). Although RNA stem–loop structures can be predicted near many eukaryotic UGA-Sec codons, there appears to be little similarity with other known aspects of the bacterial SECIS elements. The SEPN1 SRE consists of a highly conserved stem–loop structure that starts 6 nucleotides (nt) downstream from the UGA codon. Phylogenetic analysis and directed mutagenesis revealed the importance of the stem–loop structure in addition to the length and sequence of the spacer separating it from the UGA-Sec codon for efficient readthrough activity (Howard et al. 2005). The observation that the selenoprotein N SRE can stimulate readthrough of both UGA and UAG codons raised the possibility that at least one effect on readthrough could be due to stimulation of decoding by near cognate tRNAs rather than Sec tRNA[Ser]Sec.
To determine the effect of the SRE on Sec tRNA[Ser]Sec decoding of UGA codons, we have modified a rabbit reticulocyte lysate translation system for efficient selenocysteine insertion. The results indicate that the SRE has a strong effect on selenocysteine insertion at the SEPN1 UGA-Sec codon, and confirm that only very low levels of selenocysteine incorporation occur in the absence of a functional SECIS element. These results strongly support our contention that selenocysteine insertion efficiency, and possibly regulation, is affected by UGA-Sec adjacent recoding elements.
RESULTS
Efficient selenocysteine insertion in rabbit reticulocyte lysates: The SRE and polyamines
Previously published reports of UGA-Sec decoding in rabbit reticulocyte lysates (RRL) have revealed that SBP2 is the limiting factor for selenocysteine insertion, and that only the C-terminal 447 amino acids (SBP2-CT) are required for selenocysteine insertion activity (Mehta et al. 2004). Here, we adapt a similar RRL-based in vitro assay for efficient selenocysteine insertion to address the nature of the recoding event stimulated by the SEPN1 SRE at UGA-Sec codons. In short, UGA-decoding efficiency and specificity during translation of a bicistronic reporter mRNA was optimized by adjusting the levels of Mg++, spermidine, and SBP2 in Flexi-RRL obtained from Promega. A significant advantage of this system is the use of a bicistronic reporter vector, which allows for the maintenance and manipulation of the UGA and its native surrounding sequence context. A detailed protocol of the translation reaction mixture, RNA levels, incubation temperature, and determination of readthrough and selenocysteine insertion efficiency is outlined in Materials and Methods.
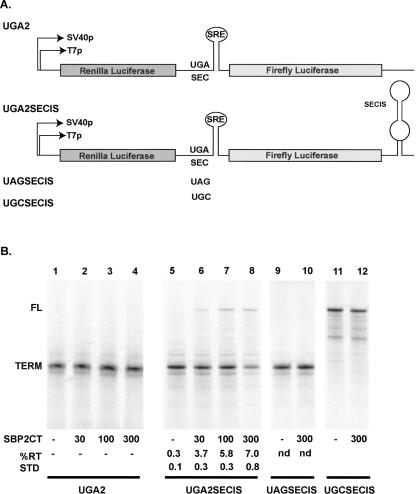
T7 RNA transcripts were produced from p2lucUGA2 (Fig. 1A). This RNA, designated UGA2, contains the SEPN1 UGA codon and flanking sequences inserted between the Renilla and Firefly luciferase reporter genes (described in Howard et al. 2005). Initially, this RNA was translated in RRL without the addition of SBP2. Analysis of [35S]methionine-labeled protein products by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) revealed that the vast majority of product was due to translational termination at the UGA codon; <0.1% readthrough product was observed relative to the termination product (Fig. 1B, lanes 1–4). The addition of the SBP2-CT had no appreciable effect on readthrough efficiency. However, when the same RNA containing an additional 3′-SECIS extension is used (UGA2SECIS) (see Materials and Methods; Howard et al. 2005), the addition of SBP2 increases readthrough levels at least 25-fold to ∼7% efficiency (Fig. 1B, lanes 5–8). It should be noted that the calculations used to determine percent readthrough measure the number of ribosomes that decode the UGA codon as a fraction of the translating ribosomes that terminate at the UGA codon (see Materials and Methods). At higher levels of SBP2-CT there is a reduction in the amount of termination product, but little change in the amount of full-length product produced. Given that there is no apparent increase in translational drop-off prior to termination (this would appear as bands below the termination product), we interpret this to mean that high levels of SBP2 may inhibit translation initiation or loading of ribosomes onto the message. As the amount of full-length protein remains high, the efficiency of UGA redefinition must proportionately be increased.
FIGURE 1.
(A) Schematic of the bicistronic 2luc reporter. UGA2 contains the SEPN1 UGA codon and surrounding sequences cloned between the renilla and firefly luciferase genes. UGA2SECIS is identical with the exception that a SECIS element is contained in the 3′ UTR. UAGSECIS and UGCSECIS indicated changes to the UGA codon only. (B) SBP2 induced readthrough of the SEPN1 UGA codon in UGA2, UGA2SECIS, the UAG codon of UAGSECIS, and standard translation of the UGC codon of UGCSECIS. SDS-PAGE analysis of [35S]methionine in vitro translation products using the respective T7 RNA transcripts. SBP2-CT was added to the indicated amounts (lanes 1–12). (FL) Full-length and (TERM) termination mark the positions where the translation products resulting from UGA readthrough and UGA termination migrate, respectively. Percent readthrough (% RT) was calculated and the means and standard deviations (STD) shown. (nd) Not detected.
Changing the UGA codon to UAG (UAGSECIS) reduced readthrough to <1% in the presence or absence of SBP2 (Fig. 1B, lanes 9,10). The very low level of readthrough observed in the absence of the SECIS or exogenously added SBP2-CT, as well as low levels of readthrough when the UGA is changed to UAG, supports the notion that readthrough is due to codon-specific selenocysteine insertion rather than nonselenocysteine readthrough of the UGA codon. The use of capped RNA to prime RRL increased overall product levels, but did not appreciably affect readthrough efficiency (data not shown).
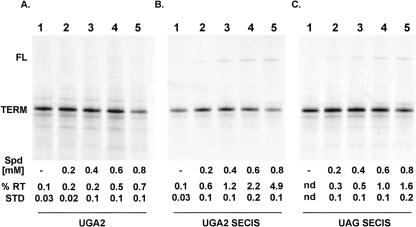
Polyamine levels have been shown to affect translational termination in several studies using RRL translations (Hryniewicz and Vonder Haar 1983; Petros et al. 2005). Based on these observations and the presumption that selenocysteine insertion is in competition with translational termination, we tested the effect of increasing spermidine levels on readthrough efficiency and decoding of UGA codons as selenocysteine. Spermidine was added at increasing concentrations to RRL translations primed with the UGA2 RNA, UGA2SECIS, and UAGSECIS. SDS-PAGE analysis of [35S]methionine-labeled products demonstrated that the addition of up to 0.8 mM spermidine increased readthrough levels in all three mRNAs to differing extents (Fig. 2A–C, cf. lanes 5); 0.7% for UGA2, 4.9% for UGA2SECIS, and 1.6% for UAGSECIS. The addition of 0.4 mM spermidine was chosen for further use, as it appears nearly optimum for overall translation levels and minimized nonspecific readthrough of the UAG codon (Fig. 2A–C, cf. lanes 3).
FIGURE 2.
The effect of spermidine addition on translational readthrough of UGA and UAG codons. (A) RNA transcribed from p2lucUGA2 (UGA2; lacking a SECIS) were translated in RRL supplemented with increasing amounts of spermidine as indicated (mM). No SBP2-CT was present in the translation reactions. (B) RNA transcribed from p2luc UGA2SECIS were translated in RRL supplemented with increasing amounts of spermidine as indicated (mM). (C) RNA transcribed from p2luc UAG2SECIS were translated in RRL supplemented with increasing amounts of spermidine as indicated (mM). [35S]methionine products were analyzed by SDS-PAGE analysis and percent readthrough determined (% RT); means and standard deviation are shown. (nd) Not detected.
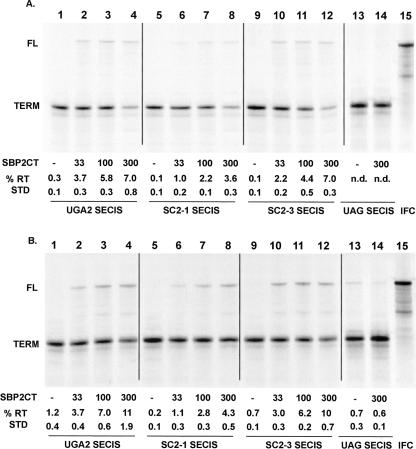
The effect of the SRE on readthrough efficiency was first analyzed by translation of T7 transcripts containing mutations in the SRE structure. The UGA2SECIS RNA and RNAs transcribed from p2lucUGA2SC2-1 (SC2-1SECIS) and p2lucUGA2SC2-3 (SC2-3SECIS) (Howard et al. 2005) were translated in RRL reactions into which increasing amounts of SBP2 were added (Fig. 3A). Changing 3 nt on the 5′ side of the SRE stem–loop, which disrupts the base-pairing potential (SC2-1SECIS), reduced the maximal SBP2 induced readthrough approximately twofold to 3.6% (Fig. 3A, lanes 5–8). Compensatory mutations on the 3′ side of the stem–loop, which reestablish the base-pairing potential (SC2-3SECIS), return the readthrough efficiency to the levels seen with the unaltered SRE (Fig. 3A, cf. lanes 1–4 and 9–12). Addition of 300 nM SBP2 to RRL translations of mRNA UAGSECIS had no effect on readthrough efficiency. These results demonstrate that SBP2-induced readthrough is inhibited by disruption of the SRE stem–loop structure at UGA codons. The addition of spermidine to 0.4 mM to each of these reactions increased readthrough levels by 1.5- to twofold over those observed in the absence of exogenously added spermidine. Spermidine also increased readthrough of the UAGSECIS UAG codon; however, this activity was not stimulated by the addition of SBP2, suggesting a general effect on termination at UAG codons.
FIGURE 3.
Effect of the SEPN1 SRE on UGA readthrough. (A) RNAs transcribed from p2lucUGA2SECIS (lanes 1–4), SC2–1SECIS (lanes 5–8), SC2–3SECIS (lanes 9–12), and UAGSECIS (lanes 13,14), each containing the SEPN1 SECIS, were translated in RRL supplemented with increasing amounts of SBP2-CT. Concentrations of SBP2-CT are indicated in nanomolar amounts. (IFC) UGCSECIS as a marker for the full-length readthrough product. (B) Same as A with the RRL translation reaction supplemented with 0.4 mM Spermidine. The [35S]methionine-labeled products were separated by SDS-PAGE and percent readthrough (% RT) determined. Means and standard deviations are shown. (nd) Not detected.
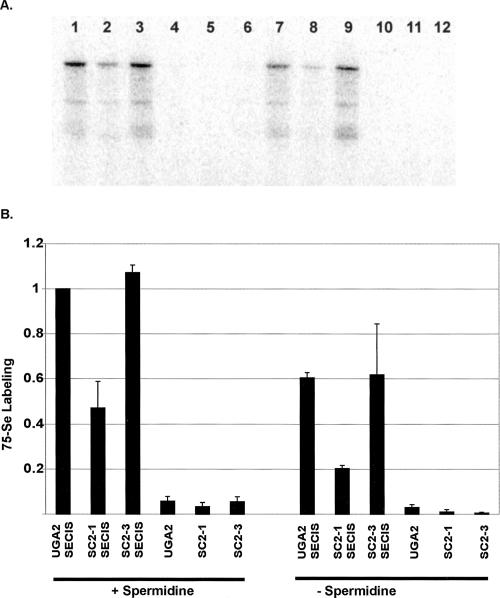
To determine if spermidine could directly affect selenocysteine insertion, [75Se] Sec tRNA[Ser]Sec (a mixture of both isoforms) was added to in vitro translations to selectively label proteins incorporating selenocysteine. The RNAs corresponding to UGA2SECIS, SC2-1SECIS, SC2-3SECIS, UGA2, SC2-1, or SC2-3 were added to selenium-labeled RRL reactions with (Fig. 4A, lanes 1–6) or without spermidine (Fig. 4A, lanes 7–12) and the resulting products resolved by SDS-PAGE. Selenium-labeling efficiency is expressed relative to that observed using the UGA2SECIS RNA in the presence of 0.4 mM spermidine (Fig. 4B). The results from this experiment make several notable points. (1) Selenocysteine incorporation during translation of RNAs containing 3′-UTR SECIS elements is at least (in most cases much greater than) 20-fold higher than insertion efficiency during decoding of RNAs lacking a SECIS. Nevertheless, some low-level selenenocysteine incorporation does occur in a SECIS-independent manner. (2) The addition of spermidine to 0.4 mM in RRL doubled selenocysteine insertion efficiency (Fig. 4, cf. lanes 1–6 and lanes 7–12). (3) Disruption of base pairing within the SRE stem–loop reduces selenocysteine insertion efficiency by at least 50%, regardless of whether spermidine is added to the reaction.
FIGURE 4.
SRE and spermidine effects on selenocysteine incorporation. RNAs were transcribed from p2lucUGA2, SC2-1, and SC2-3 linearized with either Pml-1 or Hpa-1. The former generated RNA transcripts without a SECIS element (UGA2, SC2-1, SC2-3) and latter RNAs with a SECIS element (UGA2SECIS, SC2-1SECIS, SC2-3SECIS). These RNAs were translated in RRL supplemented with 300 nM SBP2-CT and [75Se]-labeled Sec tRNA[Ser]Sec. Exogenous spermidine was added to 0.4 mM (lanes 1–6; +Spermidine) or no spermidine was added (lanes 7–12; −Spermidine). (A) [75Se]-labeled protein products were analyzed by SDS-PAGE. (B) The amount of [75Se] incorporation is expressed relative to the UGA2SECIS programmed translation reactions. Means and standard deviations are shown in bar graph form.
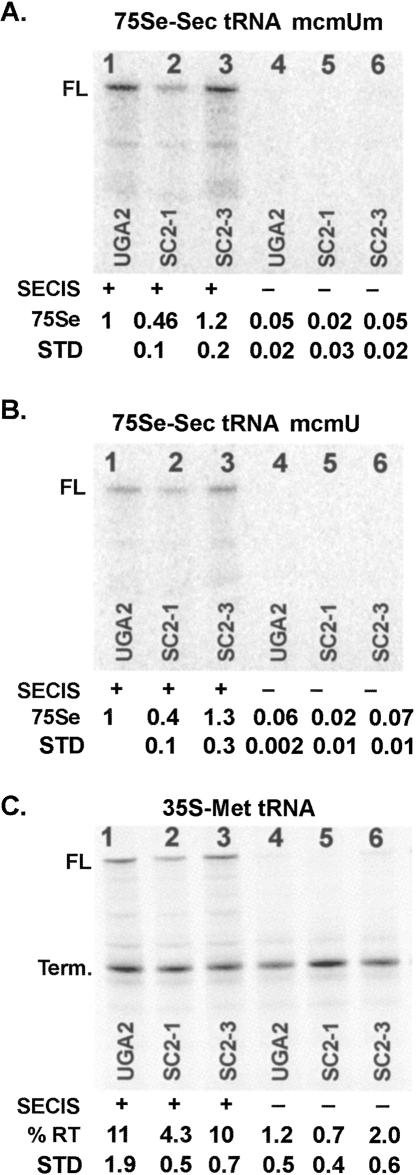
UGA decoding by two isoforms of Sec tRNA[Ser]Sec
Decoding of UGA-Sec codons is known to occur by two isoforms of Sec tRNA[Ser]Sec containing Um34 or lacking this methyl modification. These tRNAs differ from one another by the presence or absence of a methyl group on the ribosyl moiety of the anticodon wobble base, methylcarboxylmethyluridine (mcm5U), or methylcarboxymethyluridine-2′-O-methylribose (mcm5Um), respectively (for review, see Hatfield and Gladyshev 2002). The ability to separate these two isoforms by HPLC chromatography (Hatfield et al. 1991) allowed us to examine directly whether the SRE element was involved in the selection of one isoform over the other during decoding. In the above experiment (Fig. 4), a mixture of the two isoforms was utilized at roughly equal molar amounts. This experiment was repeated in RRL translation reactions supplemented with SBP2, spermidine, and highly purified HPLC fractions of the mcm5Um (Fig. 5A) or mcm5U (Fig. 5B) isoforms of [75Se]-labeled Sec tRNA[Ser]Sec. As was found using a mixture of the two isoforms, the mcm5Um and mcm5U forms of Sec tRNA[Ser]Sec each showed a strong dependence on the 3′-UTR SECIS for selenocysteine incorporation (Fig. 5A,B, cf. lanes 1–3 and 4–6), and revealed ∼50% reduction in selenocysteine incorporation when the SRE stem–loop was disrupted. The percent total readthrough was monitored using [35S]methionine as the only label in the absence of exogenously added Sec tRNA[Ser]Sec (Fig. 5C). Although overall labeling was more efficient with the methylated isoform, the lack of a differential effect on selenocysteine insertion when the SRE is disrupted argues against the notion that one function of the SRE is to select one tRNA isoform over the other during decoding of the SEPN1 UGA-Sec codon.
FIGURE 5.
The effect of Sec tRNA[Ser]Sec U34 methylation on selenocysteine insertion. RNA transcripts generated from p2lucUGA2, SC2-1, and SC2-3 with or without a SECIS element were translated in RRL supplemented with 300 nM SBP2-CT and either the [75Se]-labeled methylated isoform of Sec tRNA[Ser]Sec (mcmUm) or the unmethylated isoform (mcmU). [75Se]-labeled proteins were resolved by SDS-PAGE (A,B) and the incorporation of selenocysteine reported relative to the incorporation levels in UGA2SECIS programmed RRL reactions. Means and standard deviations are given. The percent readthrough was monitored by [35S]methionine labeling of protein products in C.
Additional cis-acting recoding elements?
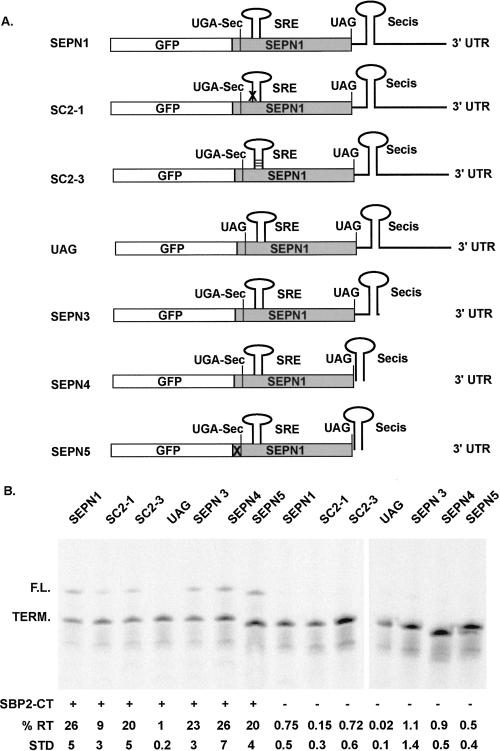
The p2luc constructs used in the above experiments contain the UGA-Sec codon and 35 nt upstream of and 46 nt downstream from SEPN1 cloned between the Firefly and Renilla luciferase genes. In addition, a minimal 100 nt SEPN1 SECIS was inserted downstream from the termination codon, but upstream of the vector polyadenylation site. To determine if additional recoding sequences may have been inadvertently missed in these experiments, the SEPN1 coding sequence starting 100 nt upstream of the UGA-Sec codon through the entire 3′ UTR was cloned downstream from GFP in the pcDNA3.1/NT-GFP expression vector (see Fig. 6A; SEPN1). The equivalents of the SC2-1 and SC2-3 mutations were made to test the role of the SRE in this expression system. As seen with the p2luc vector, when equivalent RNAs are translated in RRL supplemented with SBP2-CT, disruption of base-pairing potential in the SRE reduces readthrough levels >50%, making the requisite compensatory mutations that restore base pairing returned readthrough levels to near normal (Fig. 6; SC2-1 and SC2-3). Deletion of SEPN1 3′-UTR sequences upstream of or downstream from the SECIS had little significant effect on readthrough efficiency (Fig. 6B). Likewise, no change in readthrough efficiency was observed when SEPN1 upstream-coding sequences were deleted to within 12 nt of the UGA-Sec codon (Fig. 6; SEPN5). The omission of SBP2-CT lowered readthrough efficiency to 1% or less for all constructs. The somewhat higher overall SBP2-CT-dependent readthrough levels found in this assay compared with those found using RNAs transcribed from the p2luc expression vectors may be due to the larger size of the downstream firefly coding sequence in the latter. SDS-PAGE analysis of products translated from the luciferase-coding RNAs reveals products of intermediate size between the termination and full-length products (see Figs. 1, 2, 4, 5). These readthrough products are likely the result of ribosome drop-off and are not included in the calculation of readthrough efficiencies. Consequently, this leads to a conservative (underestimation) of readthrough efficiencies when RNA transcribed from the p2luc expression vectors are utilized in RRL translation reactions.
FIGURE 6.
Readthrough of UGA-Sec codons during translation of NTGFP-SEPN1 RNA transcripts. (A) Schematic of NTGFP mRNAs translated in RRL reactions. The exact SEPN1 sequence is indicated in the Materials and Methods. X in SC2-1 indicates a 3 nt change in the 5′ side of the SRE stem–loop, which disrupts base-pairing potential. The horizontal lines in the SRE of SC2-3 indicate compensatory changes to the 3′ side of the stem–loop, which restore base-pairing potential. (B) SDS-PAGE analysis of [35S]methionine-labeled products of RRL translation reactions programmed with RNAs described in A. The reactions were carried out in the presence or absence of 300 nM SBP2-CT, 0.4 mM spermidine, and the percent readthrough determined (% RT). Means and standard deviation are shown.
The results presented here strongly support that the UGA-Sec codon and a 3′ SECIS are the necessary cis-acting elements required to reprogram the ribosome for selenocysteine insertion, and that the SRE-recoding element acts as a modifier of UGA-Sec decoding by both isoforms of Sec tRNA[Ser]Sec.
DISCUSSION
The description of features common to eukaryotic SECIS elements has allowed for the bioinformatic recognition of probably most selenoprotein genes in humans and a number of other sequenced eukaryotic genomes (for review, see Gladyshev et al. 2004). The entire biosynthetic pathway that synthesizes selenocysteine on its tRNA has been determined recently in eukaryotes and archaea (Xu et al. 2006). Yet, despite these advances, the discovery of the selenocysteine-specific elongation factor (eEFSec) and proteins that can interact with both the ribosome and SECIS (SBP2 and L30), our understanding of the mechanism by which information is conveyed at a distance from the 3′ UTR to reprogram the ribosome during decoding of the UGA-Sec codon is incomplete. Here, we describe the ability of a second cis-acting RNA-recoding element to affect selenocysteine-insertion efficiency. The occurrence of this RNA signal nearby the UGA-Sec codon raises the intriguing possibility that this element interacts directly with components of the selenocysteine insertion machinery and/or the ribosome during UGA-Sec decoding.
The observation that SEPN1 SRE can stimulate readthrough of UAG termination codons in cultured mammalian cells, when no cis-acting SECIS is present, supports the proposition that at least one role of the SRE is to reduce termination efficiency. However, in the RRL translation system utilized here, the omission of a SECIS element or exogenously added SBP2-CT revealed only very low levels of readthrough, <0.1% (see Figs. 1, 2). It was only upon the addition of exogenous spermidine that background levels of readthrough were elevated to ∼2% (see Figs. 3, 5C); UAG readthrough levels were insensitive to the addition of SBP2-CT. The differences observed between UAG readthrough efficiencies in mammalian cells and in vitro translations may reflect differences in the abundance of accessory factors including Sec tRNA[Ser]Sec and possibly the high levels of expression obtained from the SV40 promoter in transfected cells. Nevertheless, the stimulatory effect of the SRE on selenocysteine insertion in vitro, under conditions where only negligible levels of nonselenocysteine readthrough can be measured, strongly supports the notion that the SRE has a specific role in reprogramming the ribosome to decode UGA-Sec as selenocysteine.
Another example of stop-codon redefinition occurs in the MuLV retrovirus. The gag-pol polyprotein is produced by ribosomal readthrough of the gag UAG termination codon. UAG redefinition in this case is stimulated by a pseudoknot structure located 8 nt downstream from the UAG codon (Wills et al. 1991). RNA pseudoknots with similar spacing (typically, 7–9 nt) downstream from ribosomal frameshift sites are also well-known stimulators of programmed translational frameshifting (Brierley and Pennell 2001). A recent publication by Brierley and colleagues (Namy et al. 2006) presents structural data demonstrating that the IBV frameshift-stimulating pseudoknot blocks the ribosome RNA entrance tunnel and causes conformational changes that lead to a structural deformation of the P-site tRNA. It is possible that a similar effect of the SRE stem–loop or MuLV pseudoknot may inhibit translational termination by inducing a conformation that blocks either release factor access to the A site, peptidyl tRNA hydrolysis, or both. This model provides a feasible, albeit speculative, mechanistic explanation for the ability of some downstream structures to favor decoding over translational termination at UGA or UAG codons.
The location of the SRE near the UGA codon raises the possibility that it may interact with the selenocysteine insertion machinery in addition to the ribosome during UGA decoding. In bacteria, the bSECIS stem–loop is located just 3′ to selenocysteine-encoding UGA codons, and is recognized directly by the bacterial elongation factor, SelB (Baron et al. 1993; Huttenhofer et al. 1996). Of interest is the observation that domain IV of bacterial SelB is directed to the RNA entrance cleft and, consequently, is in a position to interact directly with the RNA sequences or structures located downstream from the UGA codon (Leibundgut et al. 2005). Although the eukaryotic eEFSec equivalent structure has not been determined, this observation suggests a mechanism by which the SEPN1 SRE or other structures occurring near selenocysteine-encoding UGA codons could potentially interact directly with eEFSec. Finally, it is possible that the SRE may interact directly with the RNA-binding proteins L30 or SBP2 when they are in association with either the ribosome or SECIS. In the latter case, such interactions could potentially act as a bridge to span the distance between the UGA-Sec codon and 3′ UTR. Identifying the components of the selenocysteine insertion machinery and/or ribosome that directly or indirectly interact with the SRE may provide key insights into the mechanism by which the 3′-UTR SECIS reprograms the ribosome during UGA decoding. The in vitro reconstitution of efficient UGA-Sec decoding described here will be a valuable tool in this pursuit.
The findings presented herein clearly demonstrate a stimulatory role for the SEPN1 SRE during selenocysteine insertion. The results also preclude a model in which the SRE selectively recruits one Sec tRNA[Ser]Sec isoform over the other. The variation in occurrence and lack of obvious sequence or structural similarity between structures predicted near UGA-Sec codons suggests that these elements may play a differential role in controling selenoprotein expression at the translation level, and may even be regulatory in nature. A thorough survey of the effect on selenocysteine decoding by other potential selenoprotein SREs is underway.
MATERIALS AND METHODS
Luciferase and GFP fusion vectors
The construction of p2lucUGA2, SC2-1, and SC2-3 with and without SECIS has been previously described (Howard et al. 2005). In brief, the following sequences from the selenoprotein N gene were cloned between the BamHI and SalI sites of the p2luc plasmid (Grentzmann et al. 1998), and the SEPN1 SECIS was inserted into the NotI site downstream from the termination codon. The UGA-Sec codon is indicated in bold, and mutations in the stem–loop region are shaded.
UGA2
TCGATCCTGCTGTGGGGGGCCCTGGATGACCAGTCCTGCTGAGGTTCAGGGCGGACTCTCCGGGAGACTGTCCTGGAAAGTTCGCCCAGATC
SC2–1
TCGATCCTGCTGTGGGGGGCCCTGGATGACCAGTCCTGCTGAGGTTCAGGGGCTACTCTCCGGGAGACTGTCCTGGAAAGTTCGCCCAGATC
SC2–3
TCGATCCTGCTGTGGGGGGCCCTGGATGACCAGTCCTGCTGAGGTTCAGGGGCTACTCTCCGGGAGACTGTCCTGGAAAGTGGCCCCAGATC
SEPN1 SECIS
GGCCGCAGTGGCTTCCCCGGCAGCAGCCCCATGATGGCTGAATCCGAAATCCTCGATGGGTCCAGCTTGATGTCTTTGCAGCTGCACCTATGGGGCGGCC
SEPN1 NTGFP T7 expression vectors were constructed using pcDNA3.1/NTGFP obtained from Invitrogen. SEPN1 sequences, nucleotides 1235–4109, were PCR amplified from a cDNA clone and ligated by TA cloning in frame with GFP coding sequences of pcDNA3.1/NTGFP (numbering based on SEPN1 Refseq NM206926). This clone was designated NTGFP SEPN1. SEPN1 deletions and directed mutagenesis of the SRE was accomplished using the PCR mutagenesis Phusion kit (New England Biolabs).
NTGFP SC2-1 nucleotides 1349–1351 CGG>GCT
NTGFP SC2-3 nucleotides 1349–1351 CGG>GCT and 1379–1381 TCG>GGC
NTGFP SEPN3 contains SEPN1 nucleotides 1235–2895 (deletion of nucleotides 2896–4109 from NTGFP SEPN1)
NTGFP SEPN4 contains SEPN1 nucleotides 1235–1726 and 2808–2895 (deletion of nucleotides 1727–2807 from SEPN3)
NTGFP SEPN5 contains SEPN1 nucleotides 1324–1726 and 2808–2895 (deletion of nucleotides 1235–1324 from SEPN4)
All changes were sequenced verified at the Sequencing Core Facility (University of Utah).
T7 RNA transcription
T7 RNA transcripts were produced using the MEGAScrip kit as described by the manufacturer (Ambion). Likewise, capped T7 transcripts were produced using the mMESSAGE mMACHINE kit (Ambion). Linear DNA templates were produced by restriction digestion of p2lucUGA2, SC2-1, and SC2-1 with Pml-1 or Hpa-1. Pml-1 cuts 5′ of the SECIS and, consequently, RNAs transcribed from this template lack a SECIS. Hpa-1 cuts 3′ of the SECIS, thus allowing for SECIS containing RNA transcripts to be produced. Constructs based on pcDNA3.1/NTGFP were linearized with NotI before addition to T7 transcription reactions.
Isolation of [75Se]-labeled Sec tRNA[Ser]Sec
HL-60 cells were grown as described (Hatfield et al. 1991) in the presence of 5 × 10−7 M sodium selenite. Five grams of cells were washed and resuspended in 80 mL of fresh RPMI growth medium consisting of 1% fetal calf serum without selenium supplementation. Five millicuries of neutralized [75Se] (University of Missouri Research Reactor) were added, the cells gently shaken for 3 h at 37°C, and cyclohexamide (1 × 10−4 M) was then added and shaken for an additional 45 min. The cells were collected by centrifugation at 800 rpm for 5 min at 4°C, washed with cold medium, and the packed cells stored at −80°C until ready for use. Phenol extraction and subsequent purification of the labeled Sec tRNA[Ser]Sec isoforms by RPC-5 chromatography were carried out as described (Hatfield et al. 1991).
In vitro translation
Flexi Rabbit Reticulocyte Lysate containing 2.54 mM endogenous magnesium was obtained from Promega. The endogenous magnesium level is an important factor to consider, as increasing the amount of magnesium was shown to increase nonspecific readthrough efficiency (data not shown). In vitro translation reactions labeled with L-[35S]methionine contained 70% Flexi RRL, 0.02 mM of each standard amino acid except methionine, 6 μCi of [35S]methionine (GE HealthCare), 75 mM KCl, 2 mM DTT, 10 μg/mL RNA, and the indicated amount of exogenously added SBP2-CT and spermidine in a final volume of 10 μL. Reactions were incubated for 1.5 h at 30°C. For in vitro translation reactions labeled with [75Se], the conditions were identical, except that all 20 standard amino acids were added to 0.02 mM, and 10,000 cpm of [75Se] Sec tRNA[Ser]Sec were added in place of [35S]methionine. Reactions were stopped by the addition of RNase A to 10 μg/mL, and incubation continued for 15 min at room temperature. A total of 5 μL of each reaction was added to 25 μL of 1X SDS loading dye (50 mM Tris-Cl at pH 6.8, 2% SDS, 10% Glycerol, 100 mM DTT) and incubated at 80°C for 4 min; 10 μL were then loaded onto a 4%–12% Bis-Tris gradient gel (Invitrogen) and electrophoresis carried out as recommended by the manufacturer. Gels were fixed in 30% methanol and 5% glycerol for 15 min and dried under vacuum at 80°C. After drying under vacuum, the gels were visualized using a Storm 860 PhosphorImager (Molecular Dynamics) and radioactive bands quantified using ImageQuant software. Percent readthrough was calculated as the percentage of full-length product relative to the termination product and the full-length product combined. The value of each product was corrected for the number of methionine codons present in the coding sequence. Each experiment was repeated at least three times; a representative experiment is shown.
pET101 containing the coding sequence for SBP2-CT (amino acids 399–846) was transformed into Escherichia coli BL21 Star (Promega). Bacterial cultures were grown at 37°C to an OD600 of 0.8 in 1 L of Luria Broth containing 100 μg/mL of Ampicillin (Sigma). Protein expression was induced by the addition of isopropyl-B-D-thiogalactoside (Sigma) and incubation continued for 2 h. Cells were harvested, washed with PBS, and lysed by six rounds of sonication on ice for 10 sec each. Cell lysates were centrifuged at 12,000 rpm for 30 min. The resulting clarified lysates were purified by Ni++ affinity chromatography, desalted, and stored at −20°C in phosphate buffered saline and 50% glycerol.
ACKNOWLEDGMENTS
We thank the Donna Driscoll's laboratory for SBP2 and L30 expression vectors and helpful advice during the purification of SBP2. Dolph Hatfield, John Atkins, and Ray Gesteland provided stimulating discussions and support throughout the course of this work. This research was supported by grant R01GM077462 to M.T.H. and in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.473907.
REFERENCES
- Baron, C., Heider, J., Bock, A. Interaction of translation factor SELB with the formate dehydrogenase H selenopolypeptide mRNA. Proc. Natl. Acad. Sci. 1993;90:4181–4185. doi: 10.1073/pnas.90.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, B.L., Baron, C., Stewart, V. Nitrate-inducible formate dehydrogenase in Escherichia coli K-12. II. Evidence that a mRNA stem–loop structure is essential for decoding opal (UGA) as selenocysteine. J. Biol. Chem. 1991;266:22386–22391. [PubMed] [Google Scholar]
- Berry, M.J., Maia, A.L., Kieffer, J.D., Harney, J.W., Larsen, P.R. Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology. 1992;131:1848–1852. doi: 10.1210/endo.131.4.1396330. [DOI] [PubMed] [Google Scholar]
- Bock, A., Forchhammer, K., Heider, J., Baron, C. Selenoprotein synthesis: An expansion of the genetic code. Trends Biochem. Sci. 1991;16:463–467. doi: 10.1016/0968-0004(91)90180-4. [DOI] [PubMed] [Google Scholar]
- Brierley, I., Pennell, S. Cold Spring Harbor Symposia on Quantitative Biology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. Structure and function of the Stimulatory RNAs involved in programmed eukaryotic -1 ribosomal frameshifting; pp. 233–248. [DOI] [PubMed] [Google Scholar]
- Caban, K., Copeland, P.R. Size matters: A view of selenocysteine incorporation from the ribosome. Cell. Mol. Life Sci. 2006;63:73–81. doi: 10.1007/s00018-005-5402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, B.A., Xu, X.M., Gladyshev, V.N., Hatfield, D.L. Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J. Biol. Chem. 2005;280:5542–5548. doi: 10.1074/jbc.M411725200. [DOI] [PubMed] [Google Scholar]
- Chavatte, L., Brown, B.A., Driscoll, D.M. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat. Struct. Mol. Biol. 2005;12:408–416. doi: 10.1038/nsmb922. [DOI] [PubMed] [Google Scholar]
- Copeland, P.R., Driscoll, D.M. Purification, redox sensitivity, and RNA binding properties of SECIS-binding protein 2, a protein involved in selenoprotein biosynthesis. J. Biol. Chem. 1999;274:25447–25454. doi: 10.1074/jbc.274.36.25447. [DOI] [PubMed] [Google Scholar]
- Diamond, A.M., Choi, I.S., Crain, P.F., Hashizume, T., Pomerantz, S.C., Cruz, R., Steer, C.J., Hill, K.E., Burk, R.F., McCloskey, J.A., et al. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA[Ser]Sec . J. Biol. Chem. 1993;268:14215–14223. [PubMed] [Google Scholar]
- Gladyshev, V.N., Kryukov, G.V., Fomenko, D.E., Hatfield, D.L. Identification of trace element-containing proteins in genomic databases. Annu. Rev. Nutr. 2004;24:579–596. doi: 10.1146/annurev.nutr.24.012003.132241. [DOI] [PubMed] [Google Scholar]
- Grentzmann, G., Ingram, J.A., Kelly, P.J., Gesteland, R.F., Atkins, J.F. A dual-luciferase reporter system for studying recoding signals. RNA. 1998;4:479–486. [PMC free article] [PubMed] [Google Scholar]
- Hatfield, D., Lee, B.J., Hampton, L., Diamond, A.M. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic Acids Res. 1991;19:939–943. doi: 10.1093/nar/19.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield, D.L., Gladyshev, V.N. How selenium has altered our understanding of the genetic code. Mol. Cell. Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, M.T., Aggarwal, G., Anderson, C.B., Khatri, S., Flanigan, K.M., Atkins, J.F. Recoding elements located adjacent to a subset of eukaryal selenocysteine-specifying UGA codons. EMBO J. 2005;24:1596–1607. doi: 10.1038/sj.emboj.7600642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz, M.M., Vonder Haar, R.A. Polyamines enhance readthrough of the UGA termination codon in a mammalian messenger RNA. Mol. Gen. Genet. 1983;190:336–343. doi: 10.1007/BF00330661. [DOI] [PubMed] [Google Scholar]
- Huttenhofer, A., Westhof, E., Bock, A. Solution structure of mRNA hairpins promoting selenocysteine incorporation in Escherichia coli and their base-specific interaction with special elongation factor SELB. RNA. 1996;2:354–366. [PMC free article] [PubMed] [Google Scholar]
- Lee, B.J., Worland, P.J., Davis, J.N., Stadtman, T.C., Hatfield, D.L. Identification of a selenocysteyl-tRNASer in mammalian cells that recognizes the nonsense codon, UGA. J. Biol. Chem. 1989;264:9724–9727. [PubMed] [Google Scholar]
- Leibundgut, M., Frick, C., Thanbichler, M., Bock, A., Ban, N. Selenocysteine tRNA-specific elongation factor SelB is a structural chimaera of elongation and initiation factors. EMBO J. 2005;24:11–22. doi: 10.1038/sj.emboj.7600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, A., Rebsch, C.M., Kinzy, S.A., Fletcher, J.E., Copeland, P.R. Efficiency of mammalian selenocysteine incorporation. J. Biol. Chem. 2004;279:37852–37859. doi: 10.1074/jbc.M404639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa, M.E., Carlson, B.A., El-Saadani, M.A., Kryukov, G.V., Sun, Q.A., Harney, J.W., Hill, K.E., Combs, G.F., Feigenbaum, L., Mansur, D.B., et al. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol. Cell. Biol. 2001;21:3840–3852. doi: 10.1128/MCB.21.11.3840-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namy, O., Moran, S.J., Stuart, D.I., Gilbert, R.J., Brierley, I. A mechanical explanation of RNA pseudoknot function in programmed ribosomal frameshifting. Nature. 2006;441:244–247. doi: 10.1038/nature04735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp, L.V., Lu, J., Striebel, F., Kennedy, D., Holmgren, A., Khanna, K.K. The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Mol. Cell. Biol. 2006;26:4895–4910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, J.S., Bejerano, G., Siepel, A., Rosenbloom, K., Lindblad-Toh, K., Lander, E.S., Kent, J., Miller, W., Haussler, D. Identification and classification of conserved RNA secondary structures in the human genome. PLoS Comput. Biol. 2006;2:e33. doi: 10.1371/journal.pcbi.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros, L.M., Howard, M.T., Gesteland, R.F., Atkins, J.F. Polyamine sensing during antizyme mRNA programmed frameshifting. Biochem. Biophys. Res. Commun. 2005;338:1478–1489. doi: 10.1016/j.bbrc.2005.10.115. [DOI] [PubMed] [Google Scholar]
- Rother, M., Resch, A., Wilting, R., Bock, A. Selenoprotein synthesis in archaea. Biofactors. 2001;14:75–83. doi: 10.1002/biof.5520140111. [DOI] [PubMed] [Google Scholar]
- Small-Howard, A., Morozova, N., Stoytcheva, Z., Forry, E.P., Mansell, J.B., Harney, J.W., Carlson, B.A., Xu, X.M., Hatfield, D.L., Berry, M.J. Supramolecular complexes mediate selenocysteine incorporation in vivo. Mol. Cell. Biol. 2006;26:2337–2346. doi: 10.1128/MCB.26.6.2337-2346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q.A., Wu, Y., Zappacosta, F., Jeang, K.T., Lee, B.J., Hatfield, D.L., Gladyshev, V.N. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J. Biol. Chem. 1999;274:24522–24530. doi: 10.1074/jbc.274.35.24522. [DOI] [PubMed] [Google Scholar]
- Tujebajeva, R.M., Copeland, P.R., Xu, X.M., Carlson, B.A., Harney, J.W., Driscoll, D.M., Hatfield, D.L., Berry, M.J. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills, N.M., Gesteland, R.F., Atkins, J.F. Evidence that a downstream pseudoknot is required for translational read-through of the Moloney murine leukemia virus gag stop codon. Proc. Natl. Acad. Sci. 1991;88:6991–6995. doi: 10.1073/pnas.88.16.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X.M., Carlson, B.A., Mix, H., Zhang, Y., Saira, K., Glass, R.S., Berry, M.J., Gladyshev, V.N., Hatfield, D.L. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2006;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Gladyshev, V.N. An algorithm for identification of bacterial selenocysteine insertion sequence elements and selenoprotein genes. Bioinformatics. 2005;21:2580–2589. doi: 10.1093/bioinformatics/bti400. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Romero, H., Salinas, G., Gladyshev, V.N. Dynamic evolution of selenocysteine utilization in bacteria: A balance between selenoprotein loss and evolution of selenocysteine from redox active cysteine residues. Genome Biol. 2006;7:R94. doi: 10.1186/gb-2006-7-10-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni, F., Heider, J., Bock, A. Features of the formate dehydrogenase mRNA necessary for decoding of the UGA codon as selenocysteine. Proc. Natl. Acad. Sci. 1990;87:4660–4664. doi: 10.1073/pnas.87.12.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]