Abstract
SEPYLRFamide acts as an inhibitory modulator of acetylcholine (ACh) receptors in Helix lucorum neurones. Ouabain, a specific inhibitor of Na,K-pump, (0.1 mM, bath application) decreased the ACh-induced inward current (ACh-current) and increased the leak current. Ouabain decreased the modulatory SEPYLRFamide effect on the ACh-current. There was a correlation between the effects of ouabain on the amplitude of the ACh-current and on the modulatory peptide effect. Ouabain and SEPYLRFamide inhibited the activity of Helix aspersa brain Na,K-ATPase. Activation of Na,K-pump by intracellular injection of 3 M Na acetate or 3 M NaCl reduced the modulatory peptide effect on the ACh-current. An inhibitor of Na/Ca-exchange, benzamil (25 μM, bath application), and an inhibitor of Ca2+-pump in the endoplasmic reticulum, thapsigargin (TG, applied intracellularly), both prevented the effect of ouabain on SEPYLRFamide-mediated modulatory effect. Another inhibitor of Ca2+-pump in the endoplasmic reticulum, cyclopiazonic acid (applied intracellularly), did not prevent the effect of ouabain on SEPYLRFamide-mediated modulatory effect. These results indicate that Na,K-pump is responsible for the SEPYLRFamide-mediated inhibition of ACh receptors in Helix neurons. Na/Ca-exchange and intracellular Ca2+ released from internal pools containing TG-sensitive Ca2+-pump are involved in the Na,K-pump pathway for the SEPYLRFamide-mediated inhibition of ACh receptors.
Keywords: SEPYLRFamide; Na,K-pump; Acetylcholine receptors; Released Ca2+; Na/Ca-exchange; Helix neurons
1. Introduction
A large number of endogenous peptides with an RFamide carboxyl terminal (FMRFamide-related peptides/FaRPs/), occur in molluscs and other invertebrate phyla and vertebrates including mammals [50] which strongly consider their importance as extracellular signalling molecules. The FMRFamide family has important roles both as neurotransmitters and as neuromodulators in the central and peripheral nervous system [14,26,30,53].
Seven FMRFamide-related peptides were extracted and sequenced in the land snail, Helix aspersa [43]. There are two endogenous Helix tetrapeptides (FMRFamide, FLRFamide) and five heptapeptides (NDPFLRFamide, SDPFLRFamide, pQDPFLRFamide, NDPYLRFamide, SEPYLRFamide) [43].
Endogenous molluscan FMRFamide-related peptides (FaRPs): FMRFamide, SKPYMRFamide, SEPYLRFamide and its N-terminally modified analogues, EPYLRFamide, SEGYLRFamide, SRPYLRFamide, SKPYLRFamide and acetyl-SKPYMRFamide act as inhibitory neuromodulators of cholinergic transmission in H. aspersa [1,38,41] and Helix lucorum [36,39,40,42] parietal ganglia.
The modulation of synaptic transmission by FaRPs can occur through a change in the sensitivity of the postsynaptic membrane to transmitters since it was found that all the FaRPs reduced reversibly the inward current in H. aspersa and H. lucorum identified neurons to local acetylcholine (ACh) application onto the soma. Reduction of ACh-induced current (metabotropic effect) by SEPYLRFamide and its N-terminally modified analogues at concentrations 0.01–10.0 μM was not associated with any changes in the leak current. At concentration of 50 μM the peptides evoked an electrogenic effect, i.e., an increase (by 15–25%) of inward leak current with full recovery after wash. [39].
Elevation of free intracellular Ca2+ reduces ACh-induced inward Cl− currents in Lymnaea stagnalis [12,52] and Helix pomatia [3] dialysed neurons. This means that an artificial increase of intracellular free Ca2+ in the cytoplasm has a similar effect to that of FaRPs on somatic acetylcholine receptors. Hence FaRPs may inhibit cholinosensitivity in Helix neurons via an elevation of intracellular Ca2+. This assumption is supported by the observation that FMRFamide increases basal intracellular Ca2+ in Helix neurons since FMRFamide amplifies a fluorescent signal from H. aspersa identified neurons loaded with Ca2+-sensitive dye fluo-3 [36].
Our electrophysiological data show that intracellular free Ca2+ is involved in the intracellular mechanism for the reduction of cholinosensitivity in Helix neurons by FaRPs [36,38,41]. Ryanodine receptors and IP3 receptors may be involved in the inhibitory metabotropic effects of FaRPs on somatic cholinergic receptors of identified Helix neurons [36,38,41,42].
We searched the literature for a possible cellular regulator to explain the inhibitory metabotropic effect evoked by the FaRPs on ACh receptors. One possible regulator is the Na,K-pump. It is known from the literature that inhibition of the Na,K-pump by ouabain evokes a decrease of ACh-induced currents of H. pomatia dialysed neurons [3]. Ouabain can elevate intracellular calcium via the activation of the reverse mode of Na/Ca-exchange (Na+-efflux, Ca2+-influx) [1,11,13,15,20,31,45,46] and stimulate Ca2+ release from internal stores [4,32,46,47].
Previous work has shown that FMRFamide increases intracellular Ca2+ via inhibition in Naout-dependent Ca2+-efflux (Na/Ca-exchange in the normal mode) [16,24,51] and activation of Ca2+-mobilization via ryanodine receptors and IP3 receptors [18,19,36,38,41,54].
Ouabain reduces reversibly ACh-induced inward currents in identified H. lucorum neurons (LPa2, LPa3, RPa3, RPa2) [33,35,37] that were used in our investigations with FaRPs [36,38,39,40,42]. This ouabain-mediated metabotropic effect is Ca2+-dependent.
There are identical effects of FaRPs and ouabain on the amplitude of ACh-induced currents in Helix neurons and on intracellular Ca2+ level. This provides the basis to postulate a working hypothesis: FaRPs may inhibit postsynaptic cholinosensitivity in Helix neurons partly via initial Na,K-pump inhibition by means of G-proteins followed by elevation of intracellular free Ca2+.
In this study we investigated the participation of Na,K-pump in the modulatory effect of endogenous molluscan heptapeptide, SEPYLRFamide, on cholinosensitivity in Helix neurones. The role of the following intracellular regulators were investigated, G-proteins, Na/Ca-exchange and intracellular calcium released from internal pools in the modulatory peptide-mediated cascade involving inhibition of Na,K-pump.
2. Materials and methods
2.1. Recording of transmembrane currents
2.1.1. Animals
Snails, H. lucorum, were collected locally in the Sevastopol region, Crimea, Ukraine. Experiments were performed using an isolated ganglia preparation at room temperature (18–22 °C) during 2002–2004.
Animals were anaesthetized in cold saline and the circumoesophageal nerve ring was removed for study. Circumoesophageal ganglia were pinned down, dorsal side up, on a silicon rubber-coated flow chamber with a bath volume of 1.0 ml. The connective tissue sheath covering neurons was removed prior to the experiments after preliminary enzymatic preparation (digestase/Seatec/, 0.5%, 30–40 min at room temperature (18–22 °C). Ganglia were constantly superfused (0.5–0.8 ml/min) with normal Helix saline containing (in mM): NaCl, 100; KCl, 4; CaCl2,10;MgCl2, 4; Tris buffer, 10; adjusted to pH 7.5 with HCl.
2.1.2. Electrophysiological recording
Experiments were carried out on identified LPa2, LPa3, RPa3 and RPa2 neurons from left and right parietal ganglia of H. lucorum. These cells are command elements of withdrawal responses to noxious stimuli [22].
Single-barrel glass microelectrodes were pulled using a PUL-1 puller (World Precision Instruments) from Pyrex glass (1.5 mm outer diameter) and filled with 2 M potassium acetate; resistance 8–120 MΩ. The electrodes were connected by a Ag–AgCl microelectrode holder (World Precision Instruments design) to a Micro-Electrode Amplifier MEZ-8101 (Nihon Kohden) and Voltage Clamp Amplifier CEZ-1100 (Nihon Kohden) that were used in Virtual Ground Mode for two-electrode voltage clamp experiments. A briquette of Ag–AgCl (Medicor) was used as reference electrode. The neurons were clamped at −75 mV. Currents were entered on a PC through the analogous-digital interface L-154 (L-CARD, Moscow) and recorded using CONAN 3.5 software (InCo, Moscow, Russia).
ACh was applied ionophoretically (interstimulus interval 5 min) onto the neuronal soma using the current source isolated from the ground. The ionophoretic solution was as follows: 1 M ACh chloride (Sigma) in distilled water (pH 7.0). The resistance of the ionophoretic pipette was 14–46 MΩ. The reference pipette was filled with normal Helix saline with a resistance of 1–3 MΩ. Cationic currents (685–877 nA; 0.1–3.0 s) were used for ionophoresis. A Laboratory Electrostimulator ESL-2 (Kaunas Research Institute of Radiometrical Engineering) was used to control the duration of the ionophoretic current. Ejection of positive currents through an ionophoretic pipette filled only with distilled water had no effect on the cells. A negative retention current (10 nA) was passed continuously through the ionophoretic pipette in order to prevent the spontaneous diffusion of ACh.
For an estimation of changes of stationary membrane conductance the holding potential in voltage clamp mode was shifted periodically in a negative direction (rectangular pulses, 10 mV, 5 s) for 9 s up to the start of ionophoretic ACh application. Change of amplitude of the inward leak current evoked by the negative shift of a holding potential was directly proportional to the change in resting membrane conductance.
2.1.3. Drugs
SEPYLRFamide (Department of Biochemistry, University of Southampton; Alta Bioscience, University of Birmingham) was locally applied under pressure at a concentration of 5 mM using piston ejector KM-2 (Chernogolovka). Peptide was applied from a glass micropipette with a broken tip, inner diameter of micropipette tip was 4.3–10.6 μM. Volume of applied solution was 4–14 nL; duration of application was 5 or 10 s; speed of application was 0.4–2.8 nL/s; distance of micropipette tip from the recording cell was 2–3 cell diameter (0.2–0.3 mm) to prevent mechanical action of ejected solution: interval between repeated peptide applications was 60 min. Red dye ruthenium red (10 mM, Sigma) was locally applied under pressure to evaluate this type of application.
An inhibitor of Na,K-pump, ouabain (0.1 mM), and an inhibitor of Na/Ca-exchange, benzamil (25 μM), were bath applied. Other compounds entered the cells by passive loading (diffusion) from intracellular voltage recording microelectrodes during a sixty minute period: sodium acetate, sodium chloride, inhibitors of Ca-ATPase in endoplasmic and sarcoplasmic reticulum (thapsigargin, cyclopiazonic acid). All drugs were obtained from Sigma.
SEPYLRFamide (5 mM) was dissolved in Helix saline. Cyclopiazonic acid (0.1 M) and thapsigargin (0.1 mM) were dissolved in dimethyl sulfoxide (DMSO, 5%; Sigma) and 2 M potassium acetate. These solutions were stored at −18 °C.
Stock solutions of ouabain (10 mM) and benzamil (5 mM) were prepared using Helix saline and stored at +4 °C. Sodium acetate (3 M) and sodium chloride (3 M) were dissolved in distilled water and stored at room temperature (18–22 °C).
The results were obtained from 167 neurons in 167 preparations. The resting potential of the cells was −61.10 ± 0.62 mV. The input resistance of neurons was 4.46 ± 0.18 MΩ.
2.1.4. Data analysis
The data are expressed as means ± standard error of the mean (S.E.M.). Each amplitude of ACh-induced current was normalised to the last three responses before each peptide application. In the text and figures, n represents the number of neurons. Significant differences for comparisons between two groups (control and experimental) were evaluated using nonparametric Wilcoxon's test. Student's t-test was used for comparisons between two linear regression slopes. A computer statistical software STADIA 6.2 (InCo, Moscow, Russia) was used for statistical analysis. A P value of less than 0.05 was considered to be statistically significant.
2.2. Measurement of Na,K-ATPase activity
2.2.1. Animals
Snails (H. aspersa.L) were collected locally in Southampton. Circumoesophageal nerve rings were extracted. Experiments were performed during October–December.
2.2.2. Homogenisation
H. aspersa were dissected, and the brain removed and weighed. The brain tissue was mixed with 5 ml per gram of medium containing: 0.32 M sucrose, 1mM EDTA, 0.1% deoxycholate, 50 mM Tris/MES, 250 μM ammonium molybdate at pH 7.4 [26 with some modification]. The homogenate was processed by differential centrifugation to separate the nuclear and mitochondrial fractions. This mixture was homogenised at 4 °C using a Teflon glass homogeniser until all tissue was broken down. A crude pellet containing mitochondria, nerve ending membranes, and myelin was separated by centrifugation at 10,000 ×g for 10 min. Clear supernatant was removed and centrifuged in a Beckman ultracentrifuge at 100,000 ×g for 60 min. This double-stage homogenization was undertaken to obtain light microsomal fraction enriched by small vesicles originating from the plasma membrane and membranes of the endoplasmic reticulum.
The final sediment was resuspended in a medium containing 0.32 M sucrose, 1 mM EDTA, 50 mM Tris/MES, 250 μM ammonium molybdate at pH 7.4. The aliquots were stored at −75 °C. Brain samples were diluted in Tris/MES buffer with 250 μM ammonium molybdate at pH 7.4 for ATPase assay.
2.2.3. ATPase assay
Measurement of ATPase activity within membrane vesicles was monitored by colorimetric determination of free inorganic phosphate released (Pi) from ATP using the catalyst polyvinylpyrrolidone [34].
Diluting 3 mM KH2PO4 in Tris/MES buffer with 250 μM ammonium molybdate at pH 7.4 produced twelve standard phosphate solutions between 0 and 96 nM PO4 per 50 μl. Each of these solutions was applied in triplicate to each assay to produce a standard phosphate curve.
A 96 well Tissue Culture Testplate (TPP, Switzerland) was used to perform this assay. The reaction was started by addition of 30–40 μl of reaction mixture to each experimental well. The reaction mixture (10 ml) consisted of: 4 ml Tris/Mes buffer with 250 μM ammonium molybdate at pH 7.4, 1 ml 20 mM ATP, 1 ml 20 mM MgSO4, 1 ml 500 mM KCl, 3 ml H20. The tissue was then incubated on ice under appropriate conditions for 30 min.
After the first incubation the tissue samples were added to the experimental wells in triplicate. The total volume in each experimental well was 50 μl with reaction mixture and experimental sample.
Reaction mixture alone was added to three wells to serve as a blank. The 96 well microtitre plate was then incubated at 37 °C for 60 min, gently mixing every 20 min.
Once incubation was complete the reaction was stopped by adding 8 μM 10% sodium dodecyl sulphate to each well. Then 150 μl Ohnishi A/B reagent and 15 μl Ohnishi Colour Developer were added to each well and mixed thoroughly using a multipipette. A colour developed over a 20 min period. The plate was then read at 620 nm of wavelength to obtain values for optical density using a Spectrophotometer Dynatech MR5000 (Anthos Labtec Instruments).
In order to determinate the level of protein in the extracts a protein assay was carried out using Helix brain tissue. Samples of the extract were added in triplicate at 1:10, 1:100 and 1:1000 concentrations. A standard curve of known human serum album (HSA) concentration was also added to the plate. The standard curve consisted of values between 0 and 2 mg/ml (HSA). BAC reagent was then added to all experimental plates and colour was allowed to develop for 30 min. The plate was then read at 620 nm wavelength and protein content calculated.
2.2.4. Drugs
SEPYLRFamide was obtained from Alta Bioscience (University of Birmingham, UK) and Department of Biochemistry (University of Southampton, UK). Ouabain, an inhibitor of Na, K-ATPase; adenosine 5′-trisphosphate; non-hydrolizable GTP analogue, guanosine-5′-O-(3-thiotriphosphate) tetralithium salt (GTP-γ-S); Na azide, an inhibitor of mitochondrial ATPase, were all obtained from Sigma.
All drugs were dissolved in water medium contained 50 mM Tris/MES, 0.32 M sucrose and 1 mM EDTA, pH 7.4. Stock solutions of SEPYLRFamide (2 mM) and GTP-γ-S (2 mM) were stored at −18 °C. Stock solution of ouabain (20 mM) was stored at +4 °C. Stock solution of Na azide (8 mM) was stored at room temperature (18–22 °C).
2.2.5. Data analysis
The data are expressed as mean ± standard error of the mean (S.E.M.). Significant differences for comparisons between two groups (control and experimental) were evaluated using nonparametric Mann–Whitney rank sum test. The computer statistical software SigmaStat 3.0 was used for statistical analysis. A P value of less than 0.05 was considered to be statistically significant.
3. Results
3.1. Control experiments with pressure application of ruthenium red onto neuron soma
In control experiments (n = 4) a dye, ruthenium red (10 mM), was applied under pressure onto the H. lucorum identified neurons RPa3 or LPa3 using the same parameters of application as with SEPYLRFamide. Red solution reached the soma of the target neuron 1–2 s after the end of pressure ejection of ruthenium red. The volume of ejected coloured cone exceeded the size of the target cell. This means that there was dilution of the applied solution.
Several conclusions could be made from this. It was calculated that the degree of dilution of the applied solution adjacent to the soma was around 100 times. We can therefore assume that the concentration of the applied peptide around the neuronal soma was approximately 50 μM. Following single peptide application, the peptide concentration in the flow chamber was 0.02–0.07 μM. This concentration is smaller compared with the threshold concentrations of FMRFamide-related peptides for an inhibitory action on ACh-induced inward current (0.1–1.0 μM) [39,40]. So we can assume that SEPYLRFamide application under pressure mediates the local effect on the target neuron.
3.2. SEPYLRFamide-evoked reduction of ACh-induced inward currents
The first experiment was a control to investigate the effect of pressure on the amplitude of the ACh-induced current using Helix saline ejection. In these experiments (n = 11) pressure application of Helix saline did not change the ACh-current amplitude. 20 s after the end of application the amplitude of ACh-current was 101.00 ± 5.83% (Table 1).
Table 1.
Effects of drugs on SEPYLRFamide-mediated reduction of ACh-induced current in Helix lucorum neurons
| No | Drugs | Amplitude of ACh-current, % | Compared series | Statistical significance |
|---|---|---|---|---|
| 1 | Helix saline; n=11 | 101.00 ± 5.83 | – | – |
| 2.1 | SEPYLRFamide, 1st application (S1); n=30 | 57.73 ± 4.04 | 1 | Đ<0.001* |
| 2.2 | SEPYLRFamide, 2nd application (S2); n=16 | 54.21 ± 5.31 | 2.1 | NS |
| 2.3 | SEPYLRFamide, 3rd application (S3); n=10 | 66.87 ± 8.63 | 2.1 | NS |
| 2.2 | NS | |||
| 3.1 | S (before ouabain; control); n=18 | 51.90 ± 3.94 | – | – |
| 3.2 | OUABAIN (O)+S; n=18 | 62.96 ± 5.52 | 3.1 | Đ<0.05 |
| 3.3 | S (after wash of ouabain); n=15 | 48.09 ± 5.30 | 3.2 | P<0.05 |
| 3.1 | NS | |||
| 2.1 + 3.1 | S1 + S con; n=48 | 55.54 ± 2.93 | 1 | P<0.001 |
| 4 | Na (in) + S; n=18 | 82.38 ± 3.92 | 2.1 | Đ<0.001 |
| 5.1 | BZ + S1; n=12 | 72.48 ± 7.29 | 2.1 | P<0.05 |
| 2.1 + 3.1 | P<0.05 | |||
| 5.2 | BZ + S2; n=7 | 71.67 ± 6.43 | 5.1 | NS |
| 5.3 | BZ + S3; n=5 | 74.07 ± 7.53 | 5.2 | NS |
| 6.1 | BZ + S (before ouabain; control); n=15 | 76.13 ± 6.70 | 2.1 + 3.1 | P<0.01 |
| 6.2 | BZ + O + S; n=15 | 78.15 ± 7.47 | 6.1 | NS |
| 6.3 | BZ + S; (after wash of ouabain); n=8 | 81.96 ± 8.41 | 6.2 | NS |
| 6.1 | NS | |||
| 5.1 + 6.1 | BZ-S1 + BZ-Scon; n=27 | 74.50 ± 4.98 | 2.1 + 3.1 | P<0.001 |
| 7.1 | TG + S1; n=13 | 77.25 ± 7.27 | 2.1 + 3.1 | P<0.01 |
| 7.2 | TG + S2; n=7 | 73.40 ± 7.40 | 7.1 | NS |
| 7.3 | TG + S3; n=4 | 66.28 ± 12.88 | 7.2 | NS |
| 8.1 | TG + S (before ouabain; control); n=16 | 67.46 ± 6.08 | 2.1 + 3.1 | P<0.05 |
| 8.2 | TG + O + S; n=11 | 68.12 ± 8.50 | 8.1 | NS |
| 8.3 | TG + S (after wash of ouabain); n=9 | 77.89 ± 5.36 | 8.2 | NS |
| 8.1 | NS | |||
| 7.1 + 8.1 | TG-S1 + TG-Scon; n=29 | 71.85 ± 4.82 | 2.1 + 3.1 | P<0.01 |
| 9.1 | CPA + S1; n=16 | 67.26 ± 4.92 | 2.1 + 3.1 | Đ<0.05 |
| 9.2 | CPA + S2; n=11 | 55.64 ± 4.59 | 9.1 | NS |
| 9.1 + 10.1 | Đ<0.05 | |||
| 9.3 | CPA + S3; n=6 | 55.68 ± 5.35 | 9.2 | NS |
| 9.1 + 10.1 | Đ<0.05 | |||
| 10.1 | CPA + S (before ouabain; control); n=17 | 70.25 ± 4.52 | 2.1 + 3.1 | Đ<0.05 |
| 10.2 | CPA + O + S; n=13 | 85.07 ± 5.92 | 10.1 | Đ<0.05 |
| 10.3 | CPA + S (after wash of ouabain); n=12 | 70.51 ± 4.54 | 10.2 | P<0.05 |
| 10.1 | NS | |||
| 9.1 + 10.1 | CPA-S1 + CPA-Scon; n=33 | 68.80 ± 3.29 | 2.1 + 3.1 | P<0.01 |
Values represent mean ± S.E.M.;
Wilcoxon test, NS —nonsignificant.
This control was followed by three SEPYLRFamide applications with a 60 min interval before each application of peptide.
The first local application of SEPYLRFamide (5 mM) under pressure evoked time-dependent reversible reduction of ACh-current in all LPa2, LPa3, RPa3 and RPa2 neurons that were investigated (Fig. 1). The minimal amplitude of the ACh-current occurred 20 s following the end of the local application of SEPYLRFamide, 57.73 ± 4.04%, n = 18 cells, P < 0.001) (Table 1). Reduction of the ACh-current amplitude to the 2nd and 3rd SEPYLRFamide application did not differ significantly from the peptide effect to the 1st application (Table 1). This means that repeated peptide applications did not evoke significant changes in membrane sensitivity to SEPYLRFamide.
Fig. 1.
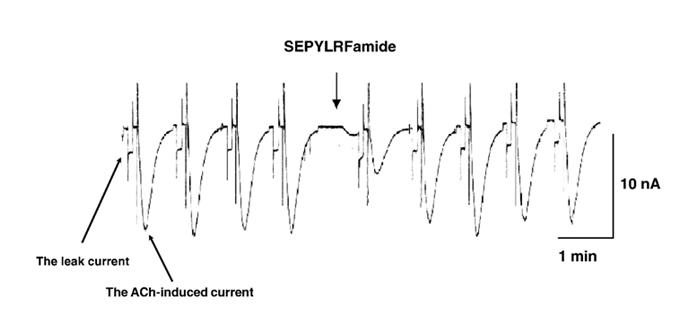
Effect of SEPYLRFamide on ACh-induced inward current and leak current in an RPa3 neuron.
3.3. Effect of ouabain on ACh-induced inward current and leak current
Before we investigated the effect of ouabain on SEPYLRFamide-evoked reduction of ACh-induced inward currents we checked for any effects of ouabain on neurons. Ouabain (0.1 mM, bath application, n = 18) evoked three clear effects in Helix neurons that developed gradually and reached stable levels after 30 min exposure: 1) the slow inward current (5–15 nA, not shown), 2) reduction of the ACh-current amplitude to 81.99 ± 5.78%, P < 0.05 (Tables 2 and 3) increase of the leak current amplitude to 150.90 ± 5.78%, P < 0.01 (Table 3).
Table 2.
Effects of OUABAIN (0.1 mM) on ACh-induced current in Helix lucorum neurons
| No | Drugs | ACh-current, (% control) |
Compared series |
Statistical significance |
|---|---|---|---|---|
| 1 | Helix saline; n=10 | 99.94 ± 7.61 | – | – |
| 2 | Ouabain (only); n=18 |
81.99 ± 5.78 | 1 | Đ<0.05* |
| 3 | Benzamil+ouabain; n=15 |
100.90 ± 4.87 | 1 | NS |
| 2 | P<0.05 | |||
| 4 | Thapsigargin+ ouabain, n=11 |
99.39 ± 5.64 | 1 | NS |
| 2 | P<0.05 | |||
| 5 | Cyclopiazonic acid+ ouabain, n=14 |
81.78 ± 5.06 | 1 | Đ<0.05 |
| 2 | NS |
Values represent mean ± S.E.M.;
Wilcoxon test, NS —nonsignificant.
Table 3.
Effects of ouabain (0.1 mM) on leak current in Helix lucorum neurons
| No | Drugs | Leak current, (% control) |
Compared series |
Statistical significance |
|---|---|---|---|---|
| 1 | Helix saline; n=10 | 93.27 ± 7.61 | – | – |
| 2 | Ouabain (only); n=18 | 150.90 ± 13.50 | 1 | Đ<0.01* |
| 3 | Benzamil+ouabain; n=15 | 136.70 ± 8.38 | 1 | P<0.01 |
| 2 | NS | |||
| 4 | Thapsigargin+ouabain; n=11 |
176.90 ± 15.65 | 1 | P<0.001 |
| 2 | NS | |||
| 5 | Cyclopiazonic acid+ ouabain; n=14 |
159.20 ± 9.20 | 1 | Đ<0.001 |
| 2 | NS |
Values represent mean ± S.E.M.;
Wilcoxon test, NS —nonsignificant.
3.4. Effect of ouabain on SEPYLRFamide-evoked reduction of ACh-induced inward currents
The experiment included three consecutive SEPYLRFamide applications with a 60 min interval: before ouabain, after 30 min of the application of ouabain and after washout.
Ouabain decreased the ability of SEPYLRFamide to reduce ACh-current amplitude (Table 1). Amplitude of the ACh-current 20 s after peptide application was 51.90 ± 3.94% before ouabain (n = 18), 62.90 ± 3.94% after the application of ouabain (n = 18) and 48.09 ± 5.30% (n = 15) after wash. There was a statistically significant reduction by ouabain of the peptide effect (P < 0.05) and recovery of peptide effect after wash (P < 0.05).
There was a significant correlation between the effects of ouabain on the amplitude of the ACh-current and on the modulatory peptide effect (r = 0.58, Pearson coefficient; P < 0.001; linear regression slope = 0.63). There was no correlation between the effects of ouabain on the amplitude of the leak current and on the modulatory peptide effect (r = 0.15, Pearson coefficient; P > 0.05; linear regression slope = 0.08).
3.5. Influence of intracellular action of sodium ions on SEPYLRFamide-evoked reduction of ACh-induced inward currents
Passive loading of the cell with sodium ions from an intracellular electrode during a 60 min period will raise the level of intracellular sodium ions and activate the Na,K-pump. Activation of Na,K-ATPase by intracellular injection of 3 M Na acetate or 3 M NaCl reduced the modulatory peptide effect on the ACh-current (Table 1). The minimal amplitude of the ACh-current 20 s after the first SEPYLRFamide pressure application following 60 min sodium injection was 82.38 ± 3.92% (n = 18 cells, P < 0.001). It is significantly smaller (P < 0.001) as compared with the reduction of the ACh-current to the first SEPYLRFamide application without injection of sodium ions (57.73 ± 4.04%, n = 30).
3.6. Effect of benzamil on the effect of ouabain on SEPYLRFamide-evoked reduction of ACh-induced inward currents
The specific inhibitor of Na/Ca-exchange in plasmalemma, benzamil (BZ, bath application, 25 μM), decreased the peptide-mediated reduction of ACh-current (Table 1). The minimal amplitude of the ACh-current 20 s after the first SEPYLRFamide pressure application following 30 min of BZ application was 72.48 ± 7.29% (n = 12 cells, P < 0.001). It is significantly smaller (P < 0.05) as compared with the reduction of the ACh-current to the first SEPYLRFamide application without the action of BZ (55.54 ± 2.93%, n = 48).
Reductions of the ACh-current amplitude to the 2nd and 3rd SEPYLRFamide application in the presence of BZ did not differ significantly from the peptide effect to the 1st application (Table 1). This means that repeated peptide application after BZ application did not change the membrane sensitivity to SEPYLRFamide.
BZ blocked the effect of ouabain on the SEPYLRFamide-mediated modulatory effect. After application of BZ, ouabain did not change the ability of SEPYLRFamide to reduce the ACh-current amplitude (Table 1). The amplitude of the ACh-current 20 s after the end of peptide application was 76.13 ± 6.70% before ouabain (n = 15), 78.15 ± 7.47% in the presence of ouabain (n = 15) and 81.96 ± 8.41% (n = 8) after wash. The differences between these peptide effects were not significant.
BZ blocked ouabain-induced reduction of the ACh-current but did not change the increase in the leak current by ouabain. In the presence of BZ the amplitude of ACh-current was 100.90 ± 4.87%, (n=15, P > 0.05, Table 2) after 30 min bath application of ouabain. Ouabain increased the leak current amplitude to 136.90 ± 8.38%, P< 0.01 (Table 3).
3.7. Effect of thapsigargin on the effect of ouabain on SEPYLRFamide-evoked reduction of ACh-induced inward currents
The specific inhibitor of endoplasmic reticulum Ca2+-ATPase, thapsigargin (TG, passive loading the cell from an intracellular electrode during 60 min period, 0.1 mM), decreased the peptide-mediated reduction of ACh-current (Table 1). The minimal amplitude of the ACh-current 20 s after the first SEPYLRFamide pressure application following 60 min of TG loading was 77.25 ± 7.27% (n =13 cells, P < 0.001). It is significantly smaller (P < 0.01) as compared with the reduction of the ACh-current to the first SEPYLRFamide application without injection of TG (55.54 ± 2.93%, n =48).
Reductions of the ACh-current amplitude to the 2nd and 3rd SEPYLRFamide application in the presence of TG did not differ from the peptide effect to the 1st application (Table 1). This means that repeated peptide application after TG injection did not change the membrane sensitivity to SEPYLRFamide.
TG blocked the effect of ouabain on the SEPYLRFamide-mediated modulatory effect. After intracellular injection of TG, ouabain did not change the ability of SEPYLRFamide to reduce the ACh-current amplitude (Table 1). The amplitude of the ACh-current 20s after the end of peptide application was 67.46 ± 6.08% before ouabain (n = 16), 68.12 ± 8.50% in the presence of ouabain (n = 11) and 77.89 ± 5.36% (n = 9) after wash. The differences between these peptide effects were not significant.
TG blocked ouabain-induced reduction of the ACh-current but did not change the increase in the leak current by ouabain. In the presence of intracellular TG the amplitude of ACh-current was 99.78 ± 5.06%, (n =11, P > 0.05, Table 2) after 30 min bath application of ouabain. Ouabain increased the leak current amplitude to 176.90 ± 15.65%, P < 0.001 (Table 3).
3.8. Influence of cyclopiazonic acid on the effect of ouabain on SEPYLRFamide-evoked reduction of ACh-induced inward currents
Another specific inhibitor of endoplasmic reticulum Ca2+-ATPase, cyclopiazonic acid (CPA, passive loading the cell from an intracellular electrode during 60 min period, 0.1 mM) decreased the peptide-mediated reduction of ACh-current (Table 1). The minimal amplitude of the ACh-current 20 s after the first SEPYLRFamide pressure application following 60 min of CPA injection was 67.26 ± 4.92% (n=16 cells, P < 0.001). It is significantly smaller (P< 0.05) as compared with the reduction of ACh-current to the first SEPYLRFamide application without injection of CPA (55.54 ±2.93%, n=48).
Reductions of the ACh-current amplitude to the 2nd and 3rd SEPYLRFamide application on the background of CPA was greater as compared with the peptide effect to the 1st application (combined group, lines 9.1 + 10.1, Table 1). This means that CPA decreased the peptide-mediated reduction of ACh-current only to the 1st peptide application.
CPA did not prevent the effect of ouabain on the SEPYLRFamide-mediated modulatory effect. After intracellular injection of CPA ouabain decreased the ability of SEPYLRFamide to reduce the ACh-current amplitude (Table 1). Amplitude of ACh-current 20 s after the end of peptide application was 70.25 ± 4.52% before ouabain (n =17), 85.07 ± 5.92% in the presence of ouabain (n =13) and 70.51 ± 4.54% (n =12) after wash. There was a statistically significant reduction by ouabain of the peptide effect (P < 0.05) and recovery of peptide effect after wash (P < 0.05).
CPA did not change the ouabain-induced reduction of the ACh-current and increase of the leak current. On the background of intracellular injection of CPA, ouabain (0.1 mM, bath application, n = 14) evoked a reduction of the ACh-current amplitude to 81.78 ± 5.06%, P < 0.05 (Table 2) and an increase in the leak current amplitude to 159.20 ± 5.06%, P < 0.001 (Table 3).
3.9. ATPase activity in Helix brain extracts
The presence of ATPase activity in the extracts was first established. It was shown that brain extracts produced 81 nM phosphate per 5 μl extract per hour. From the optical density and protein assay data, average phosphate produced was calculated. The average protein content in brain extracts was 8.23 mg/ml. The average phosphate produced by the brain was 0.561 ± 0.032 μM/ml/h or 921.76 μM of phosphate per gram protein per hour (n=9).
3.10. Effect of ouabain and SEPYLRFamide on activity of purified Na,K-ATPase
The next biochemical experiment was to demonstrate the effects of ouabain and SEPYLRFamide on purified Na,K-ATPase (2 mU/μl) activity. Ouabain (5 mM) decreased activity of pure Na,K-ATPase to 30.34 ± 5.87% (n =9, P<0.001). SEPYLRFamide (0.5 mM) did not change activity of pure Na,K-ATPase (data not shown).
These results demonstrate that ouabain reduced activity of purified Na,K-ATPase on an average by 70%. But SEPYLRFamide did not change the activity of the purified enzyme.
3.11. Effect of ouabain, SEPYLRFamide and GTP-γ-S on Na, K-ATPase activity in extracts from H. aspersa brain (circumoesophageal ganglia)
These experiments were performed using the light microsomal fraction that is enriched by small vesicles originating from plasma membrane and membranes of endoplasmic reticulum, as described in method section. Brain extracts were dissolved in the medium containing 1 mM EDTA which suppresses Ca2+-ATPase activity in the endoplasmic reticulum and therefore ATP hydrolysis will be due to only two ATPases, viz, Na,K-ATPase and ecto-ATPase. Some of these ecto-ATPases are inhibited by 1 mM azide. Therefore all the following experiments were made in the presence of 2 mM Na azide. It is reasonable to conclude that after this ATP hydrolysis was due to only Na,K-ATPase.
Ouabain, 5 mM, inhibited brain Na,K-ATPase activity by 10%. Enzyme activity in the presence of ouabain was 90.65 ± 4.23% (n=11, P=0.032) relatively to control activity without ouabain (Table 4).
Table 4.
Effects of ouabain (5 mM), SEPYLRFamide (0.5 mM) and GTP-γ-S (0.5 mM) on Na,K-ATPase activity in extracts from Helix aspersa brain
| No | Drugs | Enzyme activity, % |
|---|---|---|
| 1 | Ouabain (only)/control, n=11 | 90.65 ± 4.23 |
| P<0.05* | ||
| 2 | SEPYLRFamide (only)/control, n=10 | 87.38 ± 9.66 |
| P<0.01 | ||
| 3 | GTP-γ-S (only)/Control, n=15 | 85.14 ± 1.88 |
| P<0.001 |
Incubation medium contained: 50 mM Tris/Mes, 250 μM ammonium molybdate, 0.32 M sucrose, 2 mM ATP, 2 mM MgSO4, 50 mM KCl, 1 mM EDTA, 2 mMNa Azide; values represent mean ± S.E.M.;
Mann-Whitney rank sum test.
SEPYLRFamide, 0.5 mM, also inhibited brain Na,K-ATPase activity by about 10%. Enzyme activity in the presence of SEPYLRFamide was 87.38 ± 9.66% (n=10, P=0.003) relative to control enzyme activity without SEPYLRFamide (Table 4).
GTP-γ-S, 0.5 mM, inhibited on average by 15% brain Na,K-ATPase activity. Enzyme activity in the presence of GTP-γ-S was 85.14 ± 1.88% (n=15, P<0.001) relative to control activity without the non-hydrolysable GTP analogue (Table 4).
4. Discussion
The endogenous Helix heptapeptide SEPYLRFamide evoked a reversible reduction in the somatic membrane cholinosensitivity in identified H. lucorum neurons as it decreased acetylcholine-induced inward current.
Our data show that Na,K-ATPase may be involved in the intracellular mechanism that is responsible for the SEPYLRFamide-mediated inhibition of neuronal cholinosensitivity. Ouabain, a specific inhibitor of Na,K-ATPase, decreased the ACh-current and increased the leak current. Ouabain decreased the modulatory peptide effect on the ACh-current. There was a significant correlation between the effects of ouabain on the amplitude of the ACh-current and on the modulatory peptide effect. There was no correlation between the effects of ouabain on the amplitude of the leak current and on the modulatory peptide effect. Activation of Na,K-ATPase by intracellular injection of 3 M Na acetate or 3 M NaCl reduced the modulatory peptide effect on the ACh-current. These results indicate that SEPYLRFamide inhibits Na,K-pump. Our biochemical results support this theoretical proposition.
Why did activation of the Na,K-ATPase through a rise in intracellular Na+ reduce the modulatory peptide effect on ACh-current? We assume that extracellular SEPYLRFamide and intracellular free Na+ bind different sites of the membrane Na, K-ATPase. It is likely that in our experiments intracellular injection of Na+ evoked greater activation of Na,K-ATPase compared with the inhibition of this enzyme by SEPYLRFamide. As a result, elevation of intracellular Na+ reduced the inhibitory effect of peptide on the Na,K-ATPase activity.
Inhibition of ouabain-sensitive neuronal Na,K-ATPase by FMRFamide-related peptide, SEPYLRFamide, has been shown for the first time. Other biologically active substances are known to inhibit the activity of Na,K-ATPase. For example, this enzyme is inhibited by adrenal cortical hormone (“endogenous ouabain”) [9], mastoporan [28], 5-hydroxytrytamine [49], atrial natriuretic peptide 99–126 [5], neurotensin [29], leptin [6] and parathyroid hormone [25]. It has been shown recently that parathyroid hormone inhibits Na+, K+-ATPase activity by serine phosphorylation of the α1 subunit through protein kinase C-(PKC) and extracellular signal regulated kinase-(ERK) dependent pathways [25].
In our experiments ouabain inhibited purified Na,K-ATPase activity by 70%. SEPYLRFamide did not change the activity of the purified enzyme. This means that there are no binding sites for SEPYLRFamide on the pure Na,K-ATPase. Ouabain, SEPYLRFamide and GTP-γ-S (activator of G-proteins) inhibited the activity of H. aspersa brain Na,K-ATPase. We can propose that SEPYLRFamide inhibits plasma membrane Na,K-ATPase indirectly, probably, via activation of G-proteins.
An inhibitor of plasmalemma Na/Ca-exchange, benzamil, and a specific inhibitor of endoplasmic reticulum Ca2+-ATPase, thapsigargin, prevented the effect of ouabain on the SEPYLRFamide-mediated modulatory effect. Benzamil and TG prevented the ouabain-mediated reduction of acetylcholine-induced inward current but did not change the increase of leak current. Another specific inhibitor of the endoplasmic reticulum Ca2+-ATPase, cyclopiazonic acid, did not prevent the effect of ouabain on the SEPYLRFamide-mediated modulatory effect. CPA did not change the ouabain-mediated reduction of acetylcholine-induced inward current and increase of leak current. These results allow us to propose that Na/Ca-exchange and intracellular Ca2+ released from internal Ca2+-depots containing TG-sensitive Ca2+-ATPase may be involved in the Na,K-ATPase pathway for the SEPYLRFamide-mediated inhibition of acetylcholine receptors.
Intracellular Ca2+ released from internal pools containing CPA-sensitive Ca2+-ATPase is not involved in the Na,K-ATPase pathway for the SEPYLRFamide-mediated inhibition of acetylcholine receptors. How can these results be explained? Two different types of internal Ca2+-depots have been found in H. pomatia neurons. These two calcium pools do not overlap and at least part of the caffeine-sensitive store (with ryanodine receptors) is located close to the cellular membrane while the inositol 1,4,5-trisphosphate-sensitive pool is located in the centre of the cell [27]. We propose that caffeine-sensitive stores (with ryanodine receptors) in the Helix neurons used in this study may contain TG-sensitive Ca2+-ATPase.
Taking into account our results and those from the literature we propose the following three intracellular mechanisms for the SEPYLRFamide-mediated inhibitory modulatory effect on Helix neurone cholinosensitivity without and with Na,K-pump (Fig. 2). These mechanisms have the same final target, the elevation of free intracellular Ca2+, and are closely related. (1) SEPYLRFamide → activation of membrane receptor → activation of G-protein → elevation of inositol-1,4,5-trisphosphate (IP3, second messenger) level → activation of IP3 receptors → Ca2+ release from internal store → elevation of intracellular free Ca2+ [42]. (2) SEPYLRFamide → activation of membrane receptor → activation of G-protein → elevation of cyclic adenosine diphosphate-ribose (cADPR, second messenger) level → activation of ryanodine receptors → Ca2+ release from internal store → elevation of intracellular free Ca2+ [36]. (3) SEPYLRFamide → activation of membrane receptor → activation of G-protein → activation of neuronal signal molecule → inhibition of membrane Na,K-pump → elevation of intracellular content of Na+ → inhibition of Na/Ca-exchange in normal mode (Na+-influx, Ca2+-efflux) and activation of Na/Ca-exchange in reverse mode (Na+-efflux, Ca2+-influx) → elevation of intracellular free Ca2+ → activation of Ca2+-sensitive receptors (channels) in endoplasmic reticulum (IP3 receptors and ryanodine receptors) → Ca2+ release from internal stores → elevation of intracellular free Ca2+. Inhibition of the Na,K-ATPase must stop the hydrolysis of ATP and evoke a corresponding elevation in the intracellular ATP level that can activate ryanodine-sensitive Ca2+ release channel [48].So it is not excluded that peptide-mediated inhibition of Na,K-ATPase evokes Ca2+ release via elevated ATP and following activation of ryanodine receptors. The last pathway, including peptide-mediated inhibition of Na,K-ATPase, evokes Ca2+ release that amplifies Ca2+ signals evoked by the other two peptide-mediated pathways. This process is limited by negative feedback: high Ca2+in concentration inhibits Ca2+ release via the receptors in Ca2+-depots [7,8,10,44].
Fig. 2.
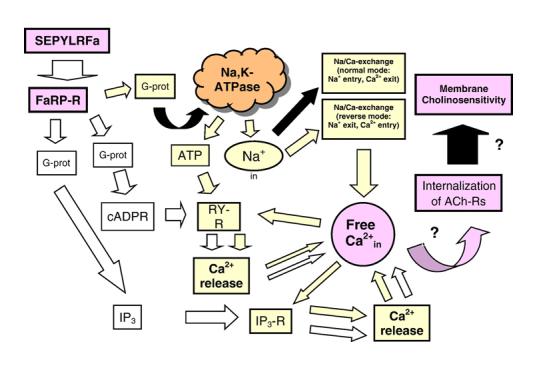
Putative intracellular mechanism of the SEPYLRFamide-mediated reduction of neuron cholinosensitivity. SEPYLRFa: SEPYLRFamide; FaRP-R: FMRFamide-related peptide receptor; G-Prot: G-protein; cADPR: cyclic adenosine diphosphate-ribose; ATP: adenosine triphosphate; IP3: inositol-1,4,5-trisphosphate; RY-R: ryanodine receptor; IP3-R: IP3 receptor; ACh-Rs: acetylcholine receptors. Activation, elevation of intracellular level: light arrows; inhibition: black arrows.
It should be noted that the scheme in Fig. 2 contains three G-protein boxes. However, this does not mean that the peptide receptor is connected with three different G-proteins. We do not have experimental evidence which would enable us to conclude that the three intracellular pathways include activation of the same G-protein. This remains an open question.
How does elevation of intracellular free Ca2+ mediate the reduction of membrane cholinosensitivity? It has been shown that regulation of AMPA receptor trafficking in hippocampal neurons results in changes in receptor number at a postsynaptic membrane, and hence modifications in synaptic strength, which are proposed to underlie learning and memory [17,21]. AMPA receptor internalization triggered by NMDA receptor activation is Ca2+-dependent, requires protein phosphatases, and is followed by rapid membrane reinsertion [17]. A novel postsynaptic Ca2+-binding protein provides a direct mechanistic link between NMDA receptor-mediated Ca2+ influx and AMPA-receptor endocytosis [21]. In consideration of these results we assume that the cytoplasmic Ca2+ which is elevated by SEPYLRF-amide activates an internalization of acetylcholine receptors via endocytosis that leads to a reversible reduction of cholinosensitivity in the non-synaptic membrane.
The postsynaptic membrane in H. lucorum neurons contains acetylcholine receptors [50]. In conclusion the above mechanism may be responsible for the reduction in cholinergic synaptic transmission to molluscan neurons by FMRFamide-related peptides [2,23,30].
Acknowledgement
This work was supported by the Wellcome Trust (grant #066177/Z/01/Z).
References
- 1.Alvarez Leefmans FJ, Merediz Alonso G, Fernandez Calderon JR. The consequences of sodium pump inhibition in the neurons and its relevance to the physiopathology of the cell damage produced by anoxic ischemia. Gac Med Mex. 1994;130:347–54. [PubMed] [Google Scholar]
- 2.Arvanov VL, Chen ML, Sharma R, Walker RJ, Ayrapetyan SN. Low concentrations of neuroactive peptides modulate cholinergic transmission and cyclic AMP levels in Helix aspersa. Acta Biol Hung. 1992;43:89–97. [PubMed] [Google Scholar]
- 3.Arvanov VL, Stepanyan AS, Ayrapetyan SN. The effects of cAMP, Ca2+, and phorbol esters on ouabain-induced depression of acetylcholine responses in Helix neurons. Cell Mol Neurobiol. 1992;12:153–61. doi: 10.1007/BF00713369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balduini W, Costa LG. Characterization of ouabain-induced phosphoinositide hydrolysis in brain slices of the neonatal rat. Neurochem Res. 1990;15:1023–9. doi: 10.1007/BF00965749. [DOI] [PubMed] [Google Scholar]
- 5.Beltowski J, Gorny D, Marciniak A. The mechanism of Na+,K+-ATPase inhibition by atrial natriuretic factor in rat renal medulla. J Physiol Pharmacol. 1998;49:271–83. [PubMed] [Google Scholar]
- 6.Beltowski J, Marciniak A, Wojcicka G. Leptin decreases renal medullary Na+,K+-ATPase activity through phosphatidylinositol 3-kinase dependent mechanism. J Physiol Pharmacol. 2004;55:391–407. [PubMed] [Google Scholar]
- 7.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 8.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32(5–6):235–49. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 9.Blaustein MP. Endogenous ouabain: role in the pathogenesis of hypertension. Kidney Int. 1996;49:1748–53. doi: 10.1038/ki.1996.260. [DOI] [PubMed] [Google Scholar]
- 10.Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: more messengers, more channels, more complexity. Curr Biol. 2002;12:R563–5. doi: 10.1016/s0960-9822(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 11.Charlemagne D. Molecular and cellular level of action of digitalis. Herz. 1993;18:79–85. [PubMed] [Google Scholar]
- 12.Chemeris NK, Kazachenko VN, Kislov AN, Kurchikov AA. Inhibition of acetylcholine responses by intracellular calcium in Lymnaea stagnalis neurons. J Physiol. 1982;323:1–19. doi: 10.1113/jphysiol.1982.sp014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condrescu M, Gardner JP, Chernaya G, Aceto JF, Kroupis C, Reeves JP. ATP-dependent regulation of sodium–calcium exchange in Chinese hamster ovary cells transfected with the bovine cardiac sodium–calcium exchanger. J Biol Chem. 1995;270:9137–46. doi: 10.1074/jbc.270.16.9137. [DOI] [PubMed] [Google Scholar]
- 14.Cottrell GA, Lin JW, Llinas R, Price DA, Sugimori M, Stanley EF. FMRFamide-related peptides potentiate transmission at the squid giant synapse. Exp Physiol. 1992;77:881–9. doi: 10.1113/expphysiol.1992.sp003655. [DOI] [PubMed] [Google Scholar]
- 15.Deitmer JW, Eckert R, Schlue WR. Changes in the intracellular free calcium concentration of Aplysia and leech neurones measured with calcium-sensitive microelectrodes. Can J Physiol Pharm. 1987;65:934–9. doi: 10.1139/y87-149. [DOI] [PubMed] [Google Scholar]
- 16.DiPolo R, Beauge L. Cardiac sarcolemmal Na/Ca-inhibiting peptides XIP and FMRF-amide also inhibit Na/Ca exchange in squid axons. Am J Physiol. 1994;267:C307–11. doi: 10.1152/ajpcell.1994.267.1.C307. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocyting sorting. Neuron. 2000;28:511–25. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 18.Ellis AM, Huddart H. Excitation evoked by FMRFamide and FLRFamide in the heart of Buccinum undatum and evidence for inositol 1,4,5-trisphosphate as an RF-tetrapeptide second messenger. J Comp Physiol B. 2000;170:351–6. doi: 10.1007/s003600000110. [DOI] [PubMed] [Google Scholar]
- 19.Falconer SW, Carter AN, Downes CP, Cottrell GA. The neuropeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) increases levels of inositol 1,4, 5-trisphosphate in the tentacle retractor muscle of Helix aspersa. Exp Physiol. 1993;78:757–66. doi: 10.1113/expphysiol.1993.sp003723. [DOI] [PubMed] [Google Scholar]
- 20.Goldman WF, Yarowsky PJ, Juhaszova M, Krueger BK, Blaustein MP. Sodium/calcium exchange in rat cortical astrocytes. J Neurosci. 1994;14:5834–43. doi: 10.1523/JNEUROSCI.14-10-05834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–78. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ierusalimskii VN, Zakharov IS, Palikhova TA, Balaban PM. The nervous system and the mapping of the neurons in the gastropod Helix lucorum L. Z Vyss Nervn Deat Im IP Pavlova. 1992;42:1075–89. [PubMed] [Google Scholar]
- 23.Keating C, Lloyd PE. Differential modulation of motor neurons that innervate the same muscle but use different excitatory transmitters in Aplysia. J Neurophysiol. 1999;82:1759–67. doi: 10.1152/jn.1999.82.4.1759. [DOI] [PubMed] [Google Scholar]
- 24.Khananshvili D, Price DC, Greenberg MJ, Sarne Y. Phe-Met-Arg-Phe-NH2 (FMRFa)-related peptides inhibit Na+–Ca2+ exchange in cardiac sarcolemma vesicles. J Biol Chem. 1993;268:200–5. [PubMed] [Google Scholar]
- 25.Khundmiri SJ, Dean WL, McLeish KR, Lederer ED. Parathyroid hormone-mediated regulation of Na+–K+-ATPase requires ERK-dependent translocation of protein kinase Calpha. J Biol Chem. 2005;280:8705–13. doi: 10.1074/jbc.M408606200. [DOI] [PubMed] [Google Scholar]
- 26.Koyabashi M, Muneoka Y. Structure and action of molluscan neuropeptides. Zool Sci. 1990;7:801–14. [Google Scholar]
- 27.Kostyuk PG, Kirischuk SI. Spatial heterogeneity of caffeine- and inositol 1,4,5-trisphosphate-induced Ca2+ transients in isolated snail neurons. Neuroscience. 1993;53:943–7. doi: 10.1016/0306-4522(93)90479-y. [DOI] [PubMed] [Google Scholar]
- 28.Langel U, Pooga M, Kairane C, Zilmer M, Bartfai T. A galanin–mastoparan chimeric peptide activates the Na+,K+-ATPase and reverses its inhibition by ouabain. Regul Pept. 1996;62:47–52. doi: 10.1016/0167-0115(96)00002-x. [DOI] [PubMed] [Google Scholar]
- 29.Lopez Ordieres MG, Rodriquez de Lores Arnaiz G. Neurotensin inhibits neuronal Na+–K+-ATPase activity through high affinity peptide receptor. Peptides. 2000;21:571–6. doi: 10.1016/s0196-9781(00)00183-2. [DOI] [PubMed] [Google Scholar]
- 30.Man-Son-Hing H, Zoran MJ, Lukowiak K, Haydon PG. A neuromodulation of synaptic transmission acts on the secretory apparatus as well as on ion channels. Nature. 1989;341:237–9. doi: 10.1038/341237a0. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Lehnert H, Backer A, Kramer HJ. Inhibitors of Na–K-ATPase in human urine: effects of ouabain-like factors and of vanadium–diascorbate on calcium mobilization in rat vascular smooth muscle cells: comparison with the effects of ouabain, angiotensin II, and arginine–vasopressin. Am J Hypertens. 2000;13:364–9. doi: 10.1016/s0895-7061(99)00197-1. [DOI] [PubMed] [Google Scholar]
- 32.Myles ME, Fain JN. Carbachol, but not norepinephrine, NMDA, ionomycin, ouabain, or phorbol myristate acetate, increases inositol 1,3,4,5-tetrakisphosphate accumulation in rat brain cortical slices. J Neurochem. 1994;62:2333–9. doi: 10.1046/j.1471-4159.1994.62062333.x. [DOI] [PubMed] [Google Scholar]
- 33.Nistratova VL, Pivovarov AS. Inositoltrisphospate receptors and ryanodine receptors in regulation of cholinosensitivity of Helix lucorum neurones by Na,K-pump during habituation. Z Vyss Nervn Deat Im IP Pavlova. 2004;54:554–64. [PubMed] [Google Scholar]
- 34.Ohnishi T, Gall RS, Mayer ML. An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem. 1975;69:261–7. doi: 10.1016/0003-2697(75)90585-0. [DOI] [PubMed] [Google Scholar]
- 35.Pivovarov AS, Boguslavsky DV. Na,K-pump regulates reduction of cholinosensitivity of Helix lucorum neurones in cellular analog of habituation: role of intracellular calcium. Z Vyss Nervn Deat Im IP Pavlova. 2000;50:855–66. [PubMed] [Google Scholar]
- 36.Pivovarov AS, Chad JE, Walker RJ. Involvement of ryanodine receptors in EPYLRFamide-mediated reduction of acetylcholine-induced inward currents in Helix lucorum identified neurones. Regul Pept. 2000;88:83–93. doi: 10.1016/s0167-0115(99)00125-1. [DOI] [PubMed] [Google Scholar]
- 37.Pivovarov AS, Nistratova VL, Boguslavskii DV. Participation of Na/Ca-exchange and intracellular mobilized Ca2+ in regulating depression of cholino-sensitive Helix lucorum neurons to a cellular analog of habituation. Z Vyss Nervn Deat Im IP Pavlova. 2001;51:723–32. [PubMed] [Google Scholar]
- 38.Pivovarov AS, Sharma R, Walker RJ. Inhibitory action of SKPYMRFamide on acetylcholine receptors of Helix aspersa neurons: role of second messengers. Gen Pharmacol. 1995;26:495–505. doi: 10.1016/0306-3623(95)94003-y. [DOI] [PubMed] [Google Scholar]
- 39.Pivovarov AS, Sharma R, Walker RJ. Structure-activity relationships of the Helix neuropeptide, SEPYLRFamide, and its N-terminally modified analogues on identified Helix lucorum neurones. Brain Res. 1999;821:294–308. doi: 10.1016/s0006-8993(99)01097-5. [DOI] [PubMed] [Google Scholar]
- 40.Pivovarov AS, Walker RJ. Direct and modulatory effects of FMRFamide, SKPYMRFamide and acety1-SKPYMRFamide on LPa2, LPa3, and RPa3 identified neurons of Helix lucorum. Regul Pept. 1996;67:169–78. doi: 10.1016/s0167-0115(96)00129-2. [DOI] [PubMed] [Google Scholar]
- 41.Pivovarov AS, Walker RJ. Effects of SEPYLRFamide on acetylcholine-induced currents of Helix aspersa neurones: role of ryanodine receptors. Invertebr neurosci. 1999;4:17–24. doi: 10.1007/pl00022365. [DOI] [PubMed] [Google Scholar]
- 42.Pivovarov AS, Walker RJ. EPYLRFamide-mediated reduction of acetylcholine-induced inward currents in Helix lucorum identified neurones: Role of NAADP-dependent and IP3-dependent Ca2+ release from internal stores, calmodulin and Ca2+/calmodulin-dependent protein kinase II. Regul Pept. 2003;111:31–9. doi: 10.1016/s0167-0115(02)00221-5. [DOI] [PubMed] [Google Scholar]
- 43.Price DA, Lesser W, Lee TD, Doble KE, Greenberg MJ. Seven FMRFamide-related and two SCP-related cardioactive peptides from Helix. J Exp Biol. 1990;154:421–37. doi: 10.1242/jeb.154.1.421. [DOI] [PubMed] [Google Scholar]
- 44.Roderick HL, Berridge MJ, Bootman MD. Calcium-induced calcium release. Curr Biol. 2003;13:R425. doi: 10.1016/s0960-9822(03)00358-0. [DOI] [PubMed] [Google Scholar]
- 45.Saghian AA, Ayrapetyan SN, Carpenter DO. Low concentrations of ouabain stimulate Na/Ca exchange in neurons. Cell Mol Neurobiol. 1996;16:489–98. doi: 10.1007/BF02150229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkozi S, Szentesi P, Jona I, Csernoch L. Effects of cardiac glycosides on excitation-contraction coupling in frog skeletal muscle fibres. J Physiol (Lond) 1996;495:611–26. doi: 10.1113/jphysiol.1996.sp021620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiebinger RJ, Cragoe EJ., Jr Ouabain. A stimulator of atrial natriuretic peptide secretion and its mechanism of action. Circ Res. 1993;72:1035–43. doi: 10.1161/01.res.72.5.1035. [DOI] [PubMed] [Google Scholar]
- 48.Sitsapesan R, McGarry SJ, Williams AJ. Cyclic ADP-ribose competes with ATP for the adenine nucleotide binding site on the cardiac ryanodine receptor Ca(2+)-release channel. Circ Res. 1994;75:596–600. doi: 10.1161/01.res.75.3.596. [DOI] [PubMed] [Google Scholar]
- 49.Stepp LR, Novakoski MA. Effect of 5-Hydroxytryptamine on sodium- and potassium-dependent adenosine triphosphatase and its reactivity toward ouabain. Arch Biochem Biophys. 1997;337:43–53. doi: 10.1006/abbi.1996.9762. [DOI] [PubMed] [Google Scholar]
- 50.Ter-Markarian AG, Palikhova TA, Sokolov EN. The action of atropine and d-tubocurarine on the monosynaptic connections between identified neurons in the central nervous system of the edible snail. Z Vyss Nervn Deat Im IP Pavlova. 1990;40:183–4. [PubMed] [Google Scholar]
- 51.Van Eylen F, Gourlet P, Vandermeers A, Lebrun P, Herchuelz A. Inhibition of Na/Ca exchange by Phe-Met-Arg-Phe-NH2 (FMRFa)-related peptides in intact rat pancreatic B-cells. Mol Cell Endocrinol. 1994;106:R1–5. doi: 10.1016/0303-7207(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 52.Vulfius CA, Ryazansky VD, Krasts IV, Ilyasov FE. Intracellular Ca2+ modulates Cl− current evoked by acetylcholine in Lymnaea neurons. Neurosci Lett. 1998;249:5–8. doi: 10.1016/s0304-3940(98)00338-3. [DOI] [PubMed] [Google Scholar]
- 53.Walker RJ. Neuroactive peptides with an RFamide or Famide carboxyl terminals. Comp Biochem Physiol. 1992;102C:213–22. doi: 10.1016/0742-8413(92)90104-f. [DOI] [PubMed] [Google Scholar]
- 54.Willoughby D, Yeoman MS, Benjamin PR. Inositol-1,4,5-trisphosphate and inositol-1,3,4,5-tetrakisphosphate are second messenger targets for cardioactive neuropeptides encoded on the FMRFamide gene. J Exp Biol. 1999;202:2581–93. doi: 10.1242/jeb.202.19.2581. [DOI] [PubMed] [Google Scholar]


