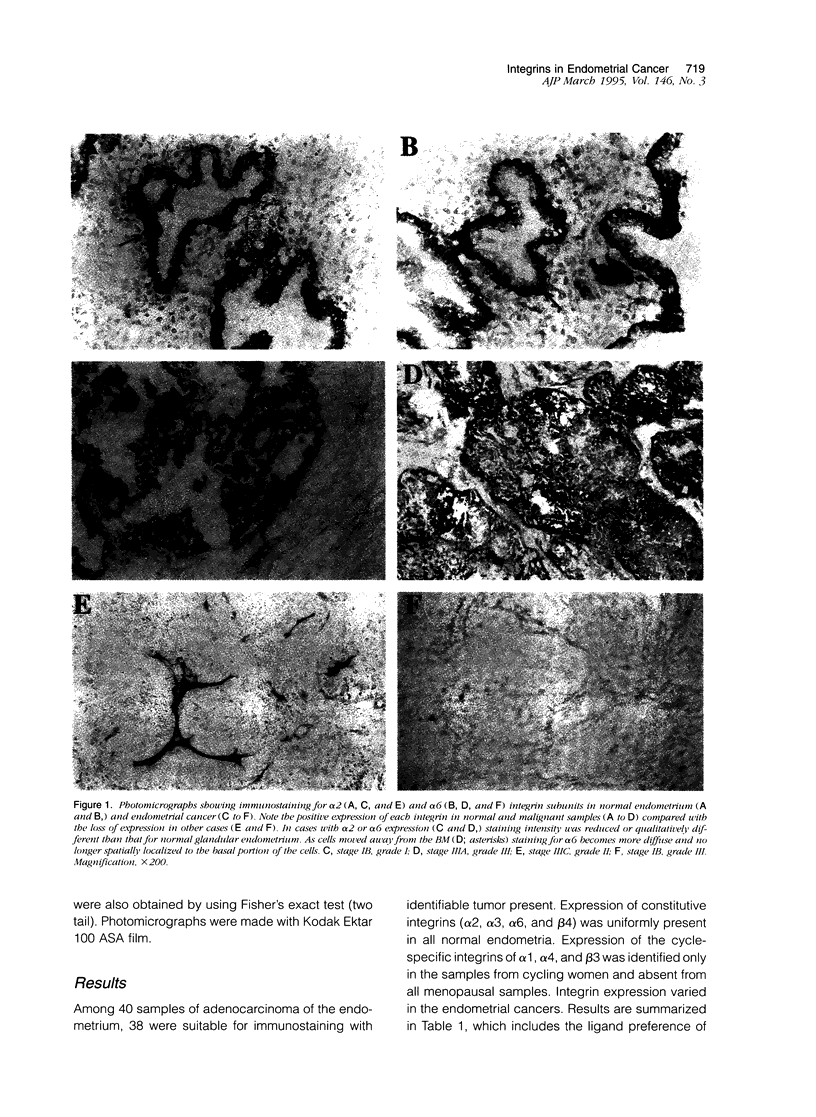
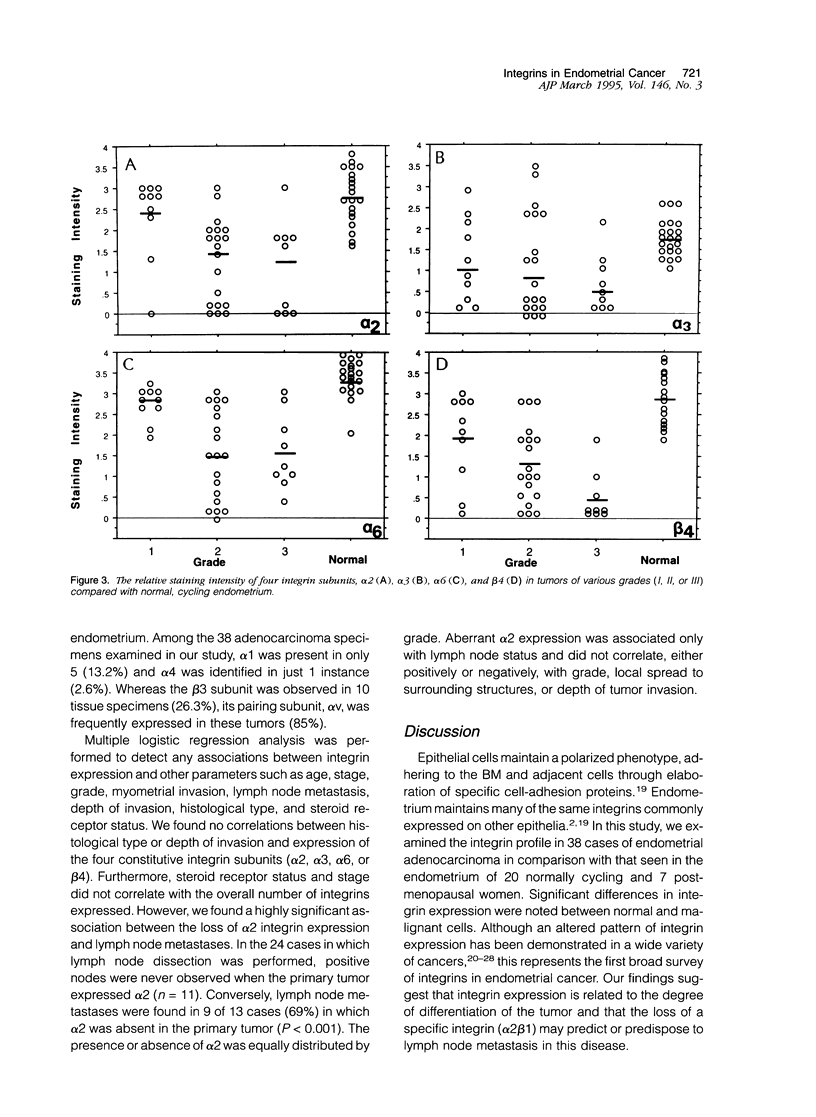
Abstract
Integrins are ubiquitous cell adhesion molecules that are involved in maintaining normal tissue morphology and have been implicated in the behavior of certain malignancies. We examined the expression of nine integrin subunits in 38 endometrial adenocarcinomas using immunohistochemistry. The pattern of integrin expression in the cancers was compared with that seen in the endometrium of 20 normal cycling women and 7 postmenopausal women. Integrin expression was correlated with grade, stage, nodal status, depth of invasion, steroid receptor status, and histological pattern. In endometrial cancers there was an inverse relationship between the number of integrins expressed and histological grade (P = 0.011). Of the normally expressed, constitutive endometrial epithelial integrin subunits (alpha 2, alpha 3, alpha 6, and beta 4), the least frequently seen in the cancers was the alpha 3 subunit (44.7%) and the most frequently found was alpha 6 (81.6%). The alpha 5 beta 1 integrin, a fibronectin receptor normally found only on endometrial stromal cells, was seen in 17.8% of cases of these epithelial cancers. In addition, a significant association was found between the loss of the alpha 2 beta 1 integrin and the presence of lymph node metastases (P < 0.001). These data suggest that a decline in integrin expression occurs more frequently in poorly differentiated endometrial cancers and that the loss of specific integrins may be associated with metastatic nodal spread.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Larjava H., Yamada K. M. Differences in the biosynthesis and localization of the fibronectin receptor in normal and transformed cultured human cells. Cancer Res. 1990 Mar 1;50(5):1601–1607. [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Albelda S. M., Daise M., Levine E. M., Buck C. A. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989 Jun;83(6):1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Albelda S. M. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993 Jan;68(1):4–17. [PubMed] [Google Scholar]
- Bonkhoff H., Stein U., Remberger K. Differential expression of alpha 6 and alpha 2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol. 1993 Mar;24(3):243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- Budwit-Novotny D. A., McCarty K. S., Cox E. B., Soper J. T., Mutch D. G., Creasman W. T., Flowers J. L., McCarty K. S., Jr Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986 Oct;46(10):5419–5425. [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Harper J. R. Arg-Gly-Asp recognition by a cell adhesion receptor requires its 130-kDa alpha subunit. J Biol Chem. 1987 Feb 5;262(4):1434–1437. [PubMed] [Google Scholar]
- Damjanovich L., Albelda S. M., Mette S. A., Buck C. A. Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am J Respir Cell Mol Biol. 1992 Feb;6(2):197–206. doi: 10.1165/ajrcmb/6.2.197. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Gray V. Isolation of a novel integrin receptor mediating Arg-Gly-Asp-directed cell adhesion to fibronectin and type I collagen from human neuroblastoma cells. Association of a novel beta 1-related subunit with alpha v. J Cell Biol. 1990 Jun;110(6):2185–2193. doi: 10.1083/jcb.110.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi P., Truffa G., Stefanuto G., Altruda F., Silengo L., Tarone G. Tumor necrosis factor alpha and interferon gamma modulate the expression of the vitronectin receptor (integrin beta 3) in human endothelial cells. J Biol Chem. 1991 Apr 25;266(12):7638–7645. [PubMed] [Google Scholar]
- Dejana E., Lampugnani M. G., Giorgi M., Gaboli M., Marchisio P. C. Fibrinogen induces endothelial cell adhesion and spreading via the release of endogenous matrix proteins and the recruitment of more than one integrin receptor. Blood. 1990 Apr 1;75(7):1509–1517. [PubMed] [Google Scholar]
- Getzenberg R. H., Pienta K. J., Coffey D. S. The tissue matrix: cell dynamics and hormone action. Endocr Rev. 1990 Aug;11(3):399–417. doi: 10.1210/edrv-11-3-399. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Coates P., Lemoine N. R., Horton M. A. Characterization of integrin chains in normal and neoplastic human pancreas. J Pathol. 1991 Sep;165(1):33–41. doi: 10.1002/path.1711650107. [DOI] [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Heino J., Massagué J. Transforming growth factor-beta switches the pattern of integrins expressed in MG-63 human osteosarcoma cells and causes a selective loss of cell adhesion to laminin. J Biol Chem. 1989 Dec 25;264(36):21806–21811. [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Sonnenberg A. Association of the VLA alpha 6 subunit with a novel protein. A possible alternative to the common VLA beta 1 subunit on certain cell lines. J Biol Chem. 1989 Apr 15;264(11):6529–6535. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Heino J., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Regulation of vitronectin receptor and LFA-1. J Biol Chem. 1989 Jan 5;264(1):389–392. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Klein C. E., Dressel D., Steinmayer T., Mauch C., Eckes B., Krieg T., Bankert R. B., Weber L. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991 Dec;115(5):1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C. E., Steinmayer T., Kaufmann D., Weber L., Bröcker E. B. Identification of a melanoma progression antigen as integrin VLA-2. J Invest Dermatol. 1991 Feb;96(2):281–284. doi: 10.1111/1523-1747.ep12464485. [DOI] [PubMed] [Google Scholar]
- Koretz K., Schlag P., Boumsell L., Möller P. Expression of VLA-alpha 2, VLA-alpha 6, and VLA-beta 1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am J Pathol. 1991 Mar;138(3):741–750. [PMC free article] [PubMed] [Google Scholar]
- Korhonen M., Laitinen L., Ylänne J., Koukoulis G. K., Quaranta V., Juusela H., Gould V. E., Virtanen I. Integrin distributions in renal cell carcinomas of various grades of malignancy. Am J Pathol. 1992 Nov;141(5):1161–1171. [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. K., Virtanen I., Korhonen M., Laitinen L., Quaranta V., Gould V. E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991 Oct;139(4):787–799. [PMC free article] [PubMed] [Google Scholar]
- Leslie K. K., Watanabe S., Lei K. J., Chou D. Y., Plouzek C. A., Deng H. C., Torres J., Chou J. Y. Linkage of two human pregnancy-specific beta 1-glycoprotein genes: one is associated with hydatidiform mole. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5822–5826. doi: 10.1073/pnas.87.15.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey B. A., Castelbaum A. J., Buck C. A., Lei Y., Yowell C. W., Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994 Sep;62(3):497–506. [PubMed] [Google Scholar]
- Lessey B. A., Castelbaum A. J., Sawin S. W., Buck C. A., Schinnar R., Bilker W., Strom B. L. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994 Aug;79(2):643–649. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- Lessey B. A., Damjanovich L., Coutifaris C., Castelbaum A., Albelda S. M., Buck C. A. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992 Jul;90(1):188–195. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert M., Washington R., Stein J., Wedemeyer G., Grossman H. B. Expression of the VLA beta 1 integrin family in bladder cancer. Am J Pathol. 1994 May;144(5):1016–1022. [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Kohn E. Cancer invasion and metastases. JAMA. 1990 Feb 23;263(8):1123–1126. [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987 Oct 9;51(1):51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Mette S. A., Pilewski J., Buck C. A., Albelda S. M. Distribution of integrin cell adhesion receptors on normal bronchial epithelial cells and lung cancer cells in vitro and in vivo. Am J Respir Cell Mol Biol. 1993 May;8(5):562–572. doi: 10.1165/ajrcmb/8.5.562. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Botti C., Mottolese M., Bigotti A., Segatto O. Changes in expression of alpha 6/beta 4 integrin heterodimer in primary and metastatic breast cancer. Br J Cancer. 1992 Aug;66(2):318–322. doi: 10.1038/bjc.1992.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Hanby A. M., Stamp G. W. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991 Sep;165(1):25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Smith M. E., Bodmer W. F. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br J Cancer. 1990 Apr;61(4):636–638. doi: 10.1038/bjc.1990.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Qian F., Vaux D. L., Weissman I. L. Expression of the integrin alpha 4 beta 1 on melanoma cells can inhibit the invasive stage of metastasis formation. Cell. 1994 May 6;77(3):335–347. doi: 10.1016/0092-8674(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Quaranta V. Epithelial integrins. Cell Differ Dev. 1990 Dec 2;32(3):361–365. doi: 10.1016/0922-3371(90)90051-w. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Noble N. A., Kagami S., Border W. A. Integrins. Kidney Int Suppl. 1994 Jan;44:S17–S22. [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Stetler-Stevenson W. G., Hendrix M. J. The 72 kDa type IV collagenase is modulated via differential expression of alpha v beta 3 and alpha 5 beta 1 integrins during human melanoma cell invasion. Cancer Res. 1993 Jul 15;53(14):3411–3415. [PubMed] [Google Scholar]
- Sheppard D., Cohen D. S., Wang A., Busk M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992 Aug 25;267(24):17409–17414. [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987 Jul 25;262(21):10376–10383. [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Daams J. H., Kennel S. J. The alpha 6 beta 1 (VLA-6) and alpha 6 beta 4 protein complexes: tissue distribution and biochemical properties. J Cell Sci. 1990 Jun;96(Pt 2):207–217. doi: 10.1242/jcs.96.2.207. [DOI] [PubMed] [Google Scholar]
- Stallmach A., von Lampe B., Matthes H., Bornhöft G., Riecken E. O. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut. 1992 Mar;33(3):342–346. doi: 10.1136/gut.33.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp G. W., Pignatelli M. Distribution of beta 1, alpha 1, alpha 2 and alpha 3 integrin chains in basal cell carcinomas. J Pathol. 1991 Apr;163(4):307–313. doi: 10.1002/path.1711630407. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Liotta L. A., Kleiner D. E., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993 Dec;7(15):1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- Stracke M. L., Murata J., Aznavoorian S., Liotta L. A. The role of the extracellular matrix in tumor cell metastasis. In Vivo. 1994 Jan-Feb;8(1):49–58. [PubMed] [Google Scholar]
- Tabibzadeh S. Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum Reprod. 1992 Jul;7(6):876–882. doi: 10.1093/oxfordjournals.humrep.a137753. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Korhonen M., Kariniemi A. L., Gould V. E., Laitinen L., Ylänne J. Integrins in human cells and tumors. Cell Differ Dev. 1990 Dec 2;32(3):215–227. doi: 10.1016/0922-3371(90)90034-t. [DOI] [PubMed] [Google Scholar]
- Weinel R. J., Rosendahl A., Neumann K., Chaloupka B., Erb D., Rothmund M., Santoso S. Expression and function of VLA-alpha 2, -alpha 3, -alpha 5 and -alpha 6-integrin receptors in pancreatic carcinoma. Int J Cancer. 1992 Nov 11;52(5):827–833. doi: 10.1002/ijc.2910520526. [DOI] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutter M. M., Mazoujian G., Santoro S. A. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol. 1990 Oct;137(4):863–870. [PMC free article] [PubMed] [Google Scholar]