Abstract
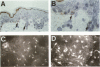
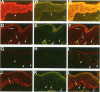
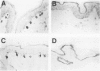
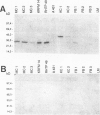
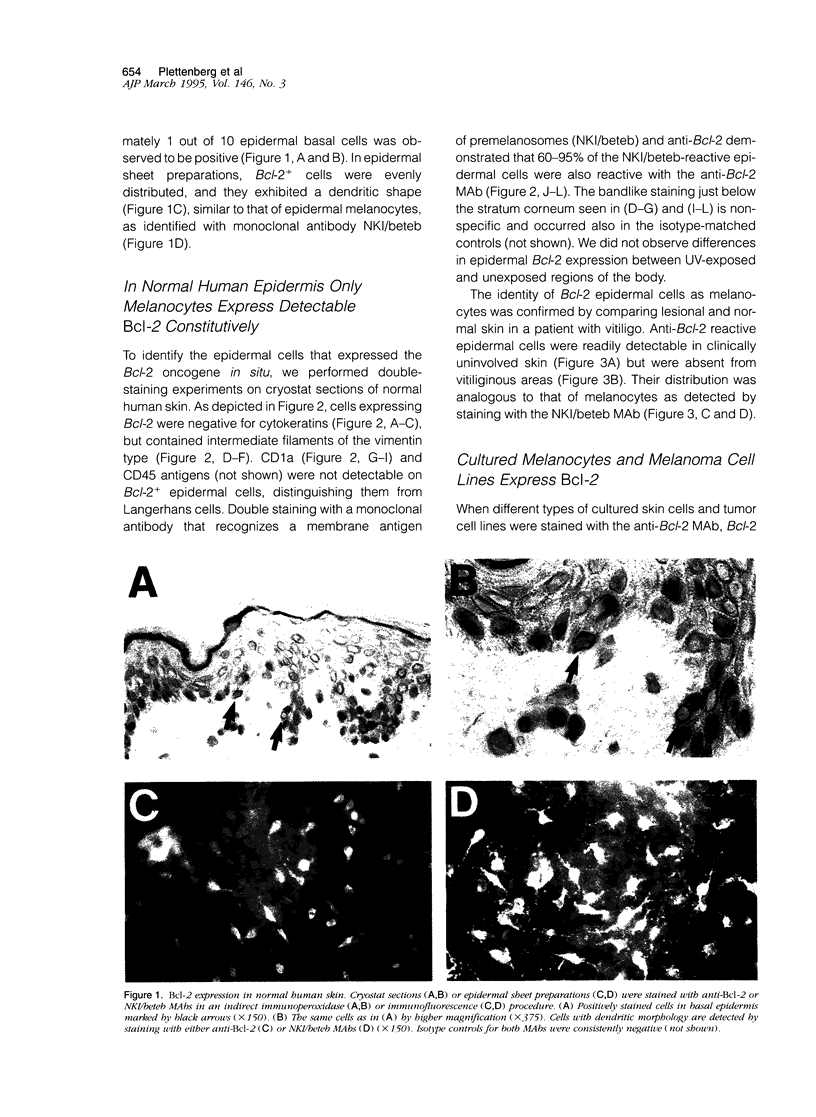
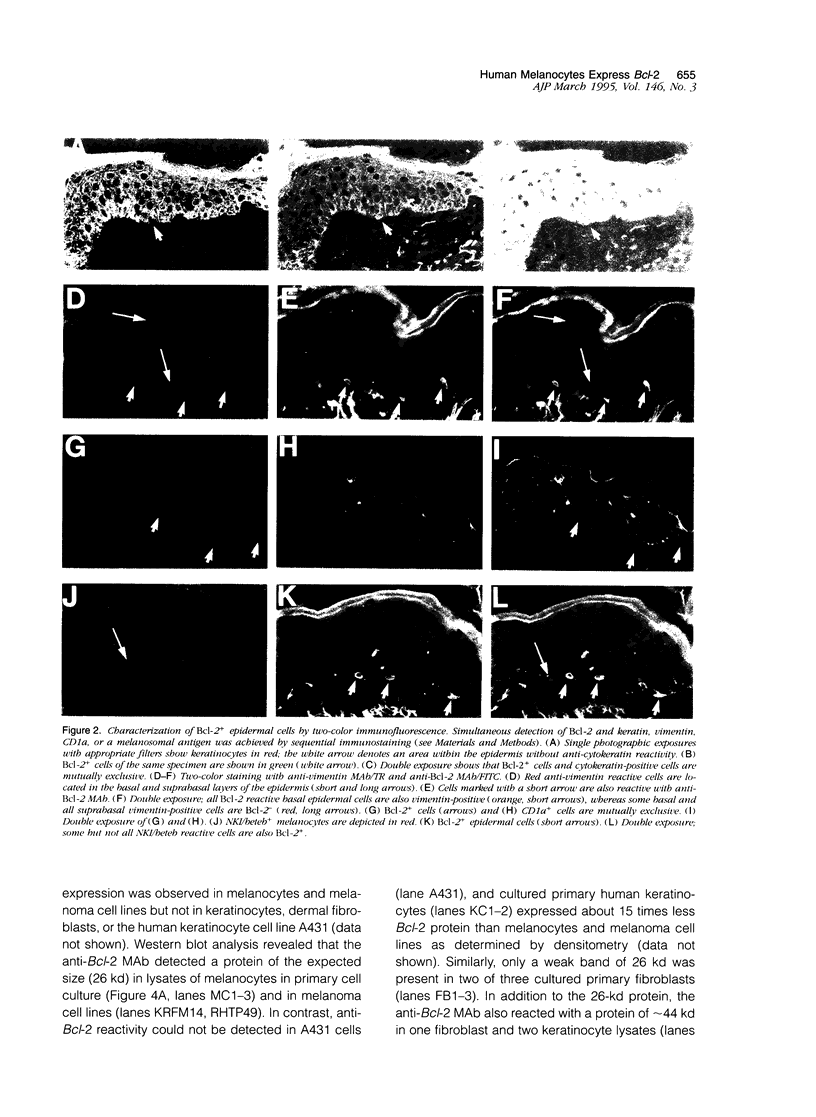
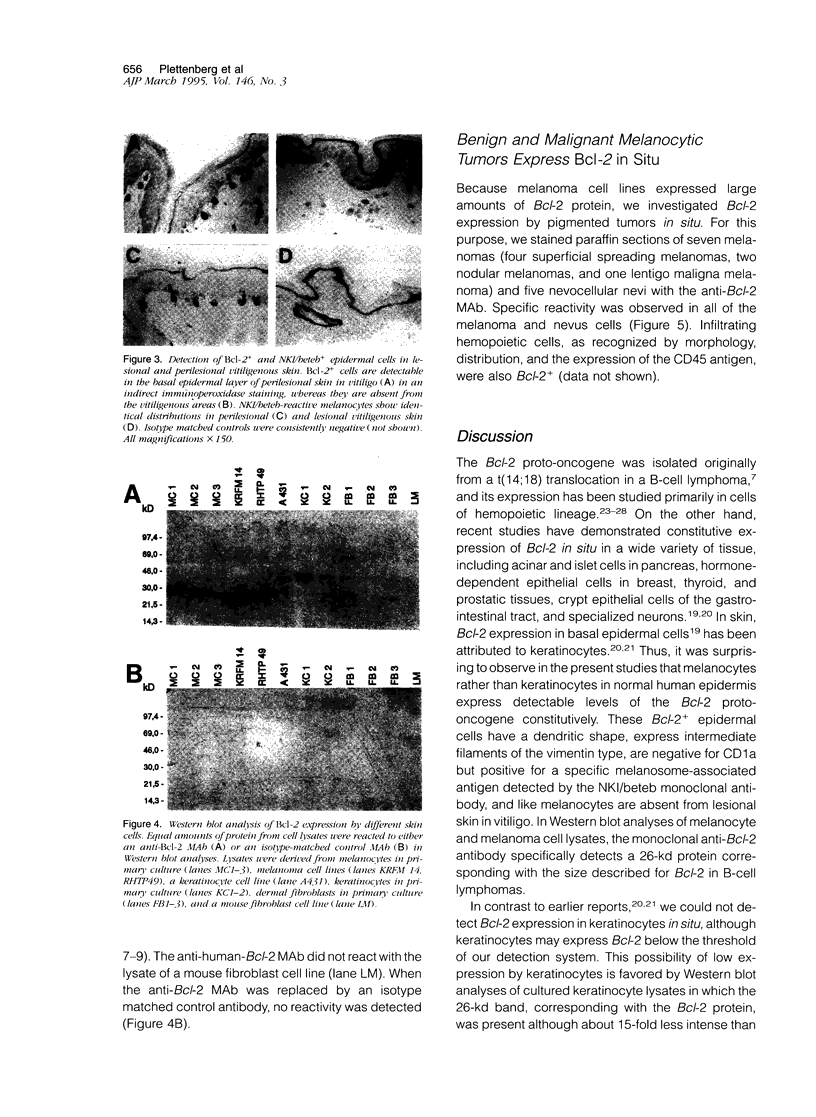
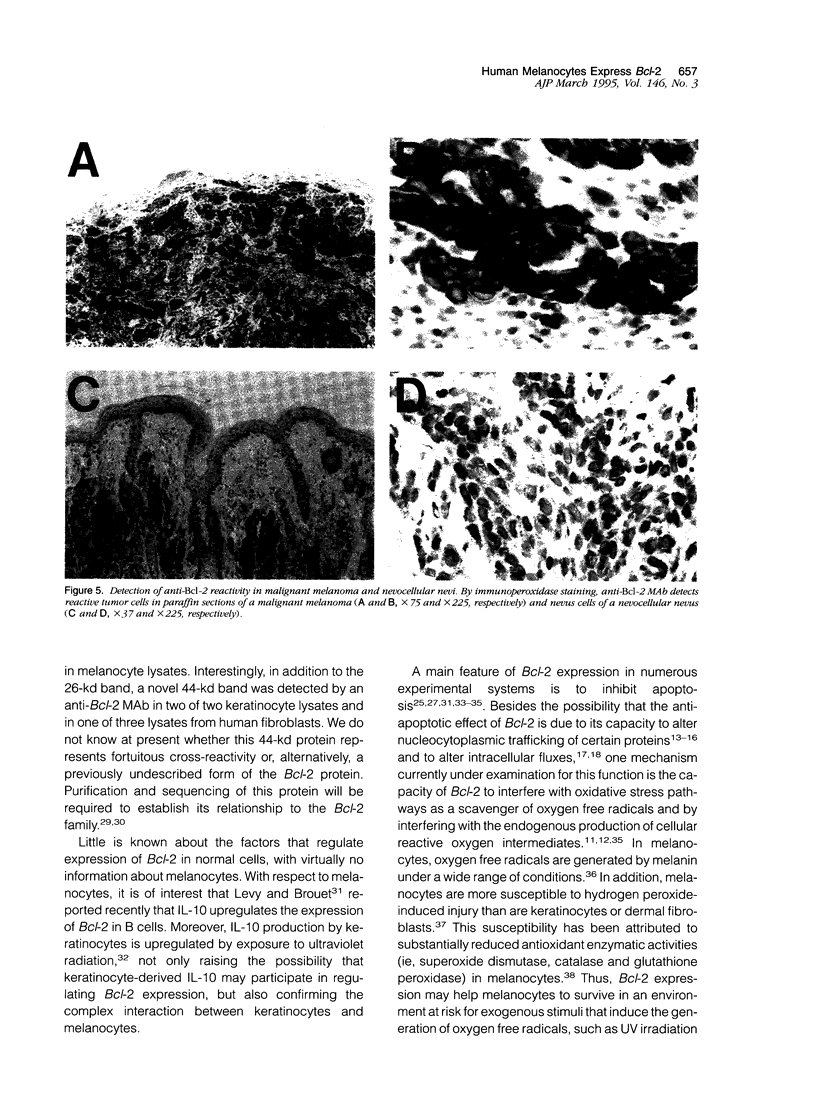
The Bcl-2 proto-oncogene regulates cell survival by antagonizing events that lead to apoptotic cell death and has been reported to be expressed in situ in lymphoid tissues, glandular epithelium, neurons, and basal epidermal cells. When we performed immunostaining on cryostat sections of normal skin, anti-Bcl-2 reactivity was confined to scattered dendritic cells in the basal epidermal layer. Double-staining experiments showed that the Bcl-2+ cells were positive for vimentin but negative for cytokeratins, CD1a, and CD45 antigens, excluding keratinocytes and Langerhans cells as possible candidates for constitutive Bcl-2 expression. Bcl-2+ epidermal cells also reacted with the monoclonal anti-melanocyte antibody NKI/beteb, and were absent from lesional skin in vitiligo, confirming that they represented epidermal melanocytes. Western blot analysis of cultured melanocytes and melanoma cell lines revealed a 26-kd protein specifically reacting with the anti-Bcl-2 monoclonal antibody. Immunostaining of pigmented lesions revealed strong expression of Bcl-2 by five of five nevocellular nevi and seven of seven melanomas. Our observations demonstrate that, within normal human epidermis, melanocytes are the only cells that express Bcl-2 constitutively and that Bcl-2 is expressed in benign and malignant pigmented tumors of the skin in situ.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Sandstrom P. A. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994 Jan;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Cerroni L., Volkenandt M., Rieger E., Soyer H. P., Kerl H. bcl-2 protein expression and correlation with the interchromosomal 14;18 translocation in cutaneous lymphomas and pseudolymphomas. J Invest Dermatol. 1994 Feb;102(2):231–235. doi: 10.1111/1523-1747.ep12371768. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Enk A. H., Katz S. I. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992 Jul 1;149(1):92–95. [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Juneja S., Lukeis R., Tan L., Cooper I., Szelag G., Parkin J. D., Ironside P., Garson O. M. Cytogenetic analysis of 147 cases of non-Hodgkin's lymphoma: non-random chromosomal abnormalities and histological correlations. Br J Haematol. 1990 Oct;76(2):231–237. doi: 10.1111/j.1365-2141.1990.tb07877.x. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Katsumata M., Siegel R. M., Louie D. C., Miyashita T., Tsujimoto Y., Nowell P. C., Greene M. I., Reed J. C. Differential effects of Bcl-2 on T and B cells in transgenic mice. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11376–11380. doi: 10.1073/pnas.89.23.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2: a repressor of lymphocyte death. Immunol Today. 1992 Aug;13(8):285–288. doi: 10.1016/0167-5699(92)90037-8. [DOI] [PubMed] [Google Scholar]
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- Lam M., Dubyak G., Chen L., Nuñez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Levy Y., Brouet J. C. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994 Jan;93(1):424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q. L., Poulsom R., Wong L., Hanby A. M. Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol. 1993 Apr;169(4):431–437. doi: 10.1002/path.1711690408. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Korsmeyer S. J. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 1991 Jan 17;349(6306):254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- Meikrantz W., Gisselbrecht S., Tam S. W., Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., Hockenbery D., McDonnell T. J., Sorensen C. M., Korsmeyer S. J. Bcl-2 maintains B cell memory. Nature. 1991 Sep 5;353(6339):71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Ryan J. J., Prochownik E., Gottlieb C. A., Apel I. J., Merino R., Nuñez G., Clarke M. F. c-myc and bcl-2 modulate p53 function by altering p53 subcellular trafficking during the cell cycle. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5878–5882. doi: 10.1073/pnas.91.13.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. M., Katsumata M., Miyashita T., Louie D. C., Greene M. I., Reed J. C. Inhibition of thymocyte apoptosis and negative antigenic selection in bcl-2 transgenic mice. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7003–7007. doi: 10.1073/pnas.89.15.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E., Schuler G., Hutterer J., Leibl H., Wolff K., Stingl G. Expression of Thy-1 antigen by murine epidermal cells. J Invest Dermatol. 1983 Sep;81(3):282–285. doi: 10.1111/1523-1747.ep12518326. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Yunis J., Onorato-Showe L., Erikson J., Nowell P. C., Croce C. M. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science. 1984 Jun 29;224(4656):1403–1406. doi: 10.1126/science.6610211. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vaux D. L. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):786–789. doi: 10.1073/pnas.90.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis D. J., Sorenson C. M., Shutter J. R., Korsmeyer S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993 Oct 22;75(2):229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Williams G. T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991 Jun 28;65(7):1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Yohn J. J., Norris D. A., Yrastorza D. G., Buno I. J., Leff J. A., Hake S. S., Repine J. E. Disparate antioxidant enzyme activities in cultured human cutaneous fibroblasts, keratinocytes, and melanocytes. J Invest Dermatol. 1991 Sep;97(3):405–409. doi: 10.1111/1523-1747.ep12480983. [DOI] [PubMed] [Google Scholar]
- de Jong D., Prins F. A., Mason D. Y., Reed J. C., van Ommen G. B., Kluin P. M. Subcellular localization of the bcl-2 protein in malignant and normal lymphoid cells. Cancer Res. 1994 Jan 1;54(1):256–260. [PubMed] [Google Scholar]