Abstract
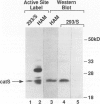
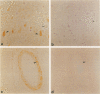
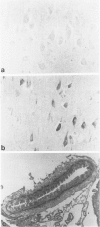
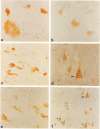
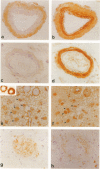
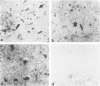
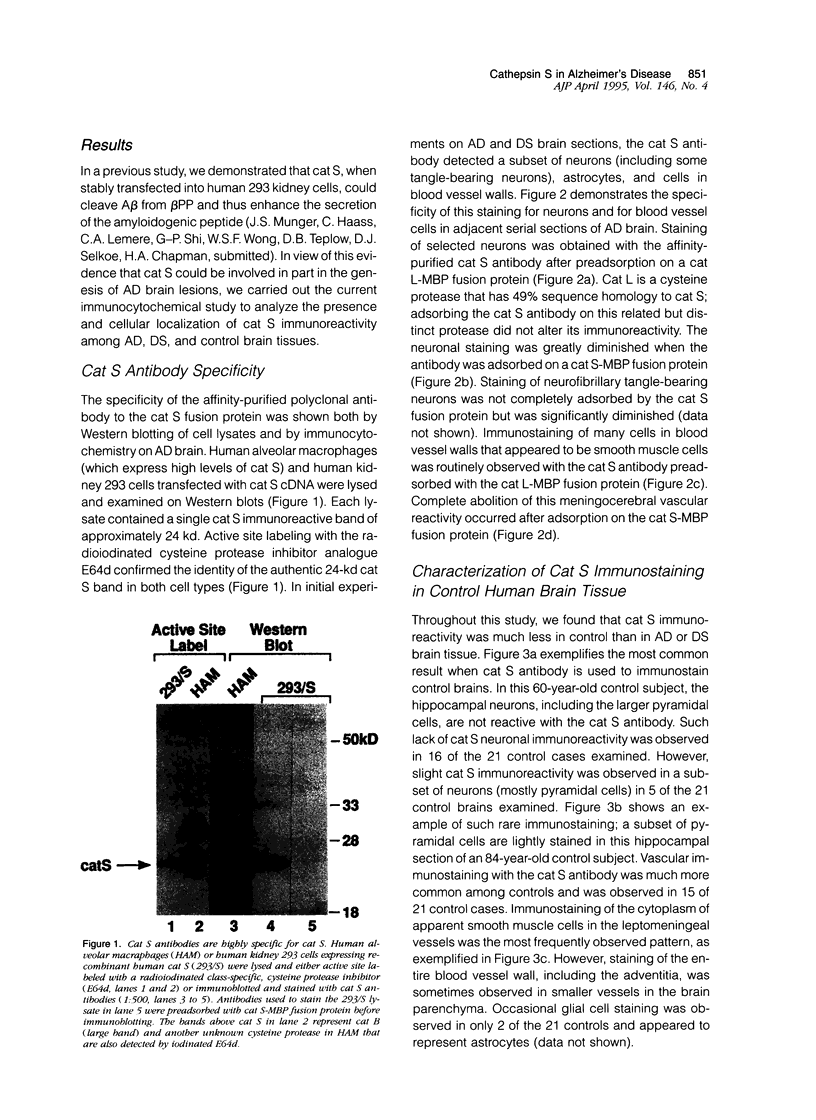
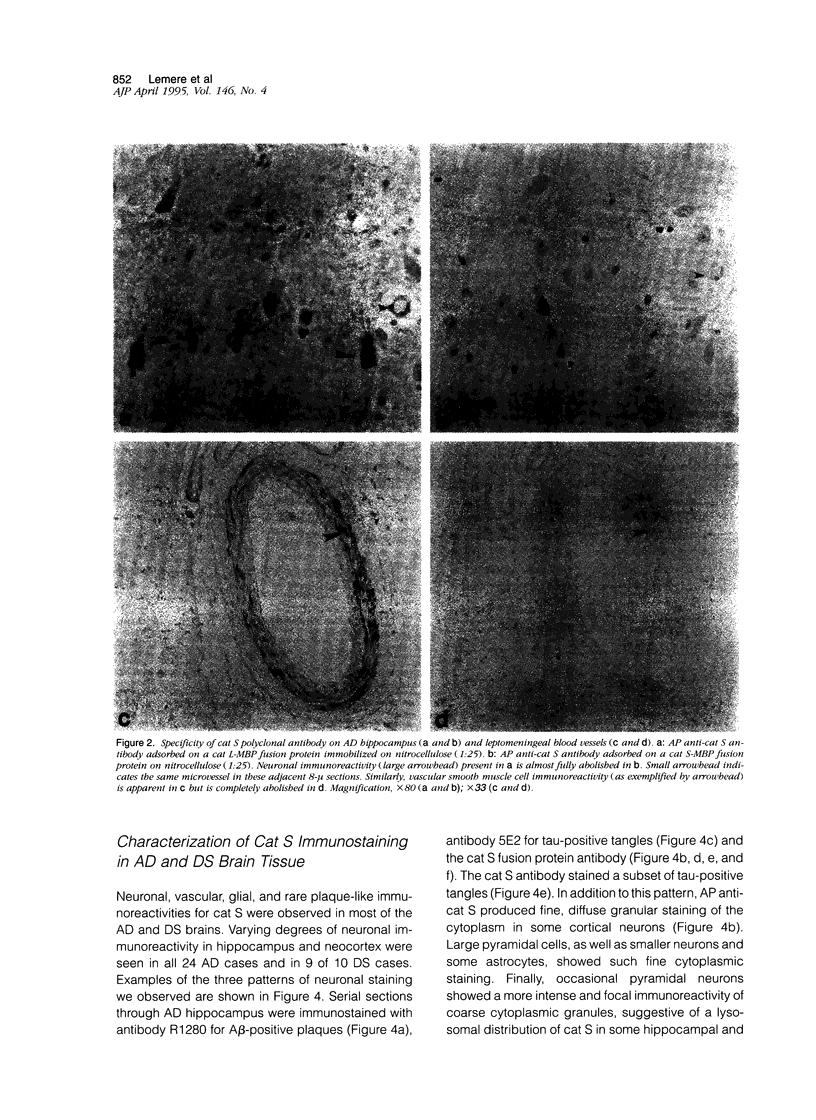
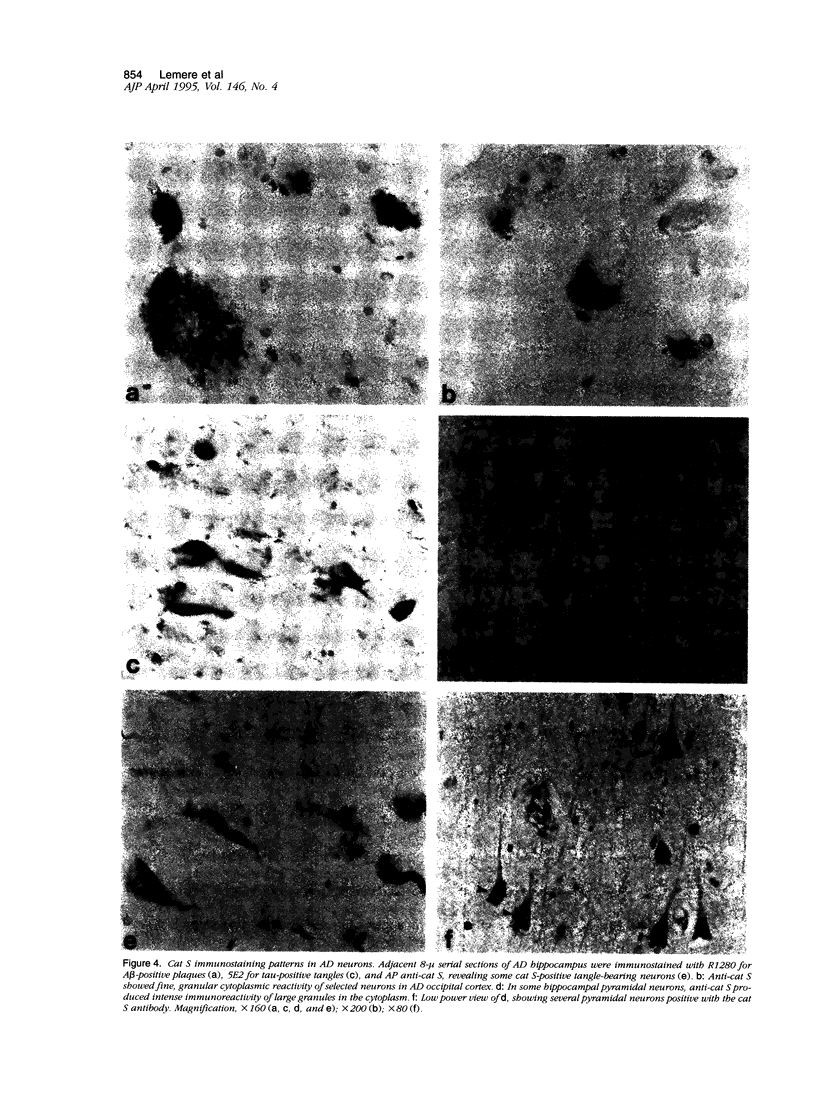
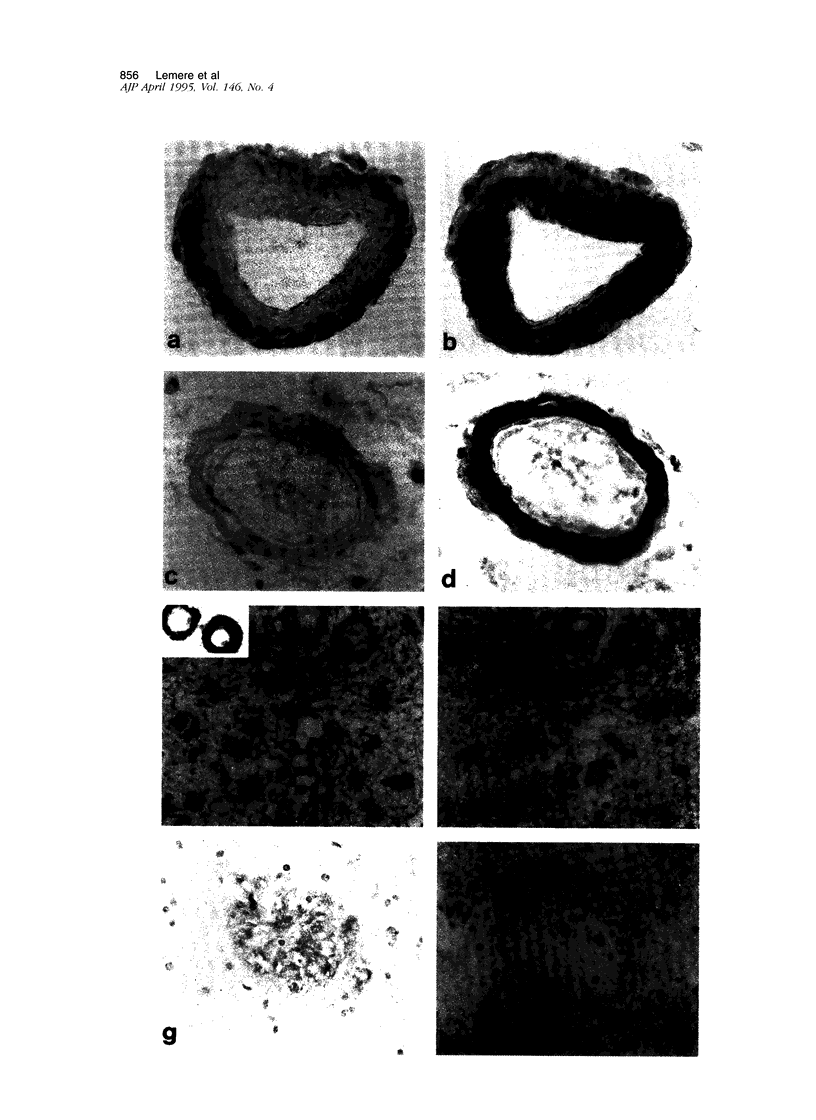
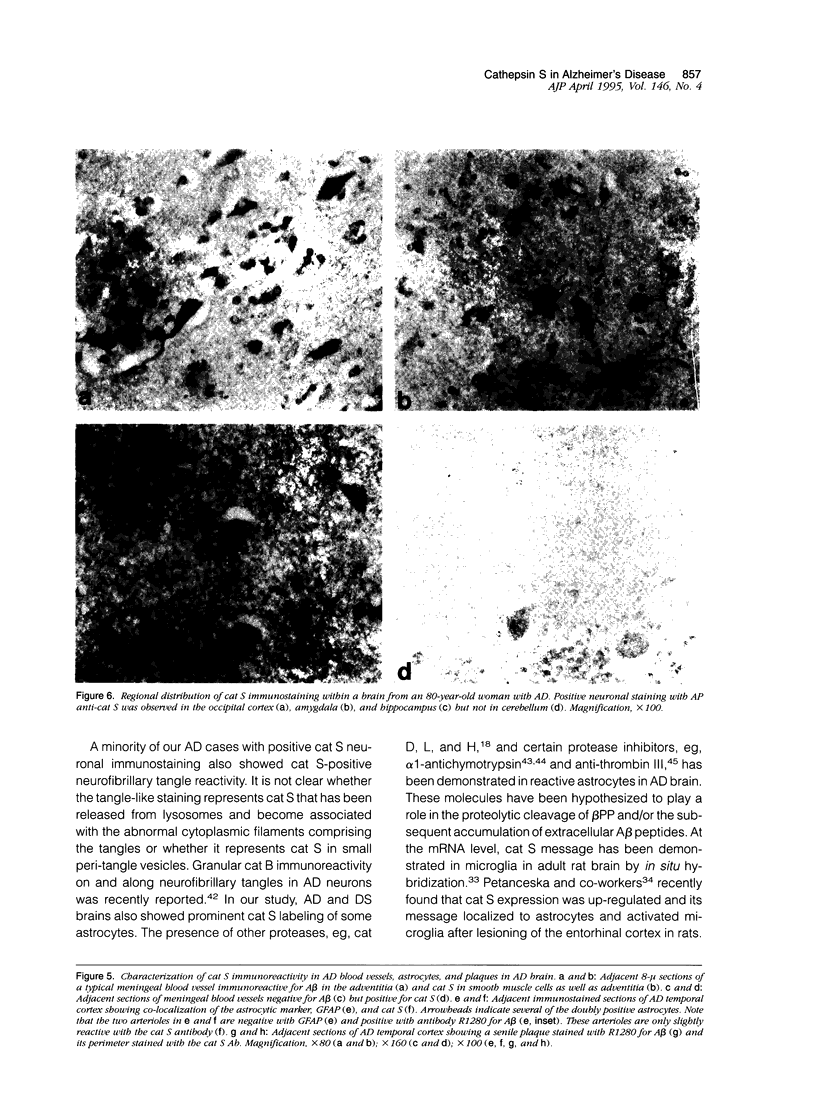
Expression of cathepsin (cat) S, a lysosomal cysteine protease, has recently been shown to cause an increase in production of amyloid beta-peptides in transfected human cells. In this study, we examined the presence and localization of cat S by immunocytochemistry in 21 control, 24 Alzheimer's disease (AD), and 10 Down syndrome (DS) postmortem brains. An antiserum to a human cat S fusion protein was affinity purified and its specificity confirmed by abolition of immunoreactivity after adsorption with cat S but not cat L fusion protein. A small minority of control cases showed light, focal staining of scattered cortical neurons. Many control cases, as well as most AD and DS cases, showed prominent staining of vascular smooth muscle cells, particularly in leptomeningeal vessels. Both AD and DS brain tissue showed increased immunoreactivity in a subset of neocortical and hippocampal neurons and glia. Cat S immunoreactivity occurred in a granular, cytoplasmic pattern in some neurons or in a more dense staining pattern in certain neurofibrillary tangle-bearing neurons. Cat S-positive neurons were also present in amygdala and basal forebrain in AD brains. A subset of astrocytes were immunoreactive with the cat S antibody in AD and DS but not in control brains. In rare AD cases, cat S immunostaining was observed in astrocytes in the periphery of amyloid-beta-containing plaques. These results suggest that cat S is up-regulated in AD and DS brain. The association of cat S immunoreactivity with tangle-bearing neurons, astrocytes, and rare senile plaques implies a role for altered cat S activity in the pathogenesis of AD.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann H. W. Hepatic neoformations. Pathol Res Pract. 1994 Jun;190(6):513–577. doi: 10.1016/S0344-0338(11)80394-8. [DOI] [PubMed] [Google Scholar]
- Ballardini G., Groff P., Badiali de Giorgi L., Schuppan D., Bianchi F. B. Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology. 1994 Feb;19(2):440–446. [PubMed] [Google Scholar]
- Bannasch P., Bloch M., Zerban H. Spongiosis hepatis. Specific changes of the perisinusoidal liver cells induced in rats by N-nitrosomorpholine. Lab Invest. 1981 Mar;44(3):252–264. [PubMed] [Google Scholar]
- Bannasch P., Zerban H. Pathogenesis of primary liver tumors induced by chemicals. Recent Results Cancer Res. 1986;100:1–15. doi: 10.1007/978-3-642-82635-1_1. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991 Mar 1;5(3):271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- Braunbeck T. A., Teh S. J., Lester S. M., Hinton D. E. Ultrastructural alterations in liver of medaka (Oryzias latipes) exposed to diethylnitrosamine. Toxicol Pathol. 1992;20(2):179–196. doi: 10.1177/019262339202000205. [DOI] [PubMed] [Google Scholar]
- Bunton T. E. Hepatopathology of diethylnitrosamine in the medaka (Oryzias latipes) following short-term exposure. Toxicol Pathol. 1990;18(2):313–323. doi: 10.1177/019262339001800210. [DOI] [PubMed] [Google Scholar]
- Burt A. D., Robertson J. L., Heir J., MacSween R. N. Desmin-containing stellate cells in rat liver; distribution in normal animals and response to experimental acute liver injury. J Pathol. 1986 Sep;150(1):29–35. doi: 10.1002/path.1711500106. [DOI] [PubMed] [Google Scholar]
- Clement B., Rissel M., Peyrol S., Mazurier Y., Grimaud J. A., Guillouzo A. A procedure for light and electron microscopic intracellular immunolocalization of collagen and fibronectin in rat liver. J Histochem Cytochem. 1985 May;33(5):407–414. doi: 10.1177/33.5.3886779. [DOI] [PubMed] [Google Scholar]
- Couch J. A., Courtney L. A. N-nitrosodiethylamine-induced hepatocarcinogenesis in estuarine sheepshead minnow (Cyprinodon variegatus): neoplasms and related lesions compared with mammalian lesions. J Natl Cancer Inst. 1987 Aug;79(2):297–321. [PubMed] [Google Scholar]
- Couch J. A. Spongiosis hepatis: chemical induction, pathogenesis, and possible neoplastic fate in a teleost fish model. Toxicol Pathol. 1991;19(3):237–250. doi: 10.1177/019262339101900306. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts A., Lazou J. M., De Bleser P., Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991 Jun;13(6):1193–1202. [PubMed] [Google Scholar]
- Geerts A., Vrijsen R., Rauterberg J., Burt A., Schellinck P., Wisse E. In vitro differentiation of fat-storing cells parallels marked increase of collagen synthesis and secretion. J Hepatol. 1989 Jul;9(1):59–68. doi: 10.1016/0168-8278(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Hendriks H. F., Brouwer A., Knook D. L. The role of hepatic fat-storing (stellate) cells in retinoid metabolism. Hepatology. 1987 Nov-Dec;7(6):1368–1371. doi: 10.1002/hep.1840070630. [DOI] [PubMed] [Google Scholar]
- Hendriks H. F., Verhoofstad W. A., Brouwer A., de Leeuw A. M., Knook D. L. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985 Sep;160(1):138–149. doi: 10.1016/0014-4827(85)90243-5. [DOI] [PubMed] [Google Scholar]
- ITO T., NEMOTO M. Uber die Kupfferschen Sternzellen und die Fettspeicherungszellen (fat storing cells) in der Blutkapillarenwand der memschlichen Leber. Okajimas Folia Anat Jpn. 1952 Oct;24(4):243–258. doi: 10.2535/ofaj1936.24.4_243. [DOI] [PubMed] [Google Scholar]
- Ito N., Moore M. A., Bannasch P. Modification of the development of N-nitrosomorpholine-induced hepatic lesions by 2-acetylaminofluorene, phenobarbital and 4,4'-diaminodiphenylmethane: a sequential histological and histochemical analysis. Carcinogenesis. 1984 Mar;5(3):335–342. doi: 10.1093/carcin/5.3.335. [DOI] [PubMed] [Google Scholar]
- Johnson S. J., Hines J. E., Burt A. D. Immunolocalization of proliferating perisinusoidal cells in rat liver. Histochem J. 1992 Feb;24(2):67–72. doi: 10.1007/BF01082441. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Suzuki J., Mukai H., Mori M. Sequential changes of extracellular matrix and proliferation of Ito cells with enhanced expression of desmin and actin in focal hepatic injury. Am J Pathol. 1986 Dec;125(3):611–619. [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Veit T., Schwögler S., Dienes H. P., Knittel T., Rieder H., Meyer zum Büschenfelde K. H. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(6):349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Zerbe O., Gressner A. M. The synthesis of proteoglycans in fat-storing cells of rat liver. Hepatology. 1987 Jul-Aug;7(4):680–687. doi: 10.1002/hep.1840070411. [DOI] [PubMed] [Google Scholar]
- Takahara T., Kojima T., Miyabayashi C., Inoue K., Sasaki H., Muragaki Y., Ooshima A. Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat. Immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest. 1988 Oct;59(4):509–521. [PubMed] [Google Scholar]
- Tsutsumi M., Takada A., Takase S. Characterization of desmin-positive rat liver sinusoidal cells. Hepatology. 1987 Mar-Apr;7(2):277–284. doi: 10.1002/hep.1840070212. [DOI] [PubMed] [Google Scholar]
- Weber E., Bannasch P. Dose and time dependence of the cellular phenotype in rat hepatic preneoplasia and neoplasia induced by continuous oral exposure to N-nitrosomorpholine. Carcinogenesis. 1994 Jun;15(6):1235–1242. doi: 10.1093/carcin/15.6.1235. [DOI] [PubMed] [Google Scholar]
- Weber E., Bannasch P. Dose and time dependence of the cellular phenotype in rat hepatic preneoplasia and neoplasia induced by single oral exposures to N-nitrosomorpholine. Carcinogenesis. 1994 Jun;15(6):1219–1226. doi: 10.1093/carcin/15.6.1219. [DOI] [PubMed] [Google Scholar]
- Weber E., Bannasch P. Dose and time dependence of the cellular phenotype in rat hepatic preneoplasia and neoplasia induced in stop experiments by oral exposure to N-nitrosomorpholine. Carcinogenesis. 1994 Jun;15(6):1227–1234. doi: 10.1093/carcin/15.6.1227. [DOI] [PubMed] [Google Scholar]
- Yokoi Y., Namihisa T., Kuroda H., Komatsu I., Miyazaki A., Watanabe S., Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984 Jul-Aug;4(4):709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]
- Zerban H., Bannasch P. Spongiosis hepatis in rats treated with low doses of hepatotropic nitrosamines. Cancer Lett. 1983 Jul;19(3):247–252. doi: 10.1016/0304-3835(83)90092-7. [DOI] [PubMed] [Google Scholar]
- Zerban H., Radig S., Kopp-Schneider A., Bannasch P. Cell proliferation and cell death (apoptosis) in hepatic preneoplasia and neoplasia are closely related to phenotypic cellular diversity and instability. Carcinogenesis. 1994 Nov;15(11):2467–2473. doi: 10.1093/carcin/15.11.2467. [DOI] [PubMed] [Google Scholar]