Abstract
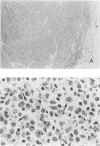
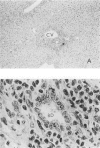
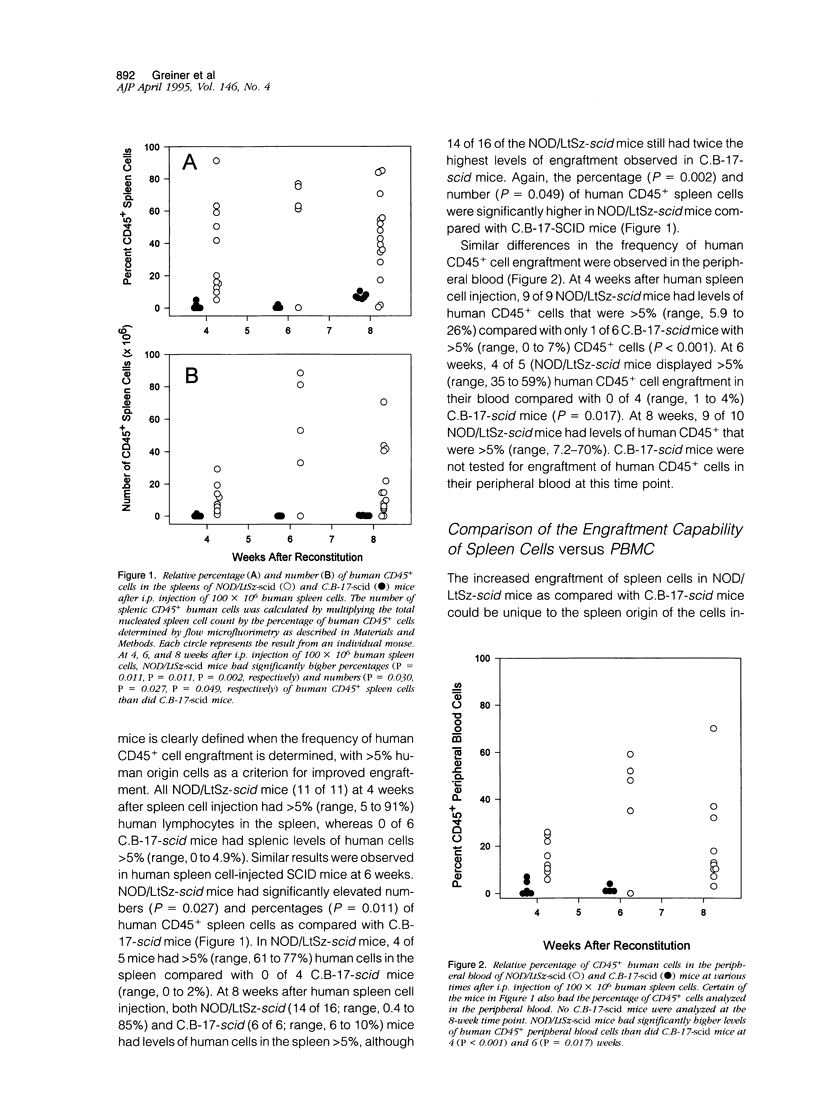
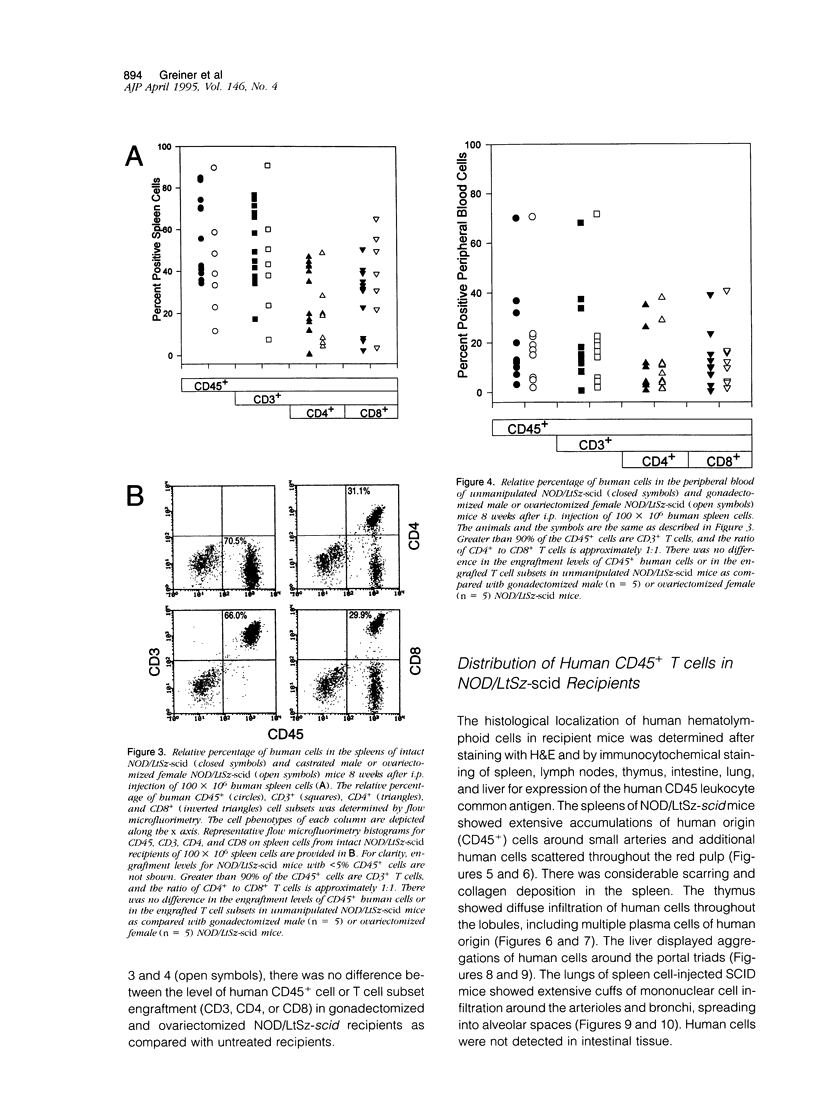
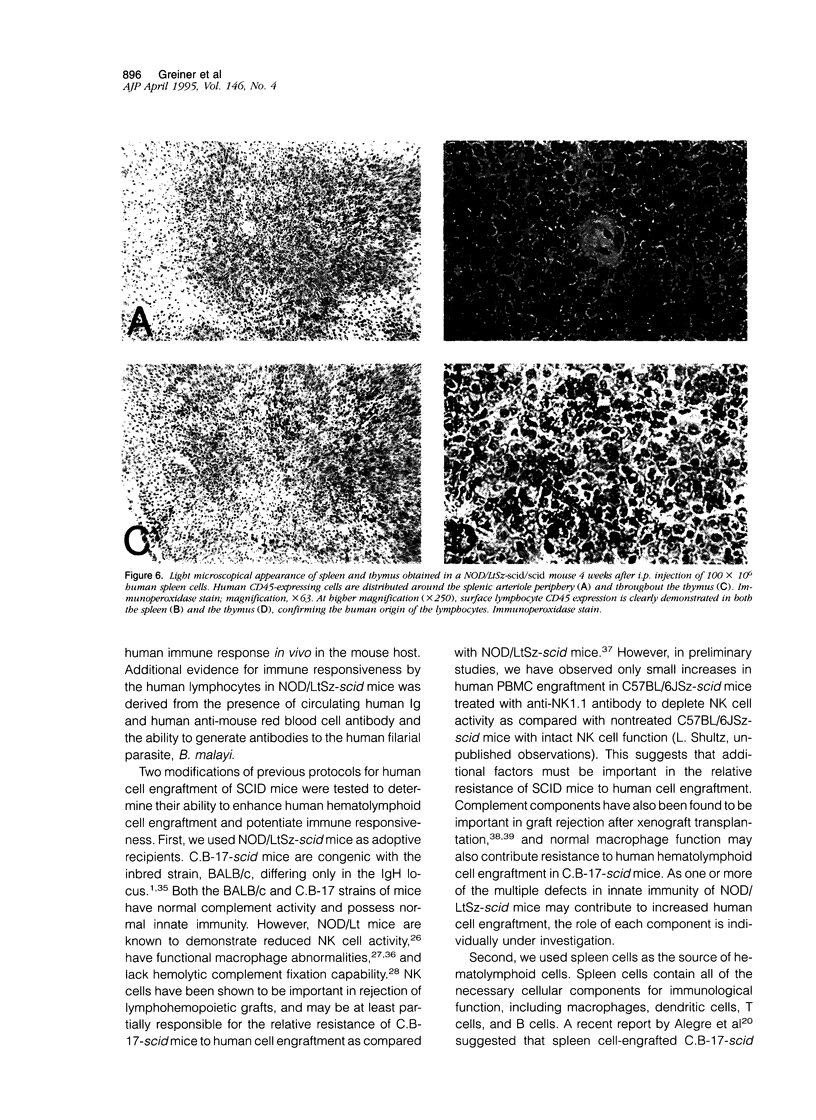
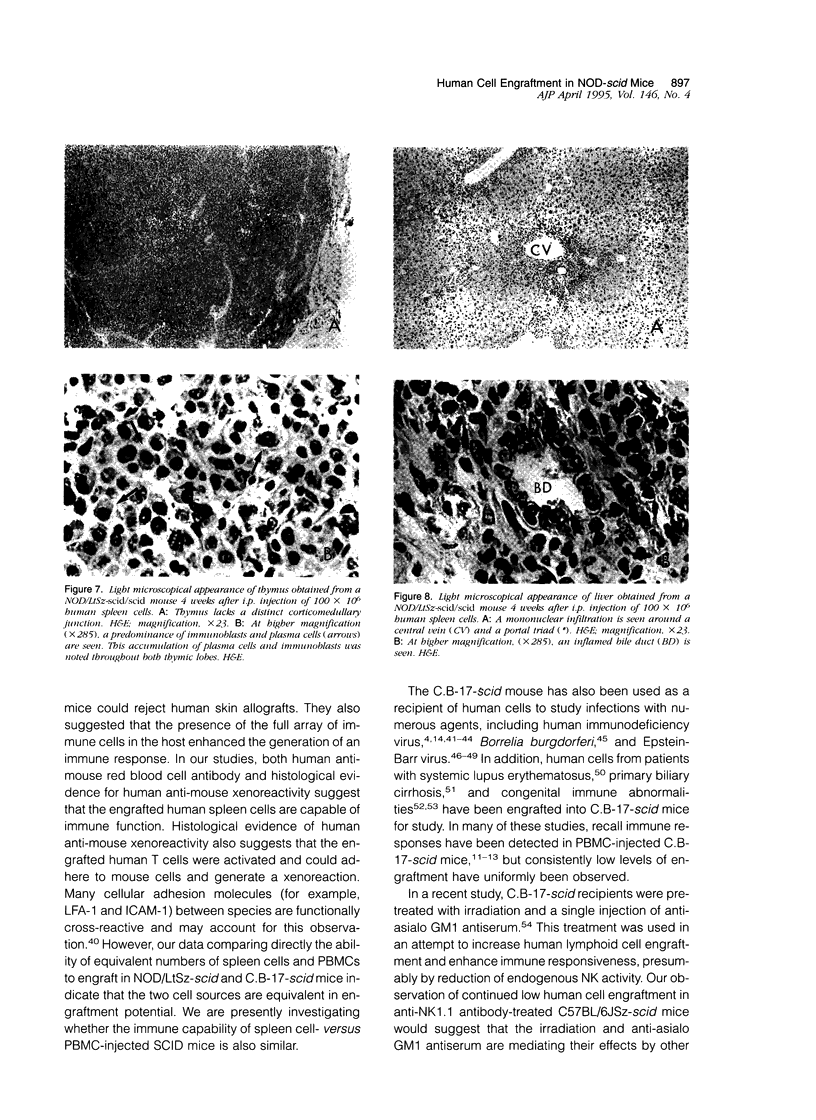
T and B lymphocyte-deficient mice homozygous for the severe combined immunodeficiency (SCID) mutation can be immunologically engrafted with human lymphocytes. However, low levels of human peripheral blood mononuclear cell engraftment are commonly observed, impeding full use of this model We now demonstrate that strain background in mice homozygous for the scid mutation is a strong determinant of levels of human lymphocyte engraftment. NOD/LtSz-scid/scid mice support higher levels of engraftment of both human spleen and peripheral blood mononuclear cells than do C.B-17-scid/scid mice. We observed, using human spleen cell injected scid mice, 1), high levels of engraftment of the host peripheral lymphoid tissues with human CD45+ (leukocytes), CD3+ (T cells), CD4+ (helper/inducer), and CD8+ (suppressor/cytotoxic) lymphoid cells for up to 24 weeks in NOD/LtSz-scid/scid mice; 2), migration of high numbers of human lymphocytes to peripheral lymphoid and nonlymphoid organs in NOD/LtSz-scid/scid, but not in C.B-17-scid/scid mice; 3), higher levels of serum immunoglobulin of human origin in NOD/LtSz-scid/scid mice than in C.B-17-scid/scid mice; 4), histological lesions character-istic of human anti-mouse xenoreactivity in NOD/LtSz-scid/scid mice; and 5), human origin antibodies against filarial antigens after engraftment with naive human spleen cells. The use of NOD/LtSz-scid/scid mice as recipients to achieve significantly enhanced human lymphopoietic cell engraftment will now enable human immunity to be more easily studied in animal models.
Full text
PDF





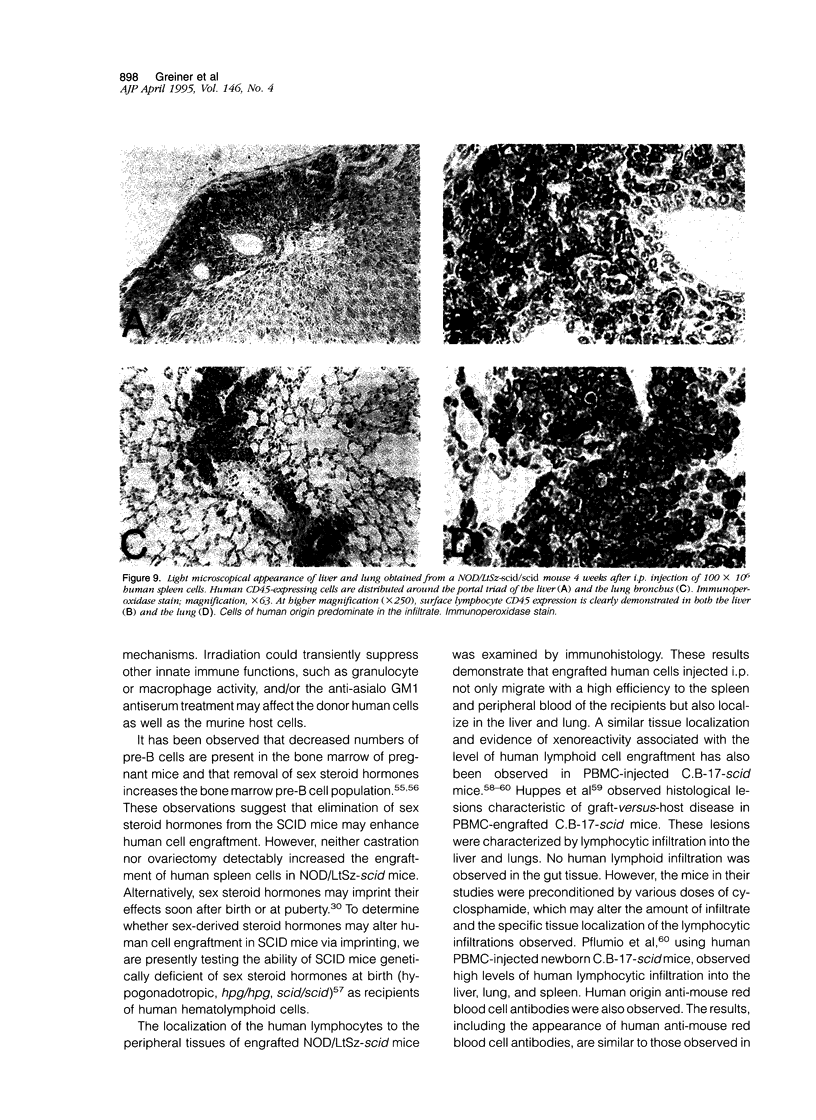
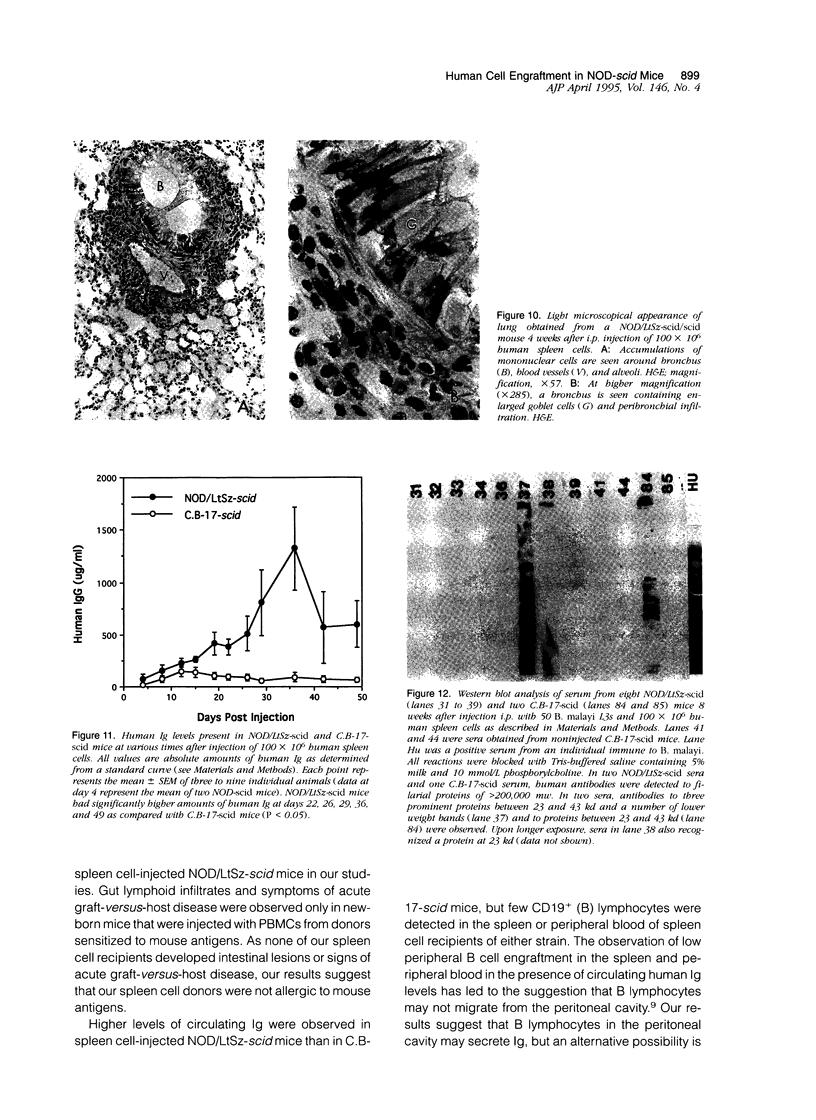



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alegre M. L., Peterson L. J., Jeyarajah D. R., Weiser M., Bluestone J. A., Thistlethwaite J. R. Severe combined immunodeficient mice engrafted with human splenocytes have functional human T cells and reject human allografts. J Immunol. 1994 Sep 15;153(6):2738–2749. [PubMed] [Google Scholar]
- Baxter A. G., Cooke A. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes. 1993 Nov;42(11):1574–1578. doi: 10.2337/diab.42.11.1574. [DOI] [PubMed] [Google Scholar]
- Baxter A. G., Mandel T. E. Hemolytic anemia in non-obese diabetic mice. Eur J Immunol. 1991 Sep;21(9):2051–2055. doi: 10.1002/eji.1830210912. [DOI] [PubMed] [Google Scholar]
- Beamer W. G., Shultz K. L., Tennent B. J., Shultz L. D. Granulosa cell tumorigenesis in genetically hypogonadal-immunodeficient mice grafted with ovaries from tumor-susceptible donors. Cancer Res. 1993 Aug 15;53(16):3741–3746. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Pisa P., Fox R. I., Cooper N. R. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J Clin Invest. 1990 Apr;85(4):1333–1337. doi: 10.1172/JCI114573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Dalmasso A. P., Platt J. L., Bach F. H. Reaction of complement with endothelial cells in a model of xenotransplantation. Clin Exp Immunol. 1991 Oct;86 (Suppl 1):31–35. [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Duchosal M. A., Eming S. A., Fischer P., Leturcq D., Barbas C. F., 3rd, McConahey P. J., Caothien R. H., Thornton G. B., Dixon F. J., Burton D. R. Immunization of hu-PBL-SCID mice and the rescue of human monoclonal Fab fragments through combinatorial libraries. Nature. 1992 Jan 16;355(6357):258–262. doi: 10.1038/355258a0. [DOI] [PubMed] [Google Scholar]
- Duchosal M. A., McConahey P. J., Robinson C. A., Dixon F. J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990 Sep 1;172(3):985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner D. L., Shultz L. D., Rossini A. A., Mordes J. P., Handler E. S., Rajan T. V. Recapitulation of normal and abnormal BB rat immune system development in scid mouse/rat lymphohemopoietic chimeras. J Clin Invest. 1991 Aug;88(2):717–719. doi: 10.1172/JCI115359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselton R. M., Koup R. A., Cromwell M. A., Graham B. S., Johns M., Sullivan J. L. Human peripheral blood xenografts in the SCID mouse: characterization of immunologic reconstitution. J Infect Dis. 1993 Sep;168(3):630–640. doi: 10.1093/infdis/168.3.630. [DOI] [PubMed] [Google Scholar]
- Huppes W., De Geus B., Zurcher C., Van Bekkum D. W. Acute human vs. mouse graft vs. host disease in normal and immunodeficient mice. Eur J Immunol. 1992 Jan;22(1):197–206. doi: 10.1002/eji.1830220129. [DOI] [PubMed] [Google Scholar]
- Johnston S. C., Dustin M. L., Hibbs M. L., Springer T. A. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990 Aug 15;145(4):1181–1187. [PubMed] [Google Scholar]
- Kamel-Reid S., Dick J. E. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988 Dec 23;242(4886):1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- Kaneshima H., Shih C. C., Namikawa R., Rabin L., Outzen H., Machado S. G., McCune J. M. Human immunodeficiency virus infection of human lymph nodes in the SCID-hu mouse. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4523–4527. doi: 10.1073/pnas.88.10.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S., Satoh J., Fujiya H., Toyota T., Suzuki R., Itoh K., Kumagai K. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes. 1983 Mar;32(3):247–253. doi: 10.2337/diab.32.3.247. [DOI] [PubMed] [Google Scholar]
- Koup R. A., Hesselton R. M., Safrit J. T., Somasundaran M., Sullivan J. L. Quantitative assessment of human immunodeficiency virus type 1 replication in human xenografts of acutely infected Hu-PBL-SCID mice. AIDS Res Hum Retroviruses. 1994 Mar;10(3):279–284. doi: 10.1089/aid.1994.10.279. [DOI] [PubMed] [Google Scholar]
- Krams S. M., Dorshkind K., Gershwin M. E. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice. J Exp Med. 1989 Dec 1;170(6):1919–1930. doi: 10.1084/jem.170.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krams S. M., Dorshkind K., Gershwin M. E. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice. J Exp Med. 1989 Dec 1;170(6):1919–1930. doi: 10.1084/jem.170.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoizumi S., Baum C. M., Kaneshima H., McCune J. M., Yee E. J., Namikawa R. Implantation and maintenance of functional human bone marrow in SCID-hu mice. Blood. 1992 Apr 1;79(7):1704–1711. [PubMed] [Google Scholar]
- Lapidot T., Pflumio F., Dick J. E. Modeling human hematopoiesis in immunodeficient mice. Lab Anim Sci. 1993 Apr;43(2):147–150. [PubMed] [Google Scholar]
- Markham R. B., Donnenberg A. D. Effect of donor and recipient immunization protocols on primary and secondary human antibody responses in SCID mice reconstituted with human peripheral blood mononuclear cells. Infect Immun. 1992 Jun;60(6):2305–2308. doi: 10.1128/iai.60.6.2305-2308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G., Anastasi J., Feng J., Mc Shan C., DeGroot L., Quintans J., Grimaldi L. M. The fate of human peripheral blood lymphocytes after transplantation into SCID mice. Eur J Immunol. 1993 May;23(5):1023–1028. doi: 10.1002/eji.1830230506. [DOI] [PubMed] [Google Scholar]
- Mazingue C., Cottrez F., Auriault C., Cesbron J. Y., Capron A. Obtention of a human primary humoral response against schistosome protective antigens in severe combined immunodeficiency mice after the transfer of human peripheral blood mononuclear cells. Eur J Immunol. 1991 Jul;21(7):1763–1766. doi: 10.1002/eji.1830210728. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Medina K. L., Smithson G., Kincade P. W. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993 Nov 1;178(5):1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Baird S. M., Kirven M. B., Gulizia R. J., Wilson D. B., Kubayashi R., Picchio G., Garnier J. L., Sullivan J. L., Kipps T. J. EBV-associated B-cell lymphomas following transfer of human peripheral blood lymphocytes to mice with severe combined immune deficiency. Curr Top Microbiol Immunol. 1990;166:317–323. doi: 10.1007/978-3-642-75889-8_39. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B., Spector D. H., Spector S. A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991 Feb 15;251(4995):791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., MacIsaac P. D., Torbett B. E., Levy J. A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993 Apr 30;260(5108):689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Kumar V., Bennett M. Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID). Evidence that natural killer cells can mediate the specificity of marrow graft rejection. J Exp Med. 1987 Apr 1;165(4):1212–1217. doi: 10.1084/jem.165.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Nanduri J., Kazura J. W. Clinical and laboratory aspects of filariasis. Clin Microbiol Rev. 1989 Jan;2(1):39–50. doi: 10.1128/cmr.2.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson F. K., Greiner D. L., Shultz L. D., Rajan T. V. The immunodeficient scid mouse as a model for human lymphatic filariasis. J Exp Med. 1991 Mar 1;173(3):659–663. doi: 10.1084/jem.173.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M., Taguchi Y., Nakamine H., Pirruccello S. J., Davis J. R., Beisel K. W., Kleveland K. L., Sanger W. G., Fordyce R. R., Purtilo D. T. Characterization of Epstein-Barr virus-induced lymphoproliferation derived from human peripheral blood mononuclear cells transferred to severe combined immunodeficient mice. Am J Pathol. 1990 Sep;137(3):517–522. [PMC free article] [PubMed] [Google Scholar]
- Ottesen E. A. Filariasis now. Am J Trop Med Hyg. 1989 Sep;41(3 Suppl):9–17. doi: 10.4269/ajtmh.1989.41.9. [DOI] [PubMed] [Google Scholar]
- Ottesen E. A. Immunological aspects of lymphatic filariasis and onchocerciasis in man. Trans R Soc Trop Med Hyg. 1984;78 (Suppl):9–18. doi: 10.1016/0035-9203(84)90309-2. [DOI] [PubMed] [Google Scholar]
- Pflumio F., Lapidot T., Murdoch B., Patterson B., Dick J. E. Engraftment of human lymphoid cells into newborn SCID mice leads to graft-versus-host disease. Int Immunol. 1993 Dec;5(12):1509–1522. doi: 10.1093/intimm/5.12.1509. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Bach F. H. The barrier to xenotransplantation. Transplantation. 1991 Dec;52(6):937–947. doi: 10.1097/00007890-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Péault B., Weissman I. L., Baum C., McCune J. M., Tsukamoto A. Lymphoid reconstitution of the human fetal thymus in SCID mice with CD34+ precursor cells. J Exp Med. 1991 Nov 1;174(5):1283–1286. doi: 10.1084/jem.174.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan T. V., Nelson F. K., Killeen N., Shultz L. D., Yates J. A., Bailis J. M., Littman D. R., Greiner D. L. CD4+ T-lymphocytes are not required for murine resistance to the human filarial parasite, Brugia malayi. Exp Parasitol. 1994 Jun;78(4):352–360. doi: 10.1006/expr.1994.1038. [DOI] [PubMed] [Google Scholar]
- Rajan T. V., Nelson F. K., Shultz L. D., Koller B. H., Greiner D. L. CD8+ T lymphocytes are not required for murine resistance to human filarial parasites. J Parasitol. 1992 Aug;78(4):744–746. [PubMed] [Google Scholar]
- Rajan T. V., Nelson F. K., Shultz L. D., Shultz K. L., Beamer W. G., Yates J., Greiner D. L. Influence of gonadal steroids on susceptibility to Brugia malayi in scid mice. Acta Trop. 1994 Apr;56(4):307–314. doi: 10.1016/0001-706x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu J., Shpitz B., Gallinger S., Hozumi N. Human primary immune response in SCID mice engrafted with human peripheral blood lymphocytes. J Immunol. 1994 Apr 15;152(8):3806–3813. [PubMed] [Google Scholar]
- Saxon A., Macy E., Denis K., Tary-Lehmann M., Witte O., Braun J. Limited B cell repertoire in severe combined immunodeficient mice engrafted with peripheral blood mononuclear cells derived from immunodeficient or normal humans. J Clin Invest. 1991 Feb;87(2):658–665. doi: 10.1172/JCI115043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Gay S., Museteanu C., Kramer M. D., Zimmer G., Eichmann K., Museteanu U., Simon M. M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990 Oct;137(4):811–820. [PMC free article] [PubMed] [Google Scholar]
- Serreze D. V., Gaedeke J. W., Leiter E. H. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: defective regulation of cytokine receptors and protein kinase C. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9625–9629. doi: 10.1073/pnas.90.20.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze D. V., Gaskins H. R., Leiter E. H. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993 Mar 15;150(6):2534–2543. [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Christianson S. W., Gott B., Schweitzer I. B., Tennent B., McKenna S., Mobraaten L., Rajan T. V., Greiner D. L. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995 Jan 1;154(1):180–191. [PubMed] [Google Scholar]
- Simpson E., Farrant J., Chandler P. Phenotypic and functional studies of human peripheral blood lymphocytes engrafted in scid mice. Immunol Rev. 1991 Dec;124:97–111. doi: 10.1111/j.1600-065x.1991.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Smith C. I., Abedi M. R., Islam K. B., Johansson M. E., Christensson B., Hammarström L. Humoral immunity in scid mice reconstituted with cells from immunoglobulin-deficient or normal humans. Immunol Rev. 1991 Dec;124:113–138. doi: 10.1111/j.1600-065x.1991.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Smithson G., Beamer W. G., Shultz K. L., Christianson S. W., Shultz L. D., Kincade P. W. Increased B lymphopoiesis in genetically sex steroid-deficient hypogonadal (hpg) mice. J Exp Med. 1994 Aug 1;180(2):717–720. doi: 10.1084/jem.180.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C. D., Gold D. P., Wiley S., Wilson D. B., Sprent J. Rat T cell response to superantigens. I. V beta-restricted clonal deletion of rat T cells differentiating in rat-->mouse chimeras. J Exp Med. 1994 Jan 1;179(1):57–62. doi: 10.1084/jem.179.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbett B. E., Picchio G., Mosier D. E. hu-PBL-SCID mice: a model for human immune function, AIDS, and lymphomagenesis. Immunol Rev. 1991 Dec;124:139–164. doi: 10.1111/j.1600-065x.1991.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Vickery A. C., Albertine K. H., Nayar J. K., Kwa B. H. Histopathology of Brugia malayi-infected nude mice after immune-reconstitution. Acta Trop. 1991 Apr;49(1):45–55. doi: 10.1016/0001-706x(91)90029-j. [DOI] [PubMed] [Google Scholar]
- Vickery A. C., Nayar J. K. Brugia pahangi in nude mice: protective immunity to infective larvae is Thy 1.2+ cell dependent and cyclosporin A resistant. J Helminthol. 1987 Mar;61(1):19–27. doi: 10.1017/s0022149x00009664. [DOI] [PubMed] [Google Scholar]
- Vickery A. C., Vincent A. L., Sodeman W. A., Jr Effect of immune reconstitution on resistance to Brugia pahangi in congenitally athymic nude mice. J Parasitol. 1983 Jun;69(3):478–485. [PubMed] [Google Scholar]
- Vincent A. L., Vickery A. C., Lotz M. J., Desai U. The lymphatic pathology of Brugia pahangi in nude (athymic) and thymic mice C3H/HeN. J Parasitol. 1984 Feb;70(1):48–56. [PubMed] [Google Scholar]