Abstract
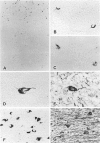
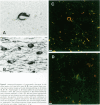
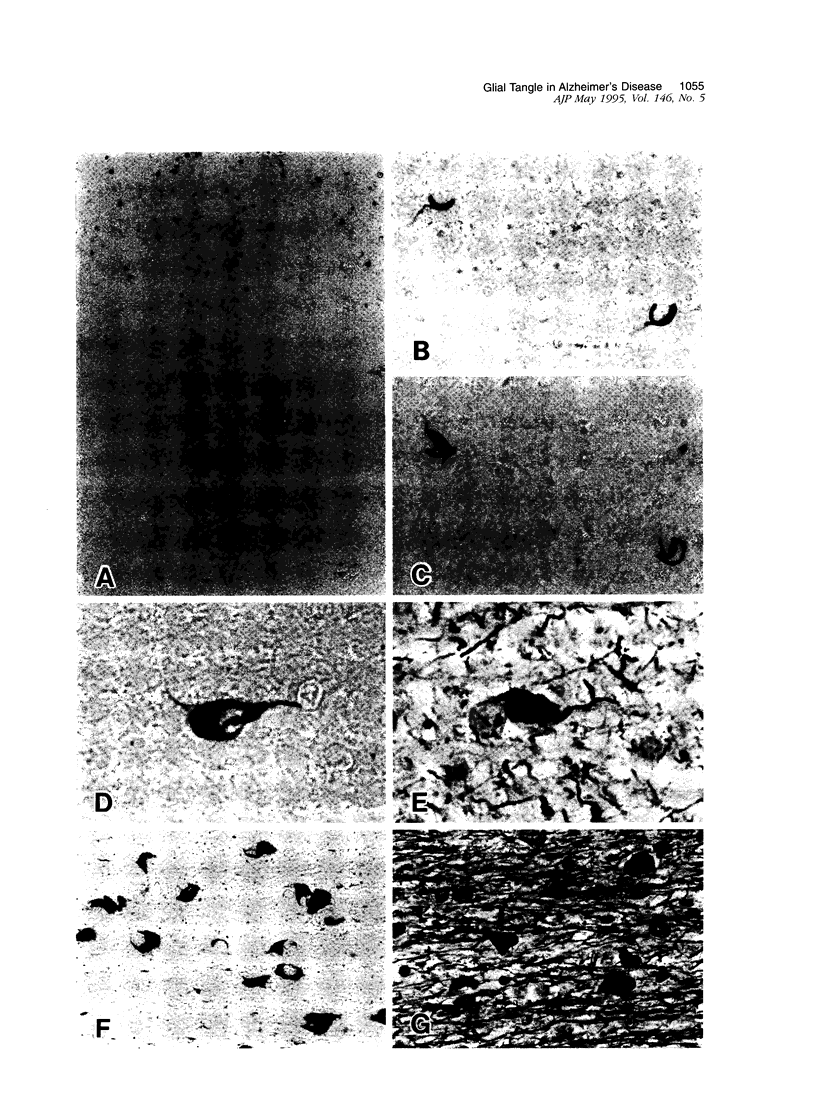
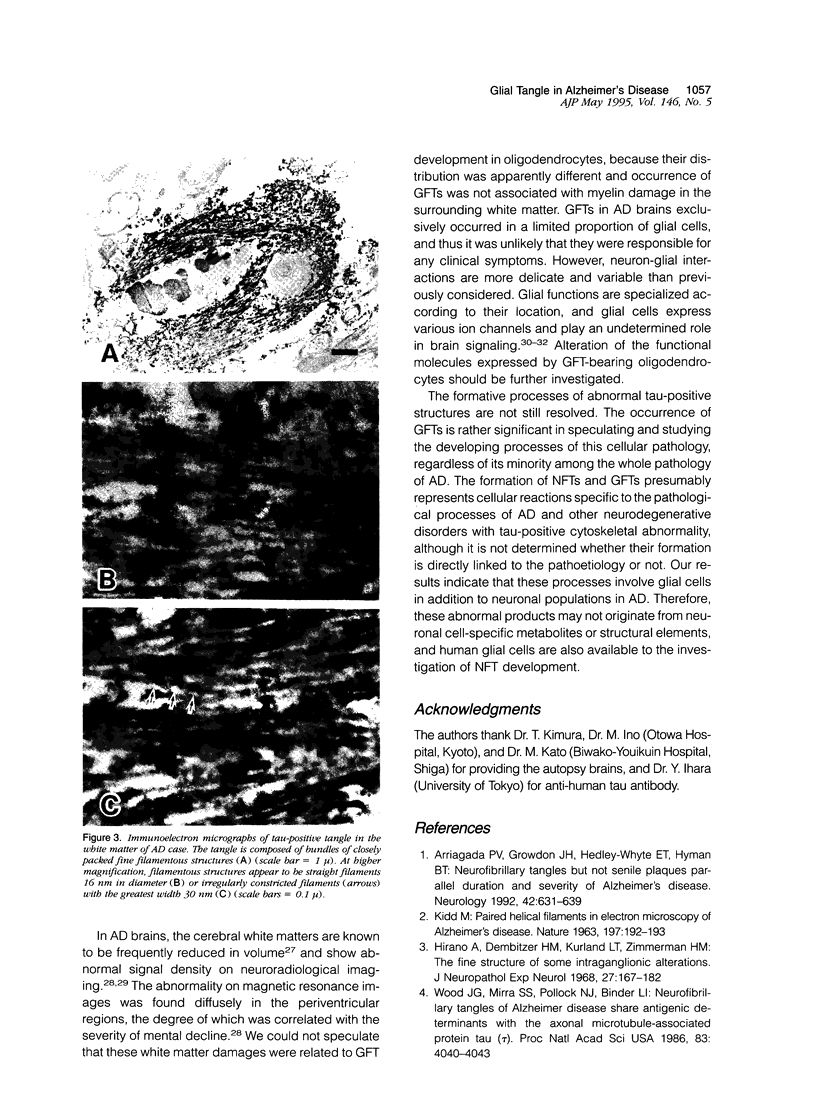
Neurofibrillary tangle is a major cytoskeletal pathology in Alzheimer's disease brains, and has been considered to develop exclusively in neuronal cells. We examined brains with Alzheimer's disease and observed argyrophilic fibrillary tangles not only in cortical neurons but also in subcortical glial cells in the frontal and temporal white matter. The tangles in glial cells were immunolabeled by antibodies against tau and ubiquitin, and double immunocytochemistry analyzed by confocal laser scanning microscopy demonstrated that the cytoplasms of tangle-bearing glia were labeled by antibodies against transferrin and 2'3'-cyclic nucleotide 3'-phosphohydrolase. Ultrastructurally, they were made up of bundles of straight filaments 16 nm in diameter and constricted filaments. These results indicate that fibrillary tangles resembling neurofibrillary tangles may develop in oligodendrocytes in brains with Alzheimer's disease and are distinguishable from glial cytoplasmic inclusions observed in multiple system atrophy brains. We referred to them as glial fibrillary tangles. Glial fibrillary tangles commonly occurred in this disease condition, and glial cells might be involved under the pathological processes similar to neuronal cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992 Mar;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Barres B. A. New roles for glia. J Neurosci. 1991 Dec;11(12):3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C., Lassmann H., Waehneldt T. V., Matthieu J. M., Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2',3'-cyclic nucleotide 3'-phosphodiesterase in the CNS of adult rats. J Neurochem. 1989 Jan;52(1):296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x. [DOI] [PubMed] [Google Scholar]
- Couchie D., Charrière-Bertrand C., Nunez J. Expression of the mRNA for tau proteins during brain development and in cultured neurons and astroglial cells. J Neurochem. 1988 Jun;50(6):1894–1899. doi: 10.1111/j.1471-4159.1988.tb02494.x. [DOI] [PubMed] [Google Scholar]
- Harrell L. E., Duvall E., Folks D. G., Duke L., Bartolucci A., Conboy T., Callaway R., Kerns D. The relationship of high-intensity signals on magnetic resonance images to cognitive and psychiatric state in Alzheimer's disease. Arch Neurol. 1991 Nov;48(11):1136–1140. doi: 10.1001/archneur.1991.00530230044019. [DOI] [PubMed] [Google Scholar]
- Hirano A., Dembitzer H. M., Kurland L. T., Zimmerman H. M. The fine structure of some intraganglionic alterations. Neurofibrillary tangles, granulovacuolar bodies and "rod-like" structures as seen in Guam amyotrophic lateral sclerosis and parkinsonism-dementia complex. J Neuropathol Exp Neurol. 1968 Apr;27(2):167–182. [PubMed] [Google Scholar]
- Ihara Y. Massive somatodendritic sprouting of cortical neurons in Alzheimer's disease. Brain Res. 1988 Aug 30;459(1):138–144. doi: 10.1016/0006-8993(88)90293-4. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Haga C., Akiyama H., Kase K., Iritani S. Coexistence of paired helical filaments and glial filaments in astrocytic processes within ghost tangles. Neurosci Lett. 1992 Dec 14;148(1-2):126–128. doi: 10.1016/0304-3940(92)90820-w. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Martin S. M., Landel H. B., Lansing A. J., Vijayan V. K. Immunocytochemical double labeling of glial fibrillary acidic protein and transferrin permits the identification of astrocytes and oligodendrocytes in the rat brain. J Neuropathol Exp Neurol. 1991 Mar;50(2):161–170. doi: 10.1097/00005072-199103000-00007. [DOI] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Mori H., Nishimura M., Namba Y., Oda M. Corticobasal degeneration: a disease with widespread appearance of abnormal tau and neurofibrillary tangles, and its relation to progressive supranuclear palsy. Acta Neuropathol. 1994;88(2):113–121. doi: 10.1007/BF00294503. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Titani K., Ihara Y. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron. 1993 Jun;10(6):1151–1160. doi: 10.1016/0896-6273(93)90063-w. [DOI] [PubMed] [Google Scholar]
- Murayama S., Arima K., Nakazato Y., Satoh J., Oda M., Inose T. Immunocytochemical and ultrastructural studies of neuronal and oligodendroglial cytoplasmic inclusions in multiple system atrophy. 2. Oligodendroglial cytoplasmic inclusions. Acta Neuropathol. 1992;84(1):32–38. doi: 10.1007/BF00427212. [DOI] [PubMed] [Google Scholar]
- Nakano I., Iwatsubo T., Otsuka N., Kamei M., Matsumura K., Mannen T. Paired helical filaments in astrocytes: electron microscopy and immunohistochemistry in a case of atypical Alzheimer's disease. Acta Neuropathol. 1992;83(3):228–232. doi: 10.1007/BF00296783. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Yamazaki H., Hirato J., Ishida Y., Yamaguchi H. Oligodendroglial microtubular tangles in olivopontocerebellar atrophy. J Neuropathol Exp Neurol. 1990 Sep;49(5):521–530. doi: 10.1097/00005072-199009000-00007. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Namba Y., Ikeda K., Oda M. Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy. Neurosci Lett. 1992 Aug 31;143(1-2):35–38. doi: 10.1016/0304-3940(92)90227-x. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Tomimoto H., Suenaga T., Nakamura S., Namba Y., Ikeda K., Akiguchi I., Kimura J. Synaptophysin and chromogranin A immunoreactivities of Lewy bodies in Parkinson's disease brains. Brain Res. 1994 Jan 21;634(2):339–344. doi: 10.1016/0006-8993(94)91940-2. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C. Tau protein immunoreactivity in dementia of the Alzheimer type. I. Morphology, evolution, distribution, and pathogenetic implications. Lab Invest. 1989 Jan;60(1):123–137. [PubMed] [Google Scholar]
- Papasozomenos S. C. Tau protein immunoreactivity in dementia of the Alzheimer type: II. Electron microscopy and pathogenetic implications. Effects of fixation on the morphology of the Alzheimer's abnormal filaments. Lab Invest. 1989 Mar;60(3):375–389. [PubMed] [Google Scholar]
- Papp M. I., Kahn J. E., Lantos P. L. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci. 1989 Dec;94(1-3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Papp M. I., Lantos P. L. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J Neurol Sci. 1992 Feb;107(2):172–182. doi: 10.1016/0022-510x(92)90286-t. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Barkhof F., Valk J., Algra P. R., van der Hoop R. G., Nauta J., Wolters E. C. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer's disease. Evidence for heterogeneity. Brain. 1992 Jun;115(Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Sato Y., Tateishi J. Massive accumulation of modified tau and severe depletion of normal tau characterize the cerebral cortex and white matter of Alzheimer's disease. Demonstration using the hydrated autoclaving method. Am J Pathol. 1992 Apr;140(4):937–945. [PMC free article] [PubMed] [Google Scholar]
- Wilkin G. P., Marriott D. R., Cholewinski A. J. Astrocyte heterogeneity. Trends Neurosci. 1990 Feb;13(2):43–46. doi: 10.1016/0166-2236(90)90065-i. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., McGeer P. L. Oligodendroglial microtubular masses: an abnormality observed in some human neurodegenerative diseases. Neurosci Lett. 1990 Dec 11;120(2):163–166. doi: 10.1016/0304-3940(90)90028-8. [DOI] [PubMed] [Google Scholar]
- de la Monte S. M. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer's disease. Ann Neurol. 1989 May;25(5):450–459. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]