Abstract
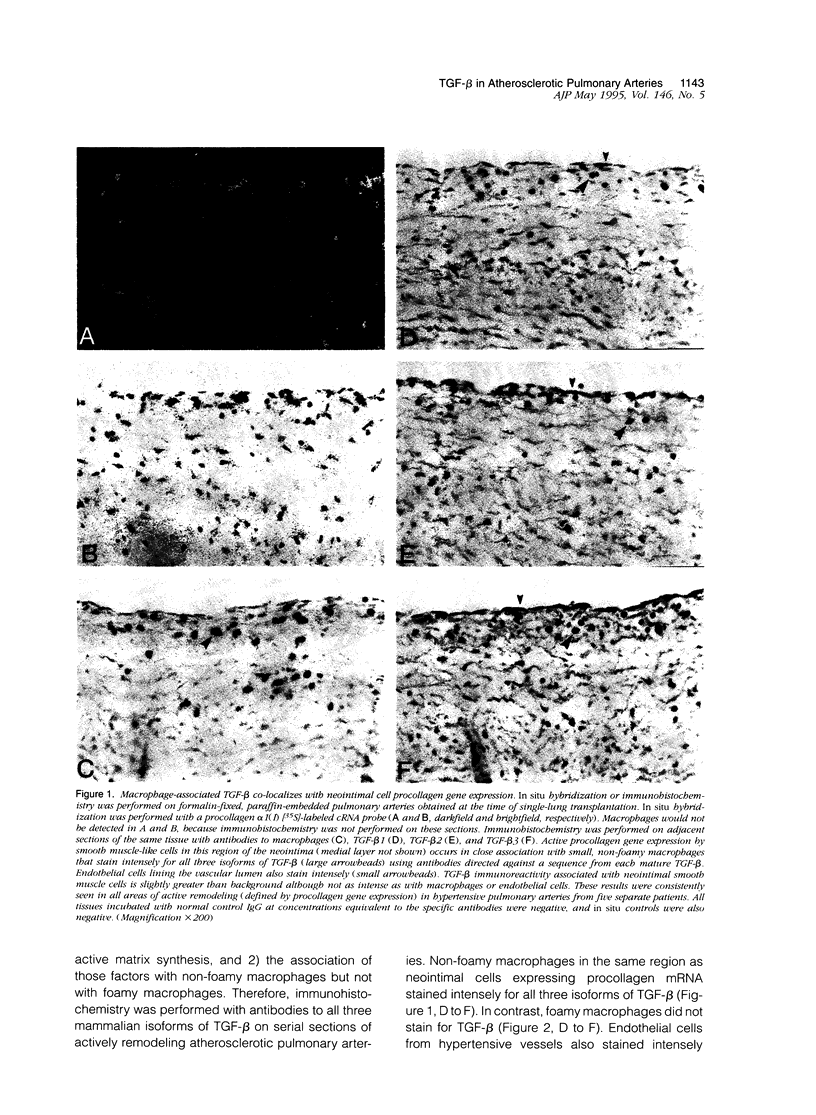
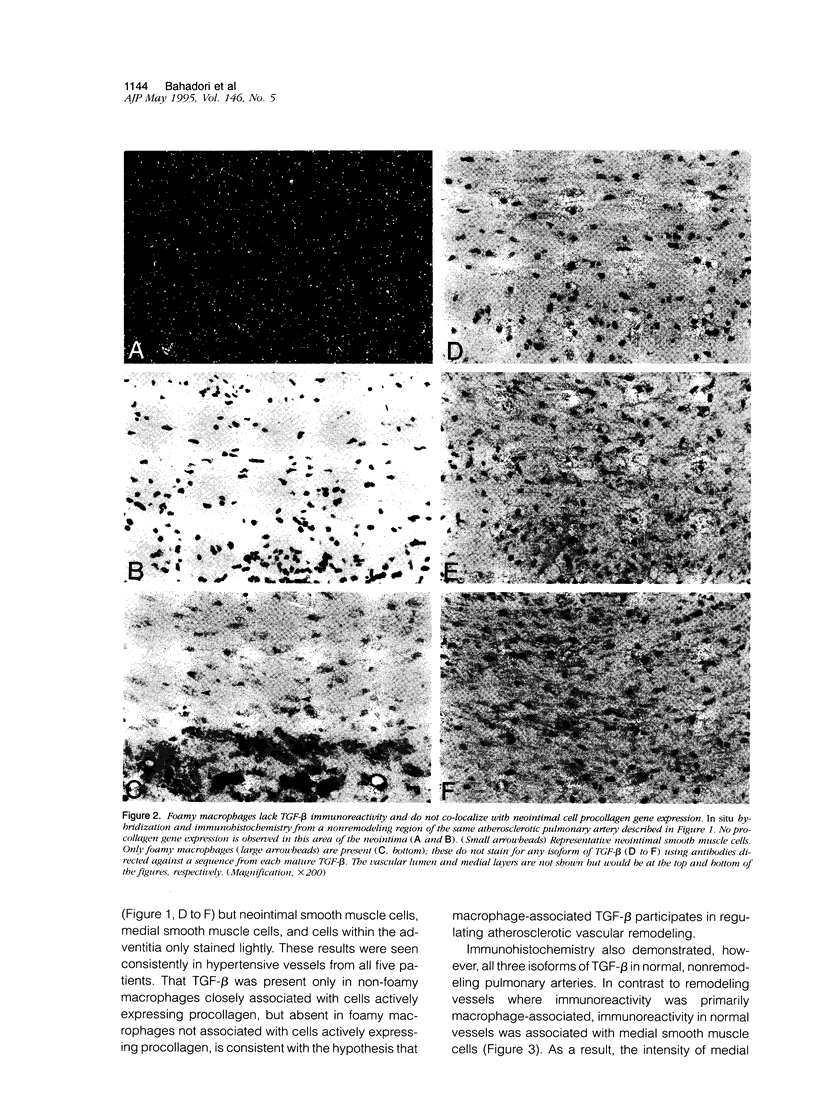
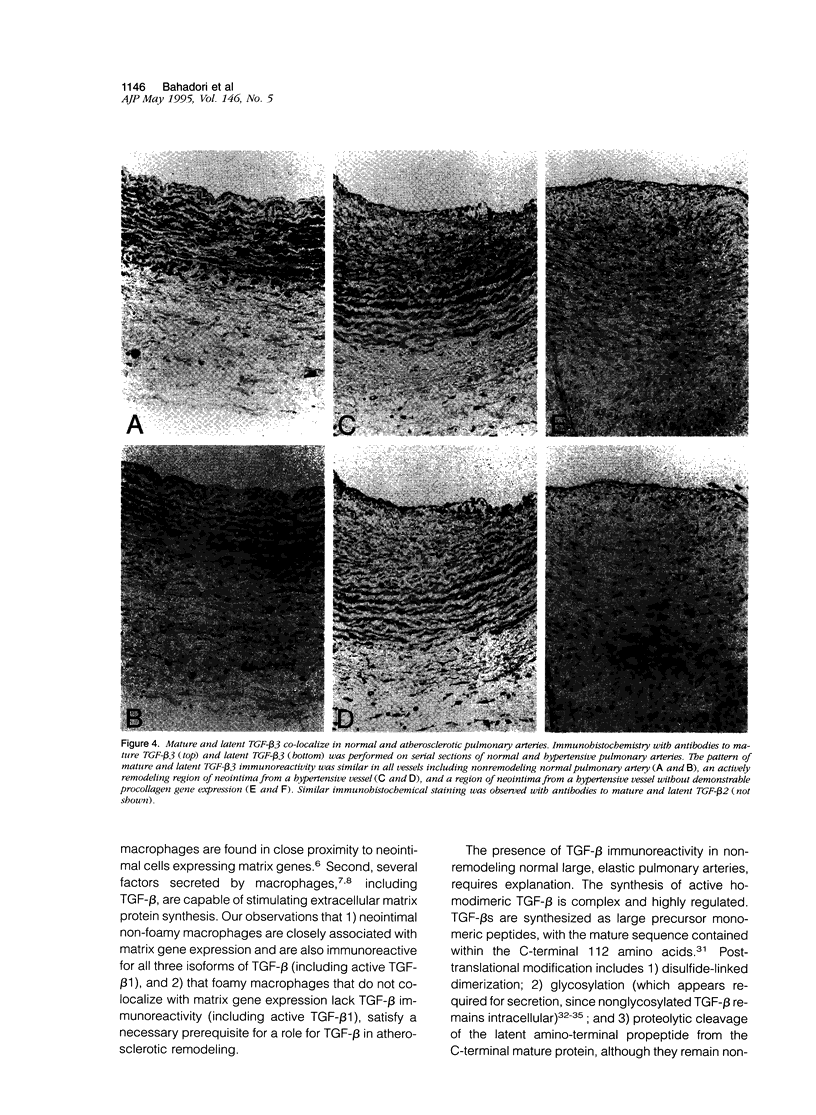
Vascular remodeling in adult atherosclerotic pulmonary arteries is characterized by discrete areas of neointimal smooth muscle cell extracellular matrix gene expression in close proximity to non-foamy macrophages, suggesting regulation by local macrophage-associated factors. The purpose of these studies was to begin addressing the role of putative macrophage-associated factors such as transforming growth factor-beta (TGF-beta), by determining the spatial relationship between TGF-beta and neointimal matrix gene expression in human atherosclerotic pulmonary arteries. For example, the participation of TGF-beta in vascular remodeling could be inferred by its colocalization with non-foamy macrophages in areas of active matrix synthesis. In situ hybridization and immunohistochemistry demonstrated focal neointimal procollagen gene expression in close association with non-foamy but not foamy macrophages. Immunohistochemistry with isoform-specific anti-TGF-beta antibodies demonstrated all three isoforms of TGF-beta associated with non-foamy macrophages, but foamy macrophages were not immunoreactive. Neointimal and medial smooth muscle cells stained lightly. In contrast, intense TGF-beta immunoreactivity was also associated with medial smooth muscle cells in normal nonremodeling vessels. Immunohistochemistry with antibodies specific for latent TGF-beta was similar to immunohistochemistry for mature TGF-beta in both remodeling and nonremodeling vessels. Finally, using an antibody specific for active TGF-beta 1, immunoreactivity was only seen in non-foamy neointimal macrophages but not in foamy macrophages or medial smooth muscle cells from hypertensive or normal vessels. These observations suggest non-foamy macrophages may participate in modulating matrix gene expression in atherosclerotic remodeling via a TGF-beta-dependent mechanism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcellos-Hoff M. H., Derynck R., Tsang M. L., Weatherbee J. A. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994 Feb;93(2):892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson J., Edwards W. D. Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc. 1985 Jan;60(1):16–25. doi: 10.1016/s0025-6196(12)65277-x. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Botney M. D., Kaiser L. R., Cooper J. D., Mecham R. P., Parghi D., Roby J., Parks W. C. Extracellular matrix protein gene expression in atherosclerotic hypertensive pulmonary arteries. Am J Pathol. 1992 Feb;140(2):357–364. [PMC free article] [PubMed] [Google Scholar]
- Botney M. D., Liptay M. J., Kaiser L. R., Cooper J. D., Parks W. C., Mecham R. P. Active collagen synthesis by pulmonary arteries in human primary pulmonary hypertension. Am J Pathol. 1993 Jul;143(1):121–129. [PMC free article] [PubMed] [Google Scholar]
- Botney M. D., Parks W. C., Crouch E. C., Stenmark K., Mecham R. P. Transforming growth factor-beta 1 is decreased in remodeling hypertensive bovine pulmonary arteries. J Clin Invest. 1992 May;89(5):1629–1635. doi: 10.1172/JCI115759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner A. M., Lioubin M. N., Marquardt H., Malacko A. R., Wang W. C., Shapiro R. A., Neubauer M., Cook J., Madisen L., Purchio A. F. Site-directed mutagenesis of glycosylation sites in the transforming growth factor-beta 1 (TGF beta 1) and TGF beta 2 (414) precursors and of cysteine residues within mature TGF beta 1: effects on secretion and bioactivity. Mol Endocrinol. 1992 Oct;6(10):1691–1700. doi: 10.1210/mend.6.10.1448117. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Eghbali M., Giambrone M. A., Eghbali M., Zern M. A. Differential effects of gamma-interferon on collagen and fibronectin gene expression. J Biol Chem. 1987 Sep 25;262(27):13348–13351. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Fine A., Goldstein R. H. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987 Mar 15;262(8):3897–3902. [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R., Abe M., Sato Y., Miyazono K., Harpel J., Heldin C. H., Rifkin D. B. Role of the latent TGF-beta binding protein in the activation of latent TGF-beta by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993 Feb;120(4):995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Jennings J. C., Mohan S. Heterogeneity of latent transforming growth factor-beta isolated from bone matrix proteins. Endocrinology. 1990 Feb;126(2):1014–1021. doi: 10.1210/endo-126-2-1014. [DOI] [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptay M. J., Parks W. C., Mecham R. P., Roby J., Kaiser L. R., Cooper J. D., Botney M. D. Neointimal macrophages colocalize with extracellular matrix gene expression in human atherosclerotic pulmonary arteries. J Clin Invest. 1993 Feb;91(2):588–594. doi: 10.1172/JCI116238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. M., Davidson J. M. The elastogenic effect of recombinant transforming growth factor-beta on porcine aortic smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):895–901. doi: 10.1016/0006-291x(88)90224-0. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Andres J., Attisano L., Cheifetz S., López-Casillas F., Ohtsuki M., Wrana J. L. TGF-beta receptors. Mol Reprod Dev. 1992 Jun;32(2):99–104. doi: 10.1002/mrd.1080320204. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., McNamer R. Transforming growth factor-beta increases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol. 1990 Oct;3(4):369–376. doi: 10.1165/ajrcmb/3.4.369. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Heldin C. H. Role for carbohydrate structures in TGF-beta 1 latency. Nature. 1989 Mar 9;338(6211):158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Olofsson A., Colosetti P., Heldin C. H. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991 May;10(5):1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K., Thyberg J., Heldin C. H. Retention of the transforming growth factor-beta 1 precursor in the Golgi complex in a latent endoglycosidase H-sensitive form. J Biol Chem. 1992 Mar 15;267(8):5668–5675. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Schultz-Cherry S., Hök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992 Feb;3(2):181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien K. D., Gordon D., Deeb S., Ferguson M., Chait A. Lipoprotein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest. 1992 May;89(5):1544–1550. doi: 10.1172/JCI115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar V. M., Vukicevic S., Reddi A. H. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991 Feb;143(2):303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Bonewald L., Mundy G. R. Characterization of the latent transforming growth factor beta complex in bone. J Bone Miner Res. 1990 Jan;5(1):49–58. doi: 10.1002/jbmr.5650050109. [DOI] [PubMed] [Google Scholar]
- Pietra G. G., Edwards W. D., Kay J. M., Rich S., Kernis J., Schloo B., Ayres S. M., Bergofsky E. H., Brundage B. H., Detre K. M. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989 Nov;80(5):1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- Prosser I. W., Stenmark K. R., Suthar M., Crouch E. C., Mecham R. P., Parks W. C. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Raghow B., Irish P., Kang A. H. Coordinate regulation of transforming growth factor beta gene expression and cell proliferation in hamster lungs undergoing bleomycin-induced pulmonary fibrosis. J Clin Invest. 1989 Dec;84(6):1836–1842. doi: 10.1172/JCI114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Kim S. J., Noma T., Glick A. B., Lafyatis R., Lechleider R., Jakowlew S. B., Geiser A., O'Reilly M. A., Danielpour D. Multiple forms of TGF-beta: distinct promoters and differential expression. Ciba Found Symp. 1991;157:7–28. doi: 10.1002/9780470514061.ch2. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Sha X., Brunner A. M., Purchio A. F., Gentry L. E. Transforming growth factor beta 1: importance of glycosylation and acidic proteases for processing and secretion. Mol Endocrinol. 1989 Jul;3(7):1090–1098. doi: 10.1210/mend-3-7-1090. [DOI] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]








