Abstract
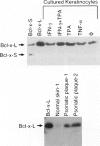
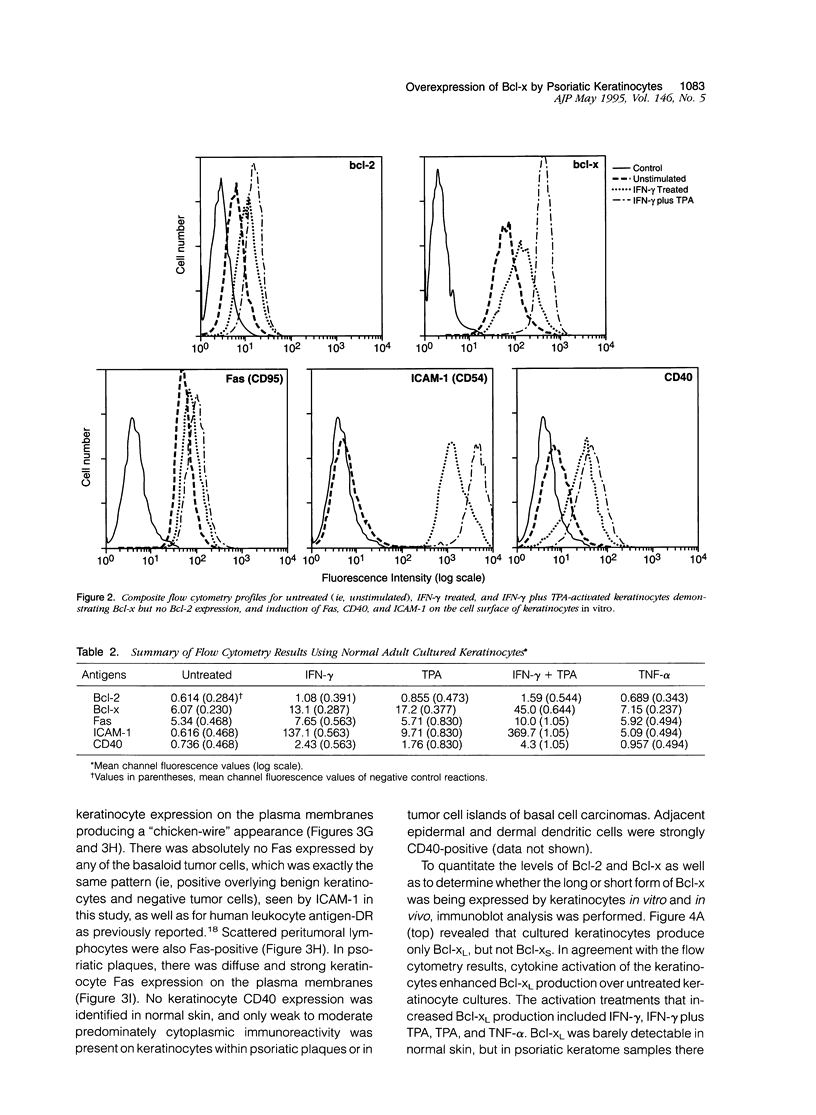
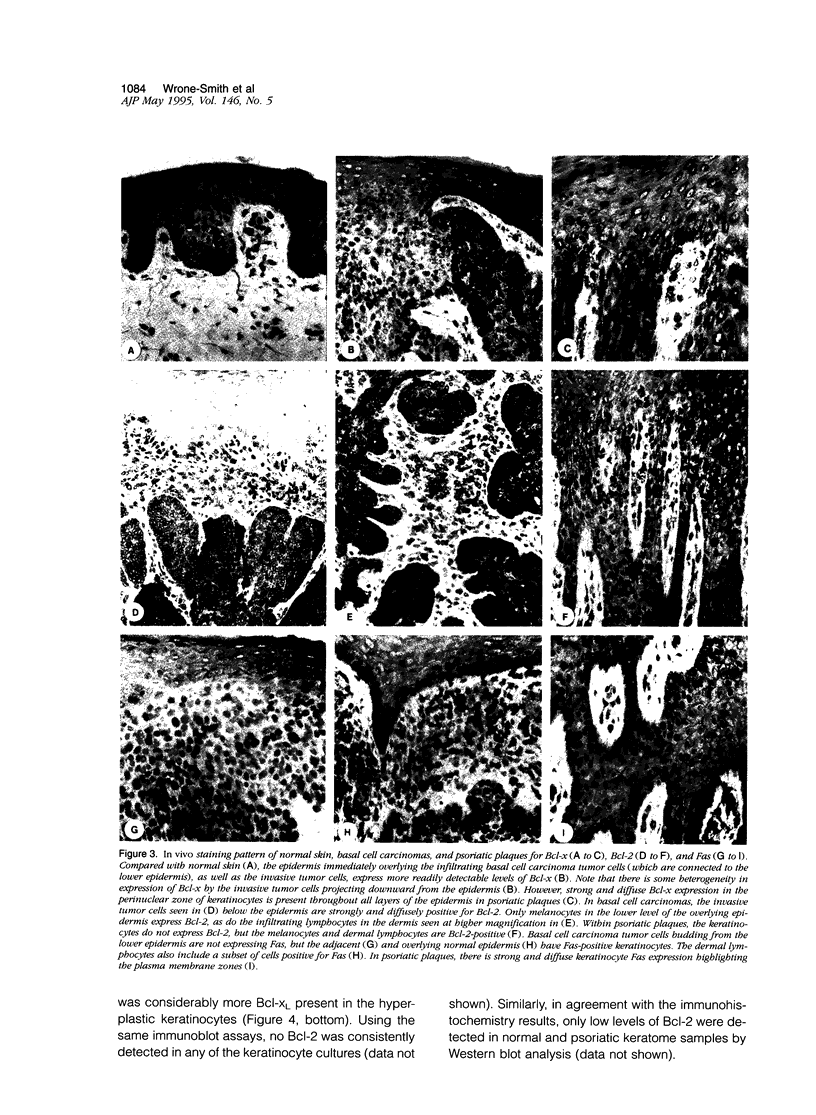
Apoptosis is a required event in maintaining kinetic homeostasis within continually renewing tissues such as skin. However, no systematic study of the apoptotic process in epidermal keratinocytes of the skin has been performed. In this report, we examined the expression of proteins associated with promoting (Fas) or preventing (Bcl-2, Bcl-x, CD40) apoptosis in the normal, psoriatic, and malignant keratinocyte. Immunohistochemical staining and flow cytometry analysis revealed that normal cultured keratinocytes express low levels of Fas, CD40, and Bcl-x that was enhanced by cytokines including gamma-interferon (IFN-gamma) and a phorbol ester tumor promoter, TPA. Only faint Bcl-2 staining was detected in cultured keratinocytes exposed to IFN-gamma and TPA compared with the prominent expression of Bcl-x. Biopsies of normal skin, psoriatic plaques, and basal cell carcinomas were examined to extend the in vitro observations. Immunohistochemical staining revealed that while keratinocytes in normal epithelium express low to absent levels of Fas and Bcl-x, psoriatic keratinocytes expressed significantly higher levels of Fas and Bcl-x. In contrast, malignant keratinocytes in basal cell carcinomas expressed high levels of Bcl-2, but minimal Bcl-x, and no Fas. Immunoblot analysis revealed that the long form of Bcl-x (Bcl-xI), which prevents apoptosis in lymphocytes, is expressed by cultured keratinocytes and psoriatic plaque keratinocytes. We conclude that normal cytokine-activated keratinocytes can express an apoptotic (Fas) and an anti-apoptotic protein (Bcl-x). The overexpression of Bcl-x in psoriasis, or Bcl-2 in basal cell carcinomas, may contribute to the longevity of these cells by blocking the normal apoptotic process involved in the terminal differentiation program of epidermal keratinocytes.
Full text
PDF



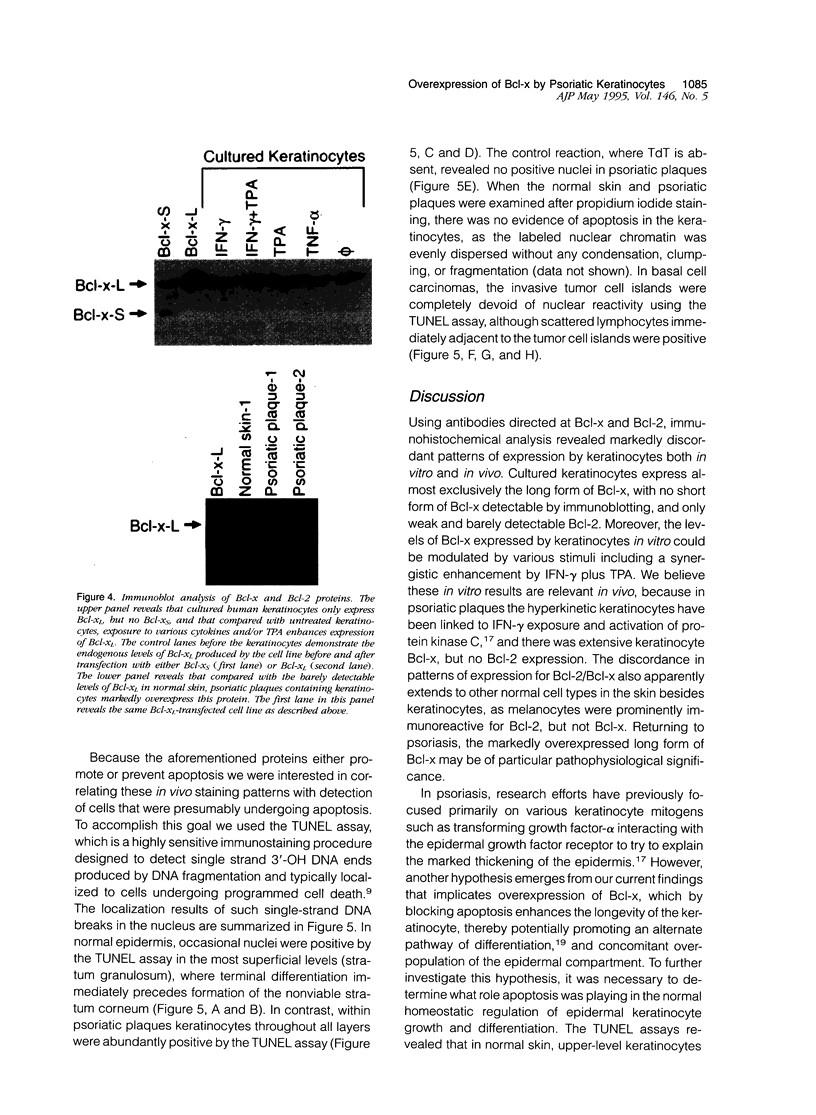
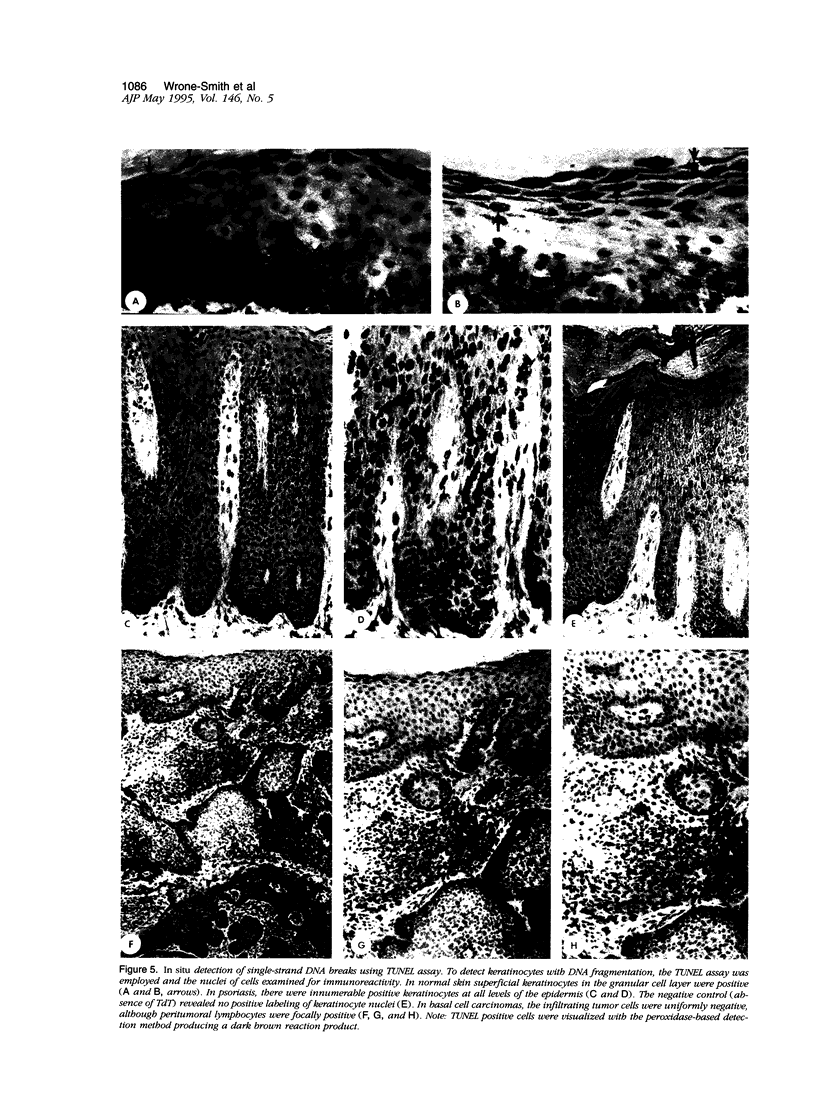


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., van Huffel C. Unraveling function in the TNF ligand and receptor families. Science. 1994 Apr 29;264(5159):667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Galy A. H., Spits H. CD40 is functionally expressed on human thymic epithelial cells. J Immunol. 1992 Aug 1;149(3):775–782. [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-García M., Pérez-Ballestero R., Ding L., Duan L., Boise L. H., Thompson C. B., Núez G. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development. 1994 Oct;120(10):3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- Gottschalk A. R., Boise L. H., Thompson C. B., Quintáns J. Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Tsujimoto Y., Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993 Jul 15;151(2):621–627. [PubMed] [Google Scholar]
- Kristensen M., Chu C. Q., Eedy D. J., Feldmann M., Brennan F. M., Breathnach S. M. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 1993 Nov;94(2):354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leithäuser F., Dhein J., Mechtersheimer G., Koretz K., Brüderlein S., Henne C., Schmidt A., Debatin K. M., Krammer P. H., Möller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993 Oct;69(4):415–429. [PubMed] [Google Scholar]
- Lu Q. L., Poulsom R., Wong L., Hanby A. M. Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol. 1993 Apr;169(4):431–437. doi: 10.1002/path.1711690408. [DOI] [PubMed] [Google Scholar]
- Mansbridge J. N., Knapp A. M. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987 Sep;89(3):253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Nakagawa K., Yamamura K., Maeda S., Ichihashi M. bcl-2 expression in epidermal keratinocytic diseases. Cancer. 1994 Sep 15;74(6):1720–1724. doi: 10.1002/1097-0142(19940915)74:6<1720::aid-cncr2820740613>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Karabin G. D., Barker J. N., Griffiths C. E., Sarma V., Mitra R. S., Elder J. T., Kunkel S. L., Dixit V. M. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol. 1991 Jan;138(1):129–140. [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Lee K., Turka L. A., Green J., Thompson C., Shimizu Y. Discordant expression of CD28 ligands, BB-1, and B7 on keratinocytes in vitro and psoriatic cells in vivo. Am J Pathol. 1993 Apr;142(4):1029–1040. [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J. The cytokine network in psoriasis. Arch Dermatol. 1991 Jun;127(6):871–884. [PubMed] [Google Scholar]
- Sayama K., Yonehara S., Watanabe Y., Miki Y. Expression of Fas antigen on keratinocytes in vivo and induction of apoptosis in cultured keratinocytes. J Invest Dermatol. 1994 Sep;103(3):330–334. doi: 10.1111/1523-1747.ep12394858. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Taylor R. S., Griffiths C. E., Brown M. D., Swanson N. A., Nickoloff B. J. Constitutive absence and interferon-gamma-induced expression of adhesion molecules in basal cell carcinoma. J Am Acad Dermatol. 1990 May;22(5 Pt 1):721–726. doi: 10.1016/0190-9622(90)70097-2. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]