Abstract
The normally developing placenta undergoes extensive but regulated noninvasive cellular proliferation. Various proto-oncogenes and growth factors have been associated with the regulation of trophoblastic placental growth. Activation of some oncogenes and altered expression of growth factors have been demonstrated in trophoblastic tumors (hydatidiform mole and choriocarcinoma). The ras proto-oncogene plays a key role in the signal transduction cascade of activated growth factors, and is known to be activated or overexpressed in multiple tumor types. Ras GTPase activating protein (RasGAP), a major down-regulator of ras activity, is present at high levels in placenta. To assess the role that Ras-GAP plays in the development of trophoblastic tumors, we performed immunohistochemical analyses with anti RasGAP antibodies of normal placentas, hydatidiform moles, invasive moles, and malignant choriocarcinomas. Normal placentas and noninvasive hydatidiform mole displayed intense positive staining confined to trophoblasts, whereas no staining was observed in the trophoblasts of invasive moles or choriocarcinomas. Thus, there was an inverse correlation between expression levels of RasGAP protein and the invasive potential and malignant phenotype in human trophoblastic tumors. The data indicate that RasGAP may play a regulatory role in trophoblast proliferation and that abolishing its activity may be associated with malignant transformation of these cells.
Full text
PDF


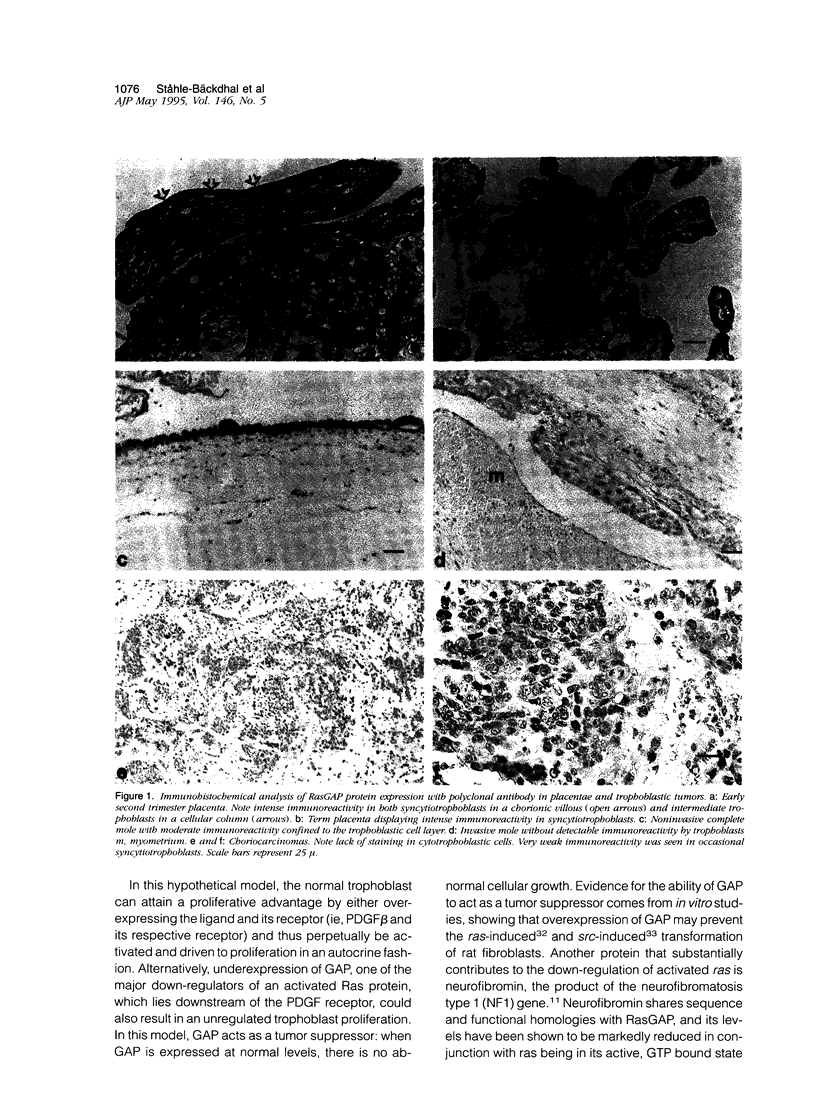


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cheung A. N., Srivastava G., Pittaluga S., Man T. K., Ngan H., Collins R. J. Expression of c-myc and c-fms oncogenes in trophoblastic cells in hydatidiform mole and normal human placenta. J Clin Pathol. 1993 Mar;46(3):204–207. doi: 10.1136/jcp.46.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Papageorge A. G., Fletcher J. A., Diehl S. R., Ratner N., Vass W. C., Lowy D. R. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992 Apr 17;69(2):265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Zhang K., Redford P., Vass W. C., Lowy D. R. Suppression of src transformation by overexpression of full-length GTPase-activating protein (GAP) or of the GAP C terminus. Mol Cell Biol. 1991 May;11(5):2819–2825. doi: 10.1128/mcb.11.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold J., Arnholdt H., Lai M. D., Löhrs U. C-myc expression in early human placenta--a critical evaluation of its localization. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61(1):65–73. doi: 10.1007/BF02890406. [DOI] [PubMed] [Google Scholar]
- Dungy L. J., Siddiqi T. A., Khan S. C-jun and jun-B oncogene expression during placental development. Am J Obstet Gynecol. 1991 Dec;165(6 Pt 1):1853–1856. doi: 10.1016/0002-9378(91)90045-s. [DOI] [PubMed] [Google Scholar]
- Elston C. W., Bagshawe K. D. The value of histological grading in the management of hydatidiform mole. J Obstet Gynaecol Br Commonw. 1972 Aug;79(8):717–724. doi: 10.1111/j.1471-0528.1972.tb12907.x. [DOI] [PubMed] [Google Scholar]
- Field J., Broek D., Kataoka T., Wigler M. Guanine nucleotide activation of, and competition between, RAS proteins from Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jun;7(6):2128–2133. doi: 10.1128/mcb.7.6.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halenbeck R., Crosier W. J., Clark R., McCormick F., Koths K. Purification, characterization, and western blot analysis of human GTPase-activating protein from native and recombinant sources. J Biol Chem. 1990 Dec 15;265(35):21922–21928. [PubMed] [Google Scholar]
- Hall J. G. How imprinting is relevant to human disease. Dev Suppl. 1990:141–148. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990 Jul;1(8):555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry I., Bonaiti-Pellié C., Chehensse V., Beldjord C., Schwartz C., Utermann G., Junien C. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature. 1991 Jun 20;351(6328):665–667. doi: 10.1038/351665a0. [DOI] [PubMed] [Google Scholar]
- Holmgren L., Flam F., Larsson E., Ohlsson R. Successive activation of the platelet-derived growth factor beta receptor and platelet-derived growth factor B genes correlates with the genesis of human choriocarcinoma. Cancer Res. 1993 Jun 15;53(12):2927–2931. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jacobs P. A., Wilson C. M., Sprenkle J. A., Rosenshein N. B., Migeon B. R. Mechanism of origin of complete hydatidiform moles. Nature. 1980 Aug 14;286(5774):714–716. doi: 10.1038/286714a0. [DOI] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Ladines-Llave C. A., Maruo T., Manalo A. M., Mochizuki M. Decreased expression of epidermal growth factor and its receptor in the malignant transformation of trophoblasts. Cancer. 1993 Jun 15;71(12):4118–4123. doi: 10.1002/1097-0142(19930615)71:12<4118::aid-cncr2820711251>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Li Y., Bollag G., Clark R., Stevens J., Conroy L., Fults D., Ward K., Friedman E., Samowitz W., Robertson M. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell. 1992 Apr 17;69(2):275–281. doi: 10.1016/0092-8674(92)90408-5. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Sasaki M., Nishida M., Wake N. [Identification of a chromosome carrying a putative tumor suppressor gene in human choriocarcinoma by microcell-mediated chromosome transfer]. Hum Cell. 1991 Mar;4(1):38–43. [PubMed] [Google Scholar]
- Mollat P., Zhang G. Y., Frobert Y., Zhang Y. H., Fournier A., Grassi J., Thang M. N. Non-neutralizing monoclonal antibodies against Ras GTPase-activating protein: production, characterization and use in an enzyme immunometric assay. Biotechnology (N Y) 1992 Oct;10(10):1151–1156. doi: 10.1038/nbt1092-1151. [DOI] [PubMed] [Google Scholar]
- Ober W. B., Edgcomb J. H., Price E. B., Jr The pathology of choriocarcinoma. Ann N Y Acad Sci. 1971 Jan 28;172(10):299–426. doi: 10.1111/j.1749-6632.1971.tb34943.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson R. Growth factors, protooncogenes and human placental development. Cell Differ Dev. 1989 Oct;28(1):1–15. doi: 10.1016/0922-3371(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Park J. S., Namkoong S. E., Lee H. Y., Kim S. J., Hong K. J., Kim I. S., Kim K. U., Shim B. S. Expression and amplification of cellular oncogenes in human developing placenta and neoplastic trophoblastic tissue. Asia Oceania J Obstet Gynaecol. 1992 Mar;18(1):57–64. doi: 10.1111/j.1447-0756.1992.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Rustin G. J., Bagshawe K. D. Gestational trophoblastic tumors. Crit Rev Oncol Hematol. 1985;3(2):103–142. doi: 10.1016/s1040-8428(85)80022-6. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Kacinski B. M., Kohorn E. I., Merino M. J., Carter D., Blakemore K. J. Demonstration of myc and ras oncogene expression by hybridization in situ in hydatidiform mole and in the BeWo choriocarcinoma cell line. Am J Obstet Gynecol. 1986 Feb;154(2):390–393. doi: 10.1016/0002-9378(86)90677-0. [DOI] [PubMed] [Google Scholar]
- Solter D. Differential imprinting and expression of maternal and paternal genomes. Annu Rev Genet. 1988;22:127–146. doi: 10.1146/annurev.ge.22.120188.001015. [DOI] [PubMed] [Google Scholar]
- Szentirmay Z., Ishizaka Y., Ohgaki H., Tahira T., Nagao M., Esumi H. Demonstration by in situ hybridization of ret proto-oncogene mRNA in developing placenta during mid-term of rat gestation. Oncogene. 1990 May;5(5):701–705. [PubMed] [Google Scholar]
- Szulman A. E., Surti U. Complete and partial hydatidiform moles: cytogenetic and morphological aspects. Adv Exp Med Biol. 1984;176:135–146. doi: 10.1007/978-1-4684-4811-5_7. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., Gibbs J. B. Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature. 1988 Sep 1;335(6185):90–93. doi: 10.1038/335090a0. [DOI] [PubMed] [Google Scholar]
- Wang D. P., Fujii S., Konishi I., Nanbu Y., Iwai T., Nonogaki H., Mori T. Expression of c-erbB-2 protein and epidermal growth factor receptor in normal tissues of the female genital tract and in the placenta. Virchows Arch A Pathol Anat Histopathol. 1992;420(5):385–393. doi: 10.1007/BF01600509. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Wolf H. K., Zarnegar R., Oliver L., Michalopoulos G. K. Hepatocyte growth factor in human placenta and trophoblastic disease. Am J Pathol. 1991 Apr;138(4):1035–1043. [PMC free article] [PubMed] [Google Scholar]
- Xu G. F., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990 Aug 10;62(3):599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- Zhang K., DeClue J. E., Vass W. C., Papageorge A. G., McCormick F., Lowy D. R. Suppression of c-ras transformation by GTPase-activating protein. Nature. 1990 Aug 23;346(6286):754–756. doi: 10.1038/346754a0. [DOI] [PubMed] [Google Scholar]




