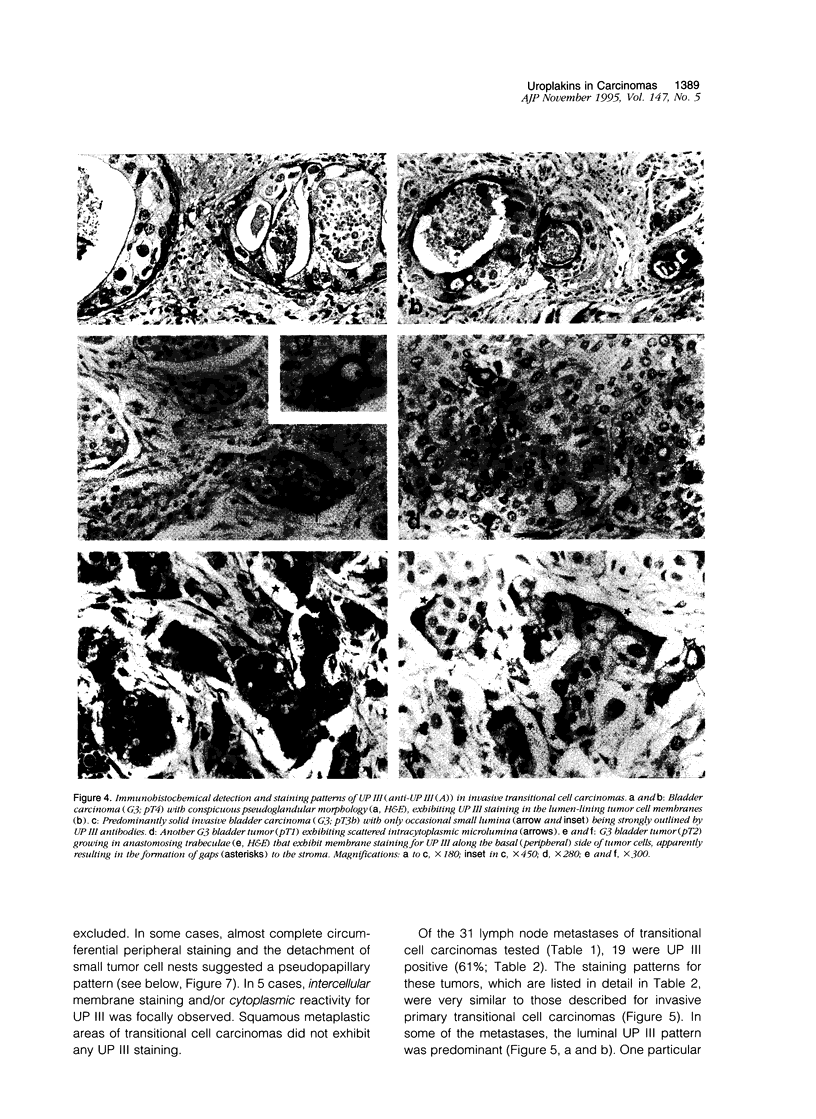
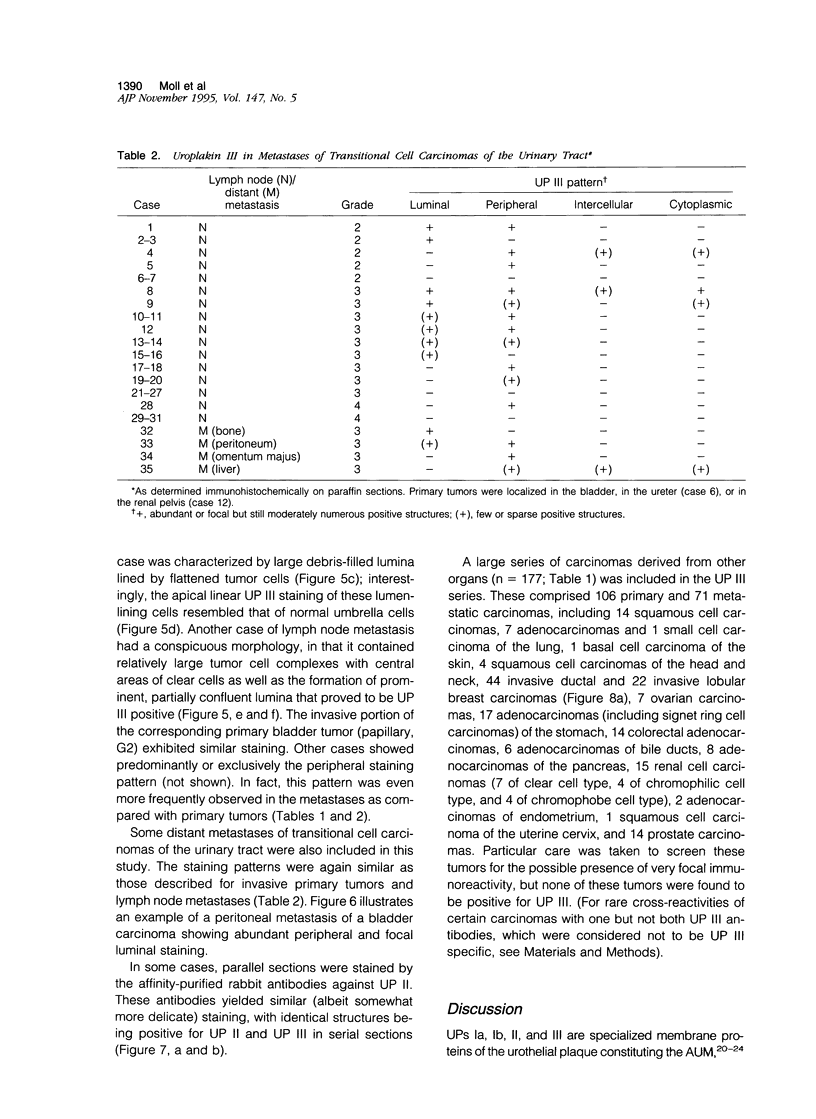
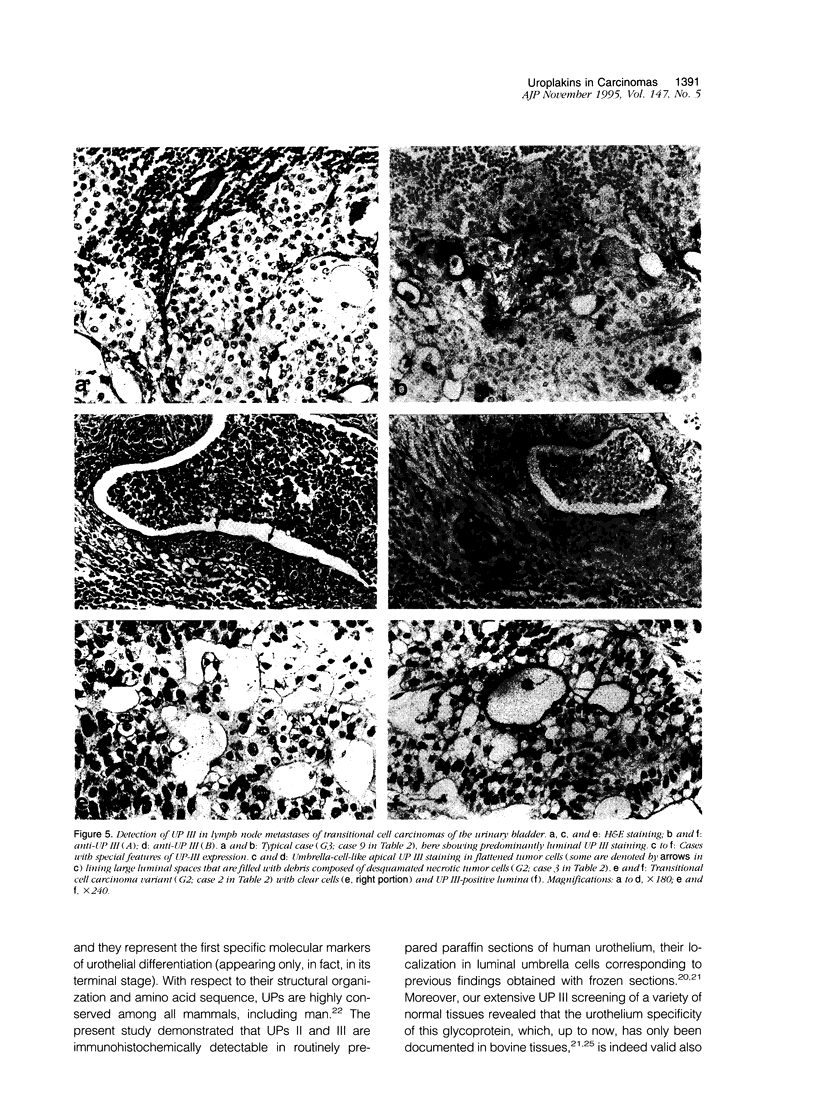
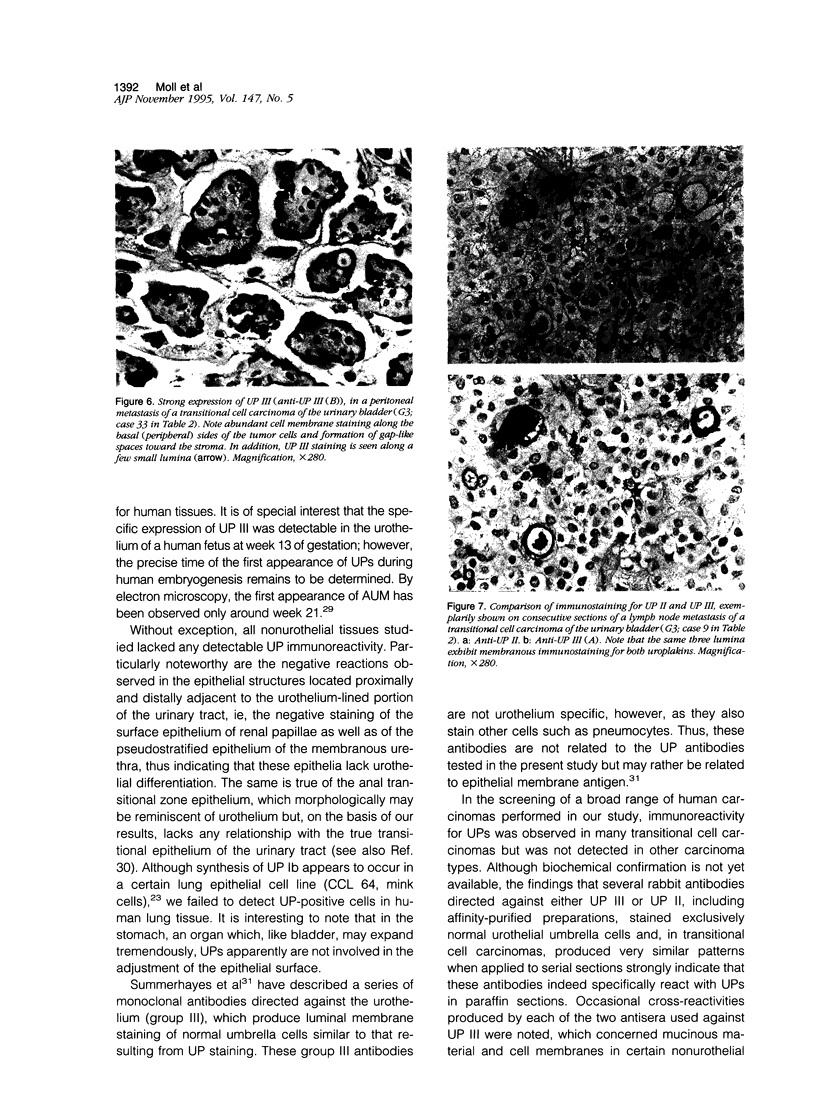
Abstract
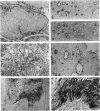
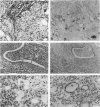
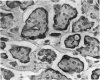
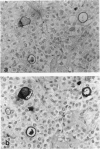
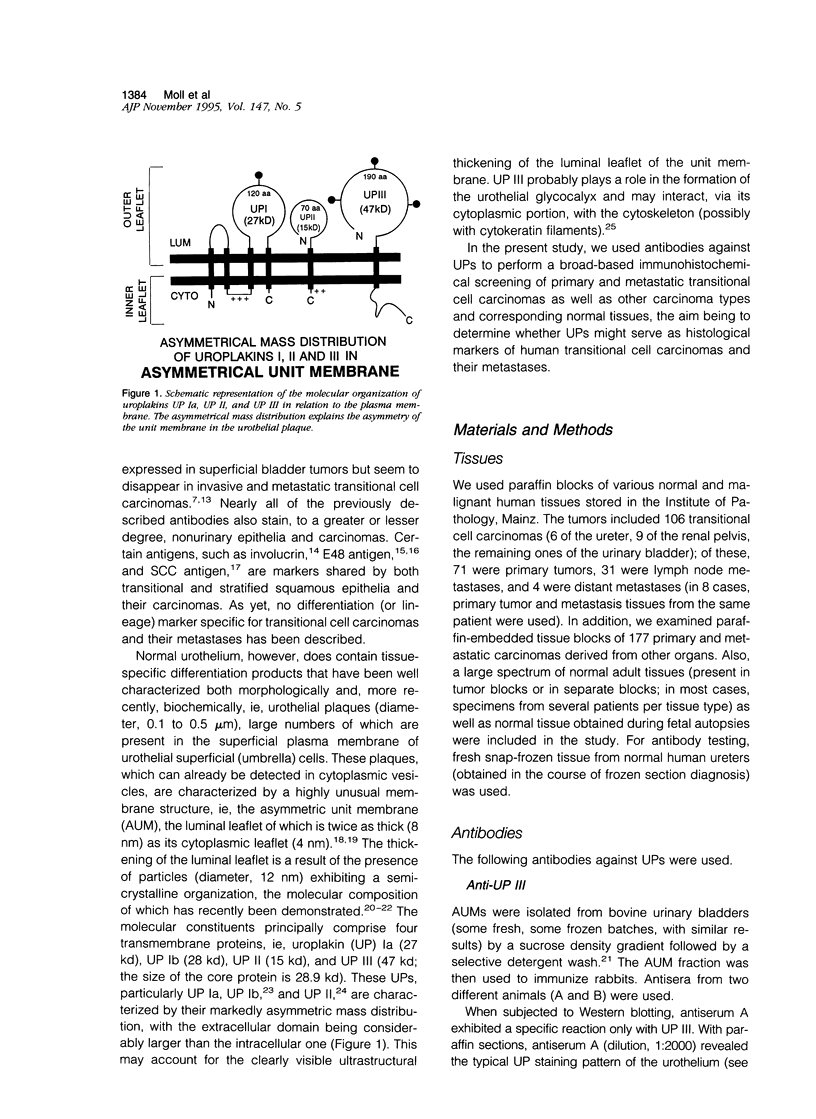
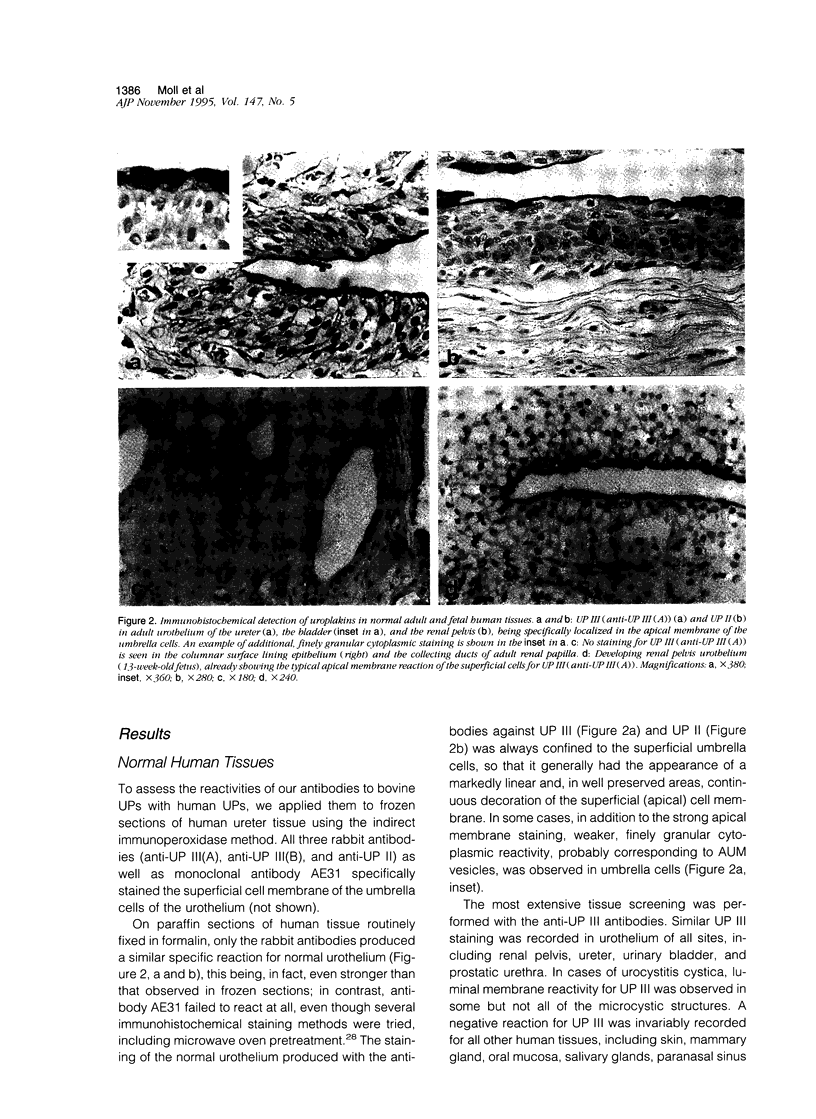
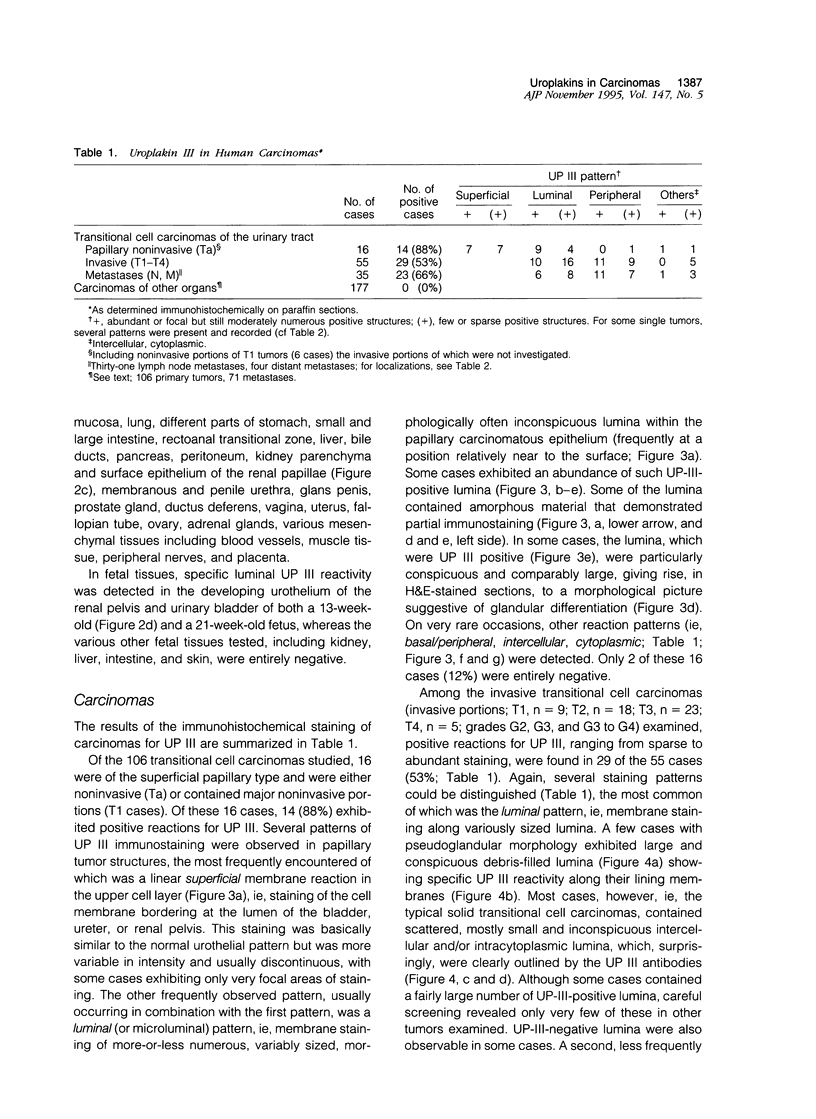
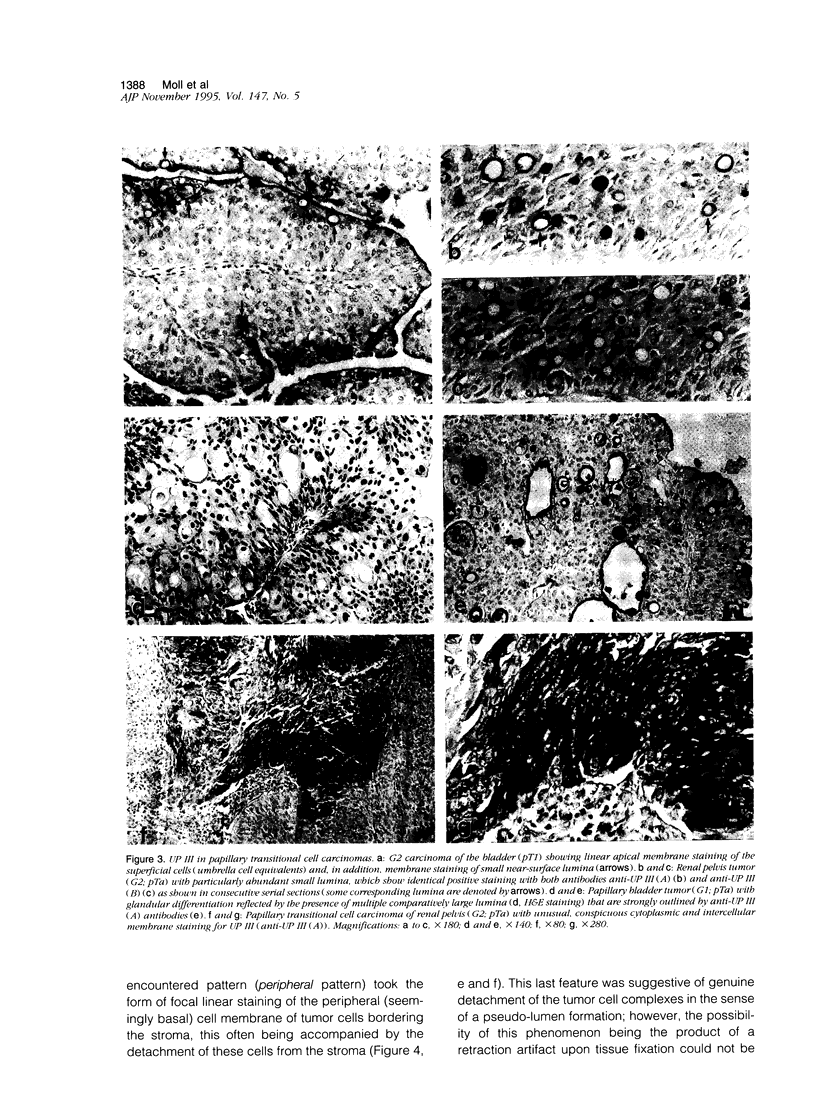
Uroplakins (UPs) Ia, Ib, II, and III, transmembrane proteins constituting the asymmetrical unit membrane of urothelial umbrella cells, are the first specific urothelial differentiation markers described. We investigated the presence and localization patterns of UPs in various human carcinomas by applying immunohistochemistry (avidin-biotin-peroxidase complex method), using rabbit antibodies against UPs II and III, to paraffin sections. Positive reactions for UP III (sometimes very focal) were noted in 14 of the 16 papillary noninvasive transitional cell carcinomas (TCCs) (88%), 29 of the 55 invasive TCCs (53%), and 23 of the 35 TCC metastases (66%). Different localization patterns of UPs could be distinguished, including superficial membrane staining like that found in normal umbrella cells (in papillary carcinoma), luminal (microluminal) membrane staining (in papillary and invasive carcinoma), and, against expectations, peripheral membrane staining (in invasive carcinoma). Non-TCC carcinomas of various origins (n = 177) were consistently negative for UPs. The presence of UPs in metastatic TCCs represents a prime example of even advanced tumor progression being compatible with the (focal) expression of highly specialized differentiation repertoires. Although of only medium-grade sensitivity, UPs do seem to be highly specific urothelial lineage markers, thus operating up interesting histodiagnostic possibilities in cases of carcinoma metastases of uncertain origin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroy J., Pauli B. U., Hayden J. E., Gould V. E. Intracytoplasmic lumina in bladder carcinomas. Hum Pathol. 1979 Sep;10(5):549–555. doi: 10.1016/s0046-8177(79)80098-2. [DOI] [PubMed] [Google Scholar]
- Alroy J., Pauli B. U., Weinstein R. S. Correlation between numbers of desmosomes and the aggressiveness of transitional cell carcinoma in human urinary bladder. Cancer. 1981 Jan 1;47(1):104–112. doi: 10.1002/1097-0142(19810101)47:1<104::aid-cncr2820470118>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Alroy J., Weinstein R. S. Intraepithelial asymmetric-unit-membrane plaques in mammalian urinary bladder. Anat Rec. 1980 May;197(1):75–83. doi: 10.1002/ar.1091970107. [DOI] [PubMed] [Google Scholar]
- Arndt R., Dürkopf H., Huland H., Donn F., Loening T., Kalthoff H. Monoclonal antibodies for characterization of the heterogeneity of normal and malignant transitional cells. J Urol. 1987 Apr;137(4):758–763. doi: 10.1016/s0022-5347(17)44205-4. [DOI] [PubMed] [Google Scholar]
- Battifora H. Intracytoplasmic lumina in breast carcinoma: a helpful histopathologic feature. Arch Pathol. 1975 Nov;99(11):614–617. [PubMed] [Google Scholar]
- Cordon-Cardo C., Wartinger D. D., Melamed M. R., Fair W., Fradet Y. Immunopathologic analysis of human urinary bladder cancer. Characterization of two new antigens associated with low-grade superficial bladder tumors. Am J Pathol. 1992 Feb;140(2):375–385. [PMC free article] [PubMed] [Google Scholar]
- Delladetsima J., Antonakopoulos G. N., Dapolla V., Kittas C. Intraepithelial lumina in urothelial bladder neoplasms. A histochemical, immunohistochemical and electron microscopy study. APMIS. 1989 May;97(5):406–412. [PubMed] [Google Scholar]
- Donhuijsen K., Schmidt U., Richter H. J., Leder L. D. Mucoid cytoplasmic inclusions in urothelial carcinomas. Hum Pathol. 1992 Aug;23(8):860–864. doi: 10.1016/0046-8177(92)90395-j. [DOI] [PubMed] [Google Scholar]
- Fenger C. The anal transitional zone. Acta Pathol Microbiol Immunol Scand Suppl. 1987;289:1–42. [PubMed] [Google Scholar]
- Fradet Y., Cordon-Cardo C., Whitmore W. F., Jr, Melamed M. R., Old L. J. Cell surface antigens of human bladder tumors: definition of tumor subsets by monoclonal antibodies and correlation with growth characteristics. Cancer Res. 1986 Oct;46(10):5183–5188. [PubMed] [Google Scholar]
- Franke W. W., Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation. 1987;36(2):145–163. doi: 10.1111/j.1432-0436.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Fulker M. J., Cooper E. H., Tanaka T. Proliferation and ultrastructure of papillary transitional cell carcinoma of the human bladder. Cancer. 1971 Jan;27(1):71–82. doi: 10.1002/1097-0142(197101)27:1<71::aid-cncr2820270112>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hicks R. M. The fine structure of the transitional epithelium of rat ureter. J Cell Biol. 1965 Jul;26(1):25–48. doi: 10.1083/jcb.26.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi A., Devonec M., Bouvier R., Escourrou G., Longin A., Perrin P., Revillard J. P. Phenotyping of 76 human bladder tumors with a panel of monoclonal antibodies: correlation between pathology, surface immunofluorescence and DNA content. Eur J Cancer Clin Oncol. 1989 May;25(5):777–783. doi: 10.1016/0277-5379(89)90120-x. [DOI] [PubMed] [Google Scholar]
- Howlett A. R., Hodges G. M., Rowlatt C. Epithelial-stromal interactions in the adult bladder: urothelial growth, differentiation, and maturation on culture facsimiles of bladder stroma. Dev Biol. 1986 Dec;118(2):403–415. doi: 10.1016/0012-1606(86)90011-4. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kanitakis J., Ramirez-Bosca A., Reano A., Viac J., Roche P., Thivolet J. Filaggrin expression in normal and pathological skin. A marker of keratinocyte differentiation. Virchows Arch A Pathol Anat Histopathol. 1988;412(4):375–382. doi: 10.1007/BF00750265. [DOI] [PubMed] [Google Scholar]
- Koss L. G. Some ultrastructural aspects of experimental and human carcinoma of the bladder. Cancer Res. 1977 Aug;37(8 Pt 2):2824–2835. [PubMed] [Google Scholar]
- Koss L. G. The asymmetric unit membranes of the epithelium of the urinary bladder of the rat. An electron microscopic study of a mechanism of epithelial maturation and function. Lab Invest. 1969 Aug;21(2):154–168. [PubMed] [Google Scholar]
- Kvedar J. C., Fewkes J., Baden H. P. Immunologic detection of markers of keratinocyte differentiation. Its use in neoplastic and preneoplastic lesions of skin. Arch Pathol Lab Med. 1986 Mar;110(3):183–188. [PubMed] [Google Scholar]
- Lin J. H., Wu X. R., Kreibich G., Sun T. T. Precursor sequence, processing, and urothelium-specific expression of a major 15-kDa protein subunit of asymmetric unit membrane. J Biol Chem. 1994 Jan 21;269(3):1775–1784. [PubMed] [Google Scholar]
- Lopez-Beltran A., Croghan G. A., Croghan I., Gaeta J. F. Cell and tumor markers' immunohistochemistry in transitional cell carcinoma of the bladder. Urol Int. 1993;50(2):61–64. doi: 10.1159/000282453. [DOI] [PubMed] [Google Scholar]
- Moll R., Löwe A., Laufer J., Franke W. W. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992 Feb;140(2):427–447. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Mitze M., Frixen U. H., Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993 Dec;143(6):1731–1742. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Schiller D. L., Franke W. W. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol. 1990 Aug;111(2):567–580. doi: 10.1083/jcb.111.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J., Antonakopoulos G. N. The fine structure of the human fetal urinary bladder. Development and maturation. A light, transmission and scanning electron microscopic study. J Anat. 1989 Oct;166:135–150. [PMC free article] [PubMed] [Google Scholar]
- Quak J. J., Balm A. J., van Dongen G. A., Brakkee J. G., Scheper R. J., Snow G. B., Meijer C. J. A 22-kd surface antigen detected by monoclonal antibody E 48 is exclusively expressed in stratified squamous and transitional epithelia. Am J Pathol. 1990 Jan;136(1):191–197. [PMC free article] [PubMed] [Google Scholar]
- Sarkis A. S., Dalbagni G., Cordon-Cardo C., Zhang Z. F., Sheinfeld J., Fair W. R., Herr H. W., Reuter V. E. Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: a marker for disease progression. J Natl Cancer Inst. 1993 Jan 6;85(1):53–59. doi: 10.1093/jnci/85.1.53. [DOI] [PubMed] [Google Scholar]
- Sato K., Moriyama M., Mori S., Saito M., Watanuki T., Terada K., Okuhara E., Akiyama T., Toyoshima K., Yamamoto T. An immunohistologic evaluation of C-erbB-2 gene product in patients with urinary bladder carcinoma. Cancer. 1992 Nov 15;70(10):2493–2498. doi: 10.1002/1097-0142(19921115)70:10<2493::aid-cncr2820701017>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sheinfeld J., Reuter V. E., Melamed M. R., Fair W. R., Morse M., Sogani P. C., Herr H. W., Whitmore W. F., Cordon-Cardo C. Enhanced bladder cancer detection with the Lewis X antigen as a marker of neoplastic transformation. J Urol. 1990 Feb;143(2):285–288. doi: 10.1016/s0022-5347(17)39935-4. [DOI] [PubMed] [Google Scholar]
- Smith A. F. An ultrastructural and morphometric study of bladder tumours (II). Virchows Arch A Pathol Anat Histol. 1982 Aug;396(3):291–301. doi: 10.1007/BF00431388. [DOI] [PubMed] [Google Scholar]
- Summerhayes I. C., McIlhinney R. A., Ponder B. A., Shearer R. J., Pocock R. D. Monoclonal antibodies raised against cell membrane components of human bladder tumor tissue recognizing subpopulations in normal urothelium. J Natl Cancer Inst. 1985 Dec;75(6):1025–1038. [PubMed] [Google Scholar]
- Takashi M., Murase T., Kinjo T., Mitsuya H., Nagura H. Epithelial membrane antigen as an immunohistochemical marker for transitional cell carcinoma of the urinary bladder. Urol Int. 1987;42(3):170–175. doi: 10.1159/000281888. [DOI] [PubMed] [Google Scholar]
- Torenbeek R., Blomjous C. E., Quak J. J., Ybema S., Meijer C. J. Use of monoclonal antibody E48 in diagnosing transitional cell carcinoma of urinary bladder. J Clin Pathol. 1992 Apr;45(4):303–307. doi: 10.1136/jcp.45.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T., Kumamoto Y., Ohmura K., Miyao N., Nammbu A., Takagi Y., Itoh N. Squamous cell carcinoma-associated antigen in uroepithelial carcinoma. Urology. 1992 Nov;40(5):477–483. doi: 10.1016/0090-4295(92)90470-h. [DOI] [PubMed] [Google Scholar]
- Walts A. E., Said J. W., Siegel M. B., Banks-Schlegel S. Involucrin, a marker of squamous and urothelial differentiation. An immunohistochemical study on its distribution in normal and neoplastic tissues. J Pathol. 1985 Apr;145(4):329–340. doi: 10.1002/path.1711450406. [DOI] [PubMed] [Google Scholar]
- Wu X. R., Lin J. H., Walz T., Häner M., Yu J., Aebi U., Sun T. T. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem. 1994 May 6;269(18):13716–13724. [PubMed] [Google Scholar]
- Wu X. R., Manabe M., Yu J., Sun T. T. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem. 1990 Nov 5;265(31):19170–19179. [PubMed] [Google Scholar]
- Wu X. R., Sun T. T. Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J Cell Sci. 1993 Sep;106(Pt 1):31–43. doi: 10.1242/jcs.106.1.31. [DOI] [PubMed] [Google Scholar]
- Yu J., Lin J. H., Wu X. R., Sun T. T. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol. 1994 Apr;125(1):171–182. doi: 10.1083/jcb.125.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Manabe M., Wu X. R., Xu C., Surya B., Sun T. T. Uroplakin I: a 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J Cell Biol. 1990 Sep;111(3):1207–1216. doi: 10.1083/jcb.111.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]