Abstract
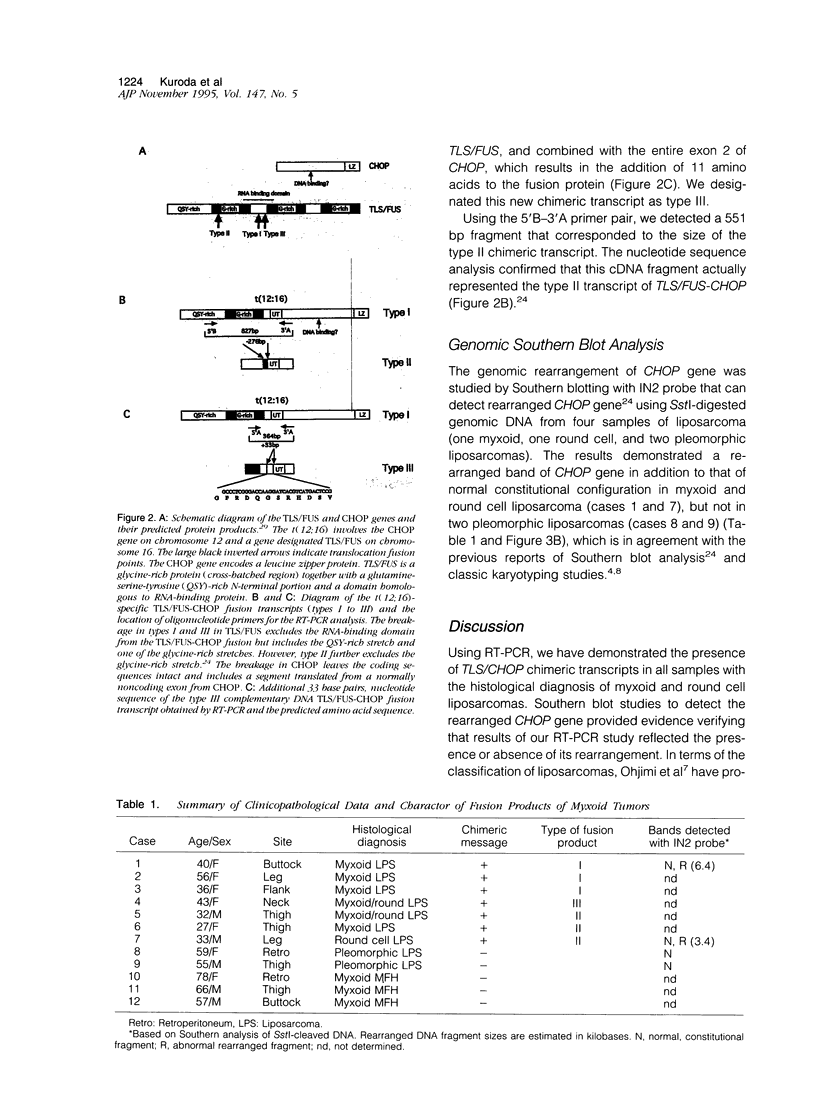
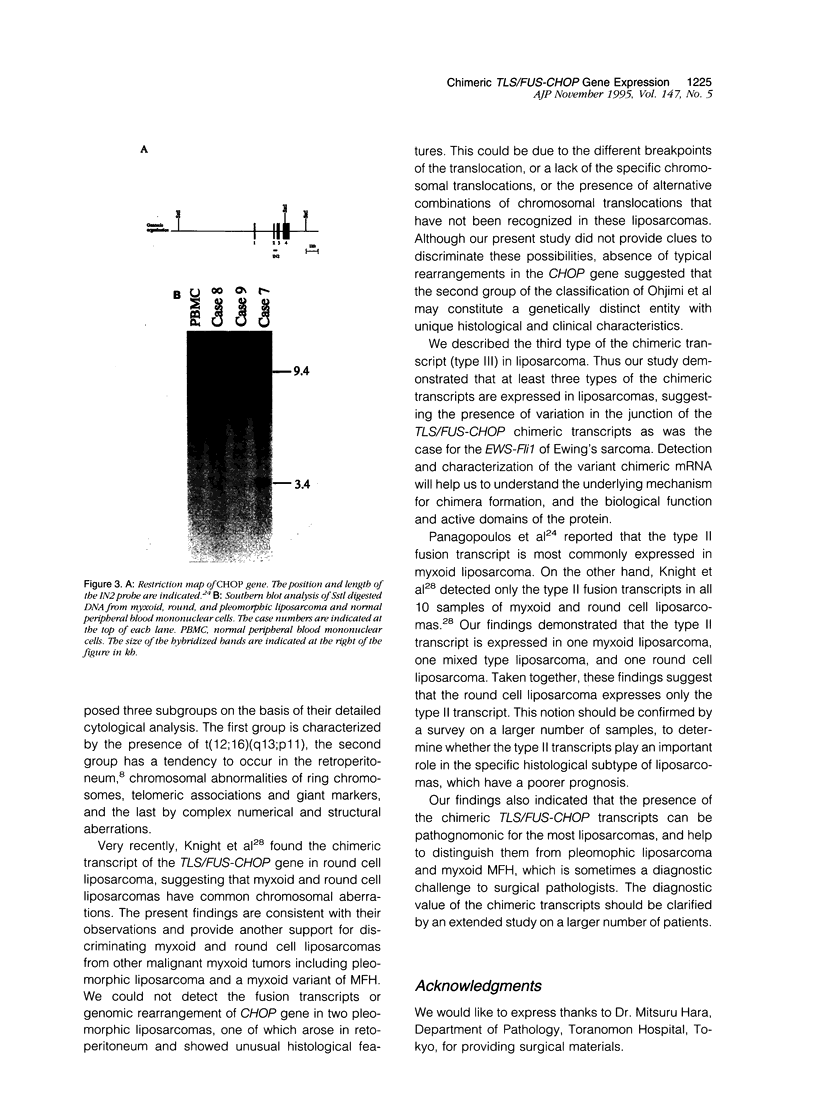
Myxoid liposarcomas have a unique and specific t(12;16)(q13;p11) chromosomal translocation. The breakpoint has recently been identified and shown to involve the TLS/FUS gene on chromosome 16 and the CHOP gene on chromosome 12. This translocation causes fusion of these genes resulting in the expression of a novel chimeric TLS/FUS-CHOP message. Using the polymerase chain reaction with primer sets derived from sequences of TLS/FUS and CHOP cDNAs, we could amplify three types of the fusion transcripts from seven of seven samples of myxoid and round cell liposarcomas. In six of the seven positive samples, two kinds of chimeric messenger RNAs were found that have been reported previously. However, the last sample had a novel chimeric message that had an extra sequence of 33 bp derived from the TLS/FUS gene. Thus, it was shown that these fusion transcripts had a varying extent of the sequence of TLS/FUS gene incorporated at the site of the fusion. However, the TLS/FUS-CHOP fusion transcripts were not detected in two pleomorphic liposarcomas or in three myxoid variants of malignant fibrous histiocytomas. Our findings indicate that in liposarcomas TLS/FUS-CHOP fusion transcripts have variations at the junction of chimeric messages, which was the case for Ewing's sarcoma. Detection of the chimeric message by reverse transcription polymerase chain reaction was also suggested to be a useful approach for the diagnosis of myxoid and round cell liposarcomas that have (12;16) translocation, and for distinguishing them from pleomorphic liposarcoma and myxoid variant of malignant fibrous histiocytomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aman P., Ron D., Mandahl N., Fioretos T., Heim S., Arheden K., Willén H., Rydholm A., Mitelman F. Rearrangement of the transcription factor gene CHOP in myxoid liposarcomas with t(12;16)(q13;p11). Genes Chromosomes Cancer. 1992 Nov;5(4):278–285. doi: 10.1002/gcc.2870050403. [DOI] [PubMed] [Google Scholar]
- Barone M. V., Crozat A., Tabaee A., Philipson L., Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994 Feb 15;8(4):453–464. doi: 10.1101/gad.8.4.453. [DOI] [PubMed] [Google Scholar]
- Clark J., Rocques P. J., Crew A. J., Gill S., Shipley J., Chan A. M., Gusterson B. A., Cooper C. S. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994 Aug;7(4):502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- Crozat A., Aman P., Mandahl N., Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993 Jun 17;363(6430):640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- Delattre O., Zucman J., Melot T., Garau X. S., Zucker J. M., Lenoir G. M., Ambros P. F., Sheer D., Turc-Carel C., Triche T. J. The Ewing family of tumors--a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994 Aug 4;331(5):294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992 Sep 10;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Downing J. R., Head D. R., Parham D. M., Douglass E. C., Hulshof M. G., Link M. P., Motroni T. A., Grier H. E., Curcio-Brint A. M., Shapiro D. N. Detection of the (11;22)(q24;q12) translocation of Ewing's sarcoma and peripheral neuroectodermal tumor by reverse transcription polymerase chain reaction. Am J Pathol. 1993 Nov;143(5):1294–1300. [PMC free article] [PubMed] [Google Scholar]
- Evans H. L. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979 Dec;3(6):507–523. doi: 10.1097/00000478-197912000-00004. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili N., Davis R. J., Fredericks W. J., Mukhopadhyay S., Rauscher F. J., 3rd, Emanuel B. S., Rovera G., Barr F. G. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993 Nov;5(3):230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- Giovannini M., Biegel J. A., Serra M., Wang J. Y., Wei Y. H., Nycum L., Emanuel B. S., Evans G. A. EWS-erg and EWS-Fli1 fusion transcripts in Ewing's sarcoma and primitive neuroectodermal tumors with variant translocations. J Clin Invest. 1994 Aug;94(2):489–496. doi: 10.1172/JCI117360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakousis C. P., Dal Cin P., Turc-Carel C., Limon J., Sandberg A. A. Chromosomal changes in soft-tissue sarcomas. A new diagnostic parameter. Arch Surg. 1987 Nov;122(11):1257–1260. doi: 10.1001/archsurg.1987.01400230043007. [DOI] [PubMed] [Google Scholar]
- Knight J. C., Renwick P. J., Dal Cin P., Van den Berghe H., Fletcher C. D. Translocation t(12;16)(q13;p11) in myxoid liposarcoma and round cell liposarcoma: molecular and cytogenetic analysis. Cancer Res. 1995 Jan 1;55(1):24–27. [PubMed] [Google Scholar]
- Mrózek K., Karakousis C. P., Bloomfield C. D. Chromosome 12 breakpoints are cytogenetically different in benign and malignant lipogenic tumors: localization of breakpoints in lipoma to 12q15 and in myxoid liposarcoma to 12q13.3. Cancer Res. 1993 Apr 1;53(7):1670–1675. [PubMed] [Google Scholar]
- Ohjimi Y., Iwasaki H., Kaneko Y., Ishiguro M., Ohgami A., Fujita C., Shinohara N., Yoshitake K., Kikuchi M. Chromosome abnormalities in liposarcomas. Cancer Genet Cytogenet. 1992 Dec;64(2):111–117. doi: 10.1016/0165-4608(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Panagopoulos I., Mandahl N., Ron D., Höglund M., Nilbert M., Mertens F., Mitelman F., Aman P. Characterization of the CHOP breakpoints and fusion transcripts in myxoid liposarcomas with the 12;16 translocation. Cancer Res. 1994 Dec 15;54(24):6500–6503. [PubMed] [Google Scholar]
- Rabbitts T. H. Chromosomal translocations in human cancer. Nature. 1994 Nov 10;372(6502):143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Larson R., Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet. 1993 Jun;4(2):175–180. doi: 10.1038/ng0693-175. [DOI] [PubMed] [Google Scholar]
- Ron D., Habener J. F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992 Mar;6(3):439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- Sorensen P. H., Lessnick S. L., Lopez-Terrada D., Liu X. F., Triche T. J., Denny C. T. A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet. 1994 Feb;6(2):146–151. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- Sreekantaiah C., Karakousis C. P., Leong S. P., Sandberg A. A. Cytogenetic findings in liposarcoma correlate with histopathologic subtypes. Cancer. 1992 May 15;69(10):2484–2495. doi: 10.1002/1097-0142(19920515)69:10<2484::aid-cncr2820691017>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Sreekantaiah C., Ladanyi M., Rodriguez E., Chaganti R. S. Chromosomal aberrations in soft tissue tumors. Relevance to diagnosis, classification, and molecular mechanisms. Am J Pathol. 1994 Jun;144(6):1121–1134. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-García I., Rabbitts T. H. Transcriptional activation by TAL1 and FUS-CHOP proteins expressed in acute malignancies as a result of chromosomal abnormalities. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7869–7873. doi: 10.1073/pnas.91.17.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turc-Carel C., Limon J., Dal Cin P., Rao U., Karakousis C., Sandberg A. A. Cytogenetic studies of adipose tissue tumors. II. Recurrent reciprocal translocation t(12;16)(q13;p11) in myxoid liposarcomas. Cancer Genet Cytogenet. 1986 Dec;23(4):291–299. doi: 10.1016/0165-4608(86)90011-7. [DOI] [PubMed] [Google Scholar]
- Zucman J., Delattre O., Desmaze C., Epstein A. L., Stenman G., Speleman F., Fletchers C. D., Aurias A., Thomas G. EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet. 1993 Aug;4(4):341–345. doi: 10.1038/ng0893-341. [DOI] [PubMed] [Google Scholar]
- Zucman J., Melot T., Desmaze C., Ghysdael J., Plougastel B., Peter M., Zucker J. M., Triche T. J., Sheer D., Turc-Carel C. Combinatorial generation of variable fusion proteins in the Ewing family of tumours. EMBO J. 1993 Dec;12(12):4481–4487. doi: 10.1002/j.1460-2075.1993.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]