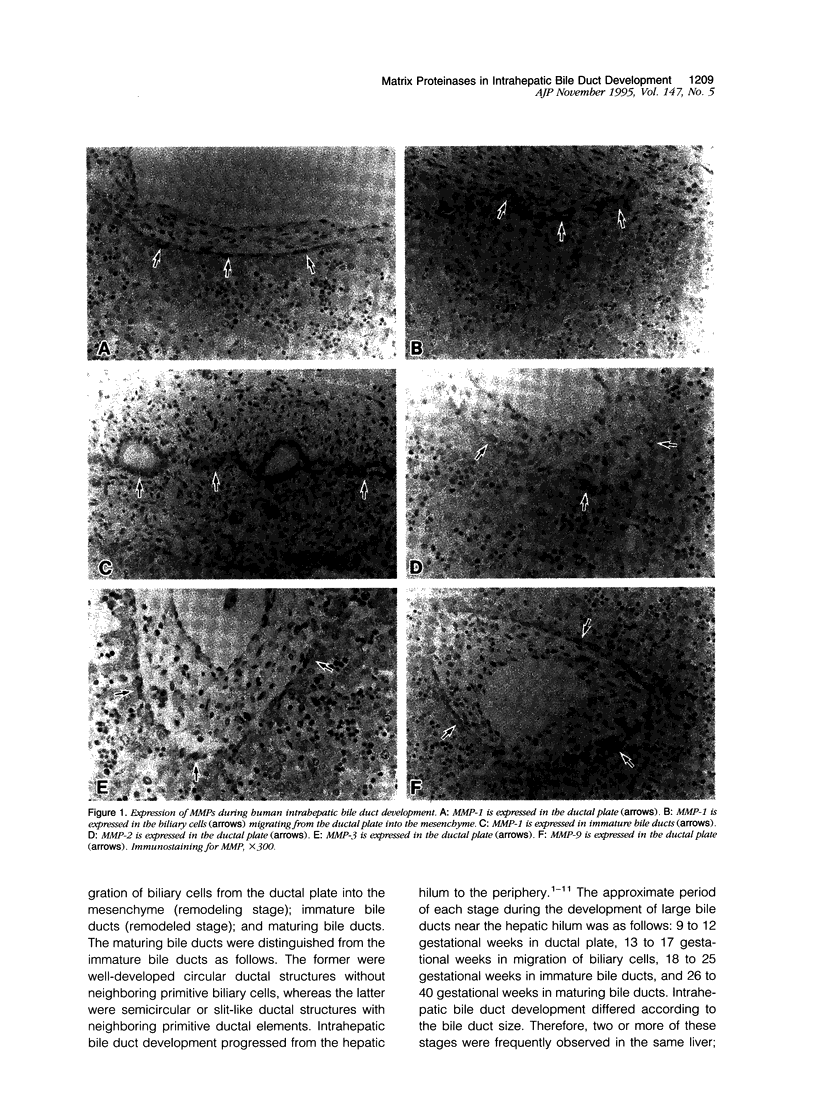
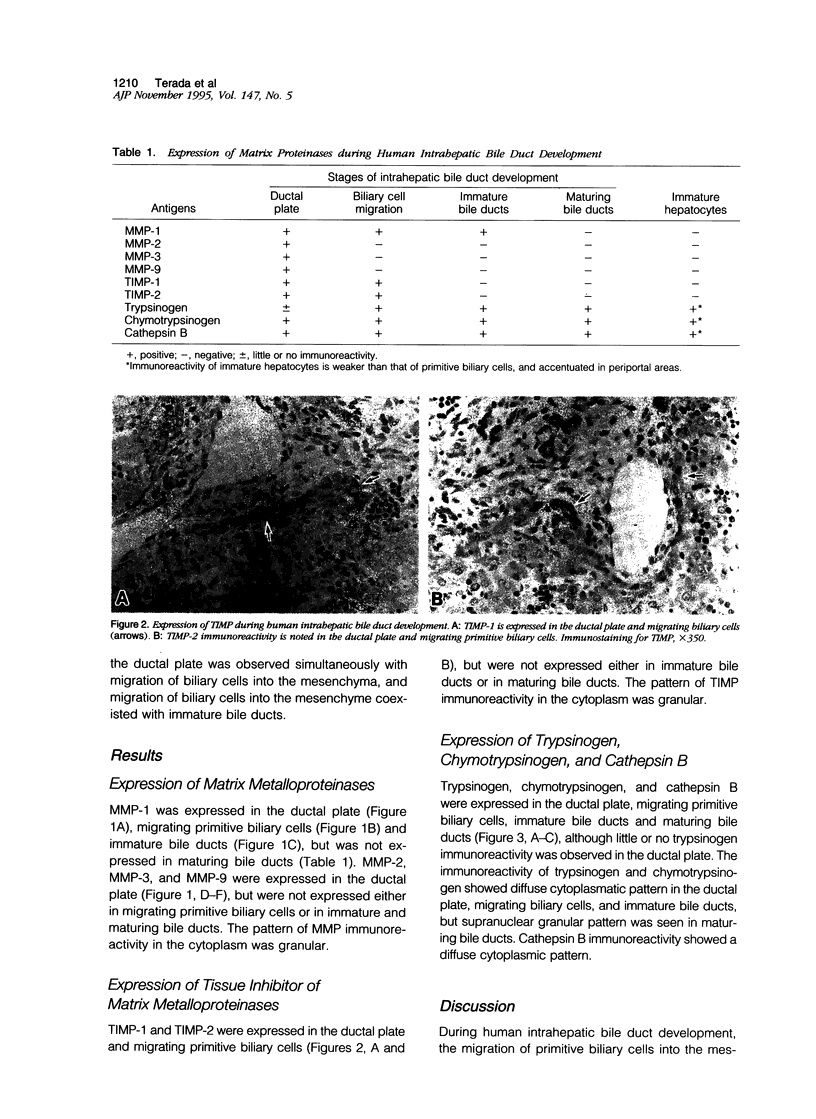
Abstract
Primitive biliary cells are known to migrate from the ductal plate into the mesenchyme during human intrahepatic bile duct development, and this migration process is essential for normal development of intrahepatic bile ducts. However, its molecular mechanism is unknown. Matrix proteinases play an important role in cell migration during cancer invasion and organ development. In this study, we therefore investigated in situ expression of matrix metalloproteinases (MMP) and tissue inhibitors of MMP (TIMP) during human intrahepatic bile duct development, using 32 human fetal livers. We also examined in situ expression of trypsinogen/trypsin, chymotrypsinogen/chymotrypsin, and cathepsin B, which are matrix proteinases and activators of MMP. MMP-1 expression was noted in the ductal plate and migrating primitive biliary cells. MMP-2, MMP-3, and MMP-9 were expressed in the ductal plate. TIMP-1 and TIMP-2 were expressed in the ductal plate and migrating primitive biliary cells. Trypsinogen/trypsin, chymotrypsinogen/chymotrypsin, and cathepsin B were also expressed in primitive biliary cells. These data suggest that MMP, trypsinogen/trypsin, chymotrypsinogen/chymotrypsin, and cathepsin B play a critical role in biliary cell migration during human intrahepatic bile duct development by degrading extracellular matrix proteins. The data also suggest that MMP inhibitors (TIMP-1 and TIMP-2) and MMP activators (trypsin, chymotrypsin, and cathepsin B) play an important role in biliary cell migration. The coordinated expression of MMP, MMP inhibitors, and MMP activators may be necessary for the normal development of human intrahepatic bile ducts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Desmet V. J. Congenital diseases of intrahepatic bile ducts: variations on the theme "ductal plate malformation". Hepatology. 1992 Oct;16(4):1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- Desmet V. J. Intrahepatic bile ducts under the lens. J Hepatol. 1985;1(5):545–559. doi: 10.1016/s0168-8278(85)80752-2. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM L. M., HIRSHKOWITZ A., SHOICHET I. The activation of trypsinogen by cathepsin B. J Biol Chem. 1959 Nov;234:2885–2890. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kodama S., Iwata K., Iwata H., Yamashita K., Hayakawa T. Rapid one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases. An application for rheumatoid arthritis serum and plasma. J Immunol Methods. 1990 Feb 20;127(1):103–108. doi: 10.1016/0022-1759(90)90345-v. [DOI] [PubMed] [Google Scholar]
- Koivunen E., Huhtala M. L., Stenman U. H. Human ovarian tumor-associated trypsin. Its purification and characterization from mucinous cyst fluid and identification as an activator of pro-urokinase. J Biol Chem. 1989 Aug 25;264(24):14095–14099. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Rifkin D. B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993 Jan;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Grappone C., Heinrichs O. E. Cellular sources of extracellular matrix proteins in normal and fibrotic liver. Studies of gene expression by in situ hybridization. J Hepatol. 1995;22(2 Suppl):71–76. [PubMed] [Google Scholar]
- Ohta T., Terada T., Nagakawa T., Tajima H., Itoh H., Fonseca L., Miyazaki I. Pancreatic trypsinogen and cathepsin B in human pancreatic carcinomas and associated metastatic lesions. Br J Cancer. 1994 Jan;69(1):152–156. doi: 10.1038/bjc.1994.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Gonoji Y., Naka K., Tomita K., Nakanishi I., Iwata K., Yamashita K., Hayakawa T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992 Oct 25;267(30):21712–21719. [PubMed] [Google Scholar]
- Okada Y., Harris E. D., Jr, Nagase H. The precursor of a metalloendopeptidase from human rheumatoid synovial fibroblasts. Purification and mechanisms of activation by endopeptidases and 4-aminophenylmercuric acetate. Biochem J. 1988 Sep 15;254(3):731–741. doi: 10.1042/bj2540731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Naka K., Kawamura K., Matsumoto T., Nakanishi I., Fujimoto N., Sato H., Seiki M. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: implications for bone resorption. Lab Invest. 1995 Mar;72(3):311–322. [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Munaut C., Thesleff I., Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994 Mar;124(6):1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H. Activation of pancreatic zymogens. Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Dig Dis Sci. 1986 Mar;31(3):314–321. doi: 10.1007/BF01318124. [DOI] [PubMed] [Google Scholar]
- Sasaguri Y., Murahashi N., Sugama K., Kato S., Hiraoka K., Satoh T., Isomoto H., Morimatsu M. Development-related changes in matrix metalloproteinase expression in human aortic smooth muscle cells. Lab Invest. 1994 Aug;71(2):261–269. [PubMed] [Google Scholar]
- Shah K. D., Gerber M. A. Development of intrahepatic bile ducts in humans. Immunohistochemical study using monoclonal cytokeratin antibodies. Arch Pathol Lab Med. 1989 Oct;113(10):1135–1138. [PubMed] [Google Scholar]
- Shah K. D., Gerber M. A. Development of intrahepatic bile ducts in humans. Possible role of laminin. Arch Pathol Lab Med. 1990 Jun;114(6):597–600. [PubMed] [Google Scholar]
- Sloane B. F. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990 Apr;1(2):137–152. [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V. Cysteine proteinases and metastasis. Cancer Metastasis Rev. 1984;3(3):249–263. doi: 10.1007/BF00048388. [DOI] [PubMed] [Google Scholar]
- Terada T., Morita T., Hoso M., Nakanuma Y. Pancreatic enzymes in the epithelium of intrahepatic large bile ducts and in hepatic bile in patients with extrahepatic bile duct obstruction. J Clin Pathol. 1994 Oct;47(10):924–927. doi: 10.1136/jcp.47.10.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T., Nakanuma Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol. 1995 Jan;146(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Terada T., Nakanuma Y. Development of human intrahepatic peribiliary glands. Histological, keratin immunohistochemical, and mucus histochemical analyses. Lab Invest. 1993 Mar;68(3):261–269. [PubMed] [Google Scholar]
- Terada T., Nakanuma Y. Expression of pancreatic enzymes (alpha-amylase, trypsinogen, and lipase) during human liver development and maturation. Gastroenterology. 1995 Apr;108(4):1236–1245. doi: 10.1016/0016-5085(95)90225-2. [DOI] [PubMed] [Google Scholar]
- Terada T., Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology. 1994 Aug;25(2):143–150. doi: 10.1111/j.1365-2559.1994.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Terada T., Nakanuma Y. Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation: a lectin-histochemical and immunohistochemical study. Hepatology. 1994 Aug;20(2):388–397. [PubMed] [Google Scholar]
- Terada T., Ohta T., Minato H., Nakanuma Y. Expression of pancreatic trypsinogen/trypsin and cathepsin B in human cholangiocarcinomas and hepatocellular carcinomas. Hum Pathol. 1995 Jul;26(7):746–752. doi: 10.1016/0046-8177(95)90222-8. [DOI] [PubMed] [Google Scholar]
- Terada T., Ohta T., Nakanuma Y. Expression of transforming growth factor-alpha and its receptor during human liver development and maturation. Virchows Arch. 1994;424(6):669–675. doi: 10.1007/BF00195783. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Callea F., Van der Steen K., Moerman P., Desmet V. J. The development of the intrahepatic bile ducts in man: a keratin-immunohistochemical study. Hepatology. 1988 Nov-Dec;8(6):1586–1595. doi: 10.1002/hep.1840080619. [DOI] [PubMed] [Google Scholar]